Abstract
Autoimmune diseases are characterized by tissue damage and loss of function due to an immune response that is directed against specific organs. This review is focused on celiac disease (CD), an autoimmune enteropathy, and type 1 diabetes (T1D), a hyperglycosaemia caused by a destructive autoimmune process targeting the insulin-producing pancreatic islet cells. Even if environmental factors and genetic susceptibility are clearly involved in the pathogenesis of autoimmunity, for most autoimmune disorders there is no or little knowledge about the causing agent or genetic makeup underlying the disease. In this respect, CD represents a unique autoimmune disorder because a close genetic association with HLA-DQ2 or HLA-DQ8 haplotypes and, more importantly, the environmental trigger (the gliadin fraction of gluten-containing grains wheat, barley, and rye) are known. Conversely, the trigger for autoimmune destruction of pancreatic ß cells in T1D is unclear. Interestingly, recent data suggest that gliadin is also involved in the pathogenesis of T1D. There is growing evidence that increased intestinal permeability plays a pathogenic role in various autoimmune diseases including CD and T1D. Therefore, we hypothesize that besides genetic and environmental factors, loss of intestinal barrier function is necessary to develop autoimmunity. In this review, each of these components will be briefly reviewed.
Keywords: tight junctions, intestinal permeability, zonulin, celiac disease, type 1 diabetes, gliadin
Introduction
It is generally accepted that the interplay between environmental factors and specific susceptibility genes underlies the aberrant immune response responsible for the onset of autoimmune diseases. Fewer than 10% of those individuals with an increased genetic susceptibility actually develop clinical disease. This suggests a strong environmental trigger in the preautoimmune process. Environmental factors are also likely to affect the outcome of the process and the rate of progression to disease in those individuals who develop autoimmunity. One of the current theories is that intestinal luminal antigens absorbed through the gut may be involved. The intestinal epithelium is the largest mucosal surface and provides an interface between the external environment and the mammalian host. Healthy, mature gut mucosa with their intact tight junctions (TJ) serve as the main barrier to the passage of macromolecules (Fig. 1). In a physiological state, quantitatively small but immunologically active antigens may cross the mucosal barrier. These antigens are absorbed across the mucosa via two functional pathways. The vast majority of absorbed proteins (up to 90%) cross the intestinal barrier through the transcellular pathway followed by lysosomal degradation that converts the proteins into smaller, nonimmunogenic peptides. The remaining portion of proteins is transported as intact proteins, resulting in antigen-specific immune responses. This latter phenomenon utilizes the Microfold (M) cell pathway or the paracellular pathway, which involves a subtle but sophisticated regulation of intercellular TJ that leads to antigenic tolerance.1,2 When the integrity of the gut barrier is compromised (TJ disassembly), as is seen during prematurity or exposure to radiation, chemotherapy and/or toxins, an immune response to environmental antigens that crossed the gut mucosa may develop, leading to autoimmune diseases or food allergies.2,3 The cells that play a key role in this immune response lie in close proximity to the intestinal epithelial barrier.4,5 Another critical factor for intestinal immunological responsiveness is the human leukocyte antigen (HLA) system. HLA class I and II genes encode antigen presenting cell (APC) glycoprotein receptors that present antigens to T cells in the intestinal mucosa.6-8 Susceptibility to at least 50 diseases, including celiac disease (CD) and type 1 diabetes (T1D), has been associated with specific HLA class I or class II alleles. A common denominator of these diseases is the presence of several preexisting conditions that lead to an autoimmune process. The first is a genetic susceptibility for the host immune system to recognize, and potentially misinterpret, an environmental antigen presented within the gastrointestinal tract. Second, the host must be exposed to the antigen. Finally, the antigen must be presented to the gastrointestinal mucosal immune system following its M-cell passage or paracellular passage (normally prevented by TJ competency) from the intestinal lumen to access the gut submucosa.2,3,9-11 In all cases, increased permeability precedes disease and causes an abnormality in antigen delivery that triggers immune events, eventually leading to a multiorgan process and autoimmunity.1 We therefore hypothesize that genes, environment, and loss of intestinal barrier function are all necessary to develop autoimmunity, especially CD and T1D.
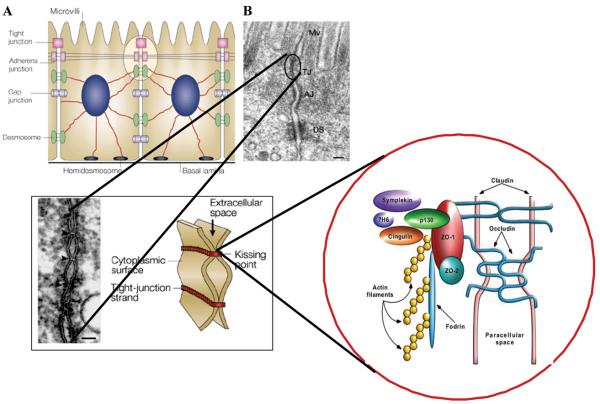
Figure 1.
Macroscopic arrangement (A) and microscopic composition (B) of intercellular tight junctions. The structural components of intercellular tight junctions can be classified as integral membrane proteins (occludin, claudins, and JAM), junctional complex proteins (ZO-1, ZO-2, p130 or ZO-3, 7H6, Symplekin, and cingulin), and cell cytoskeleton structures (microtubules, intermediate filaments and microfilaments).
Genetic Component of Autoimmunity
Celiac Disease
The complex interplay between genetic and environmental factors leading to the lack (or loss) of gluten tolerance is still poorly understood. Prevalence studies performed in Europe12 and our recent data generated in the U.S.A.13 both demonstrate a higher prevalence of CD among asymptomatic first-degree relatives of CD patients than among the general population. Of 24 reported pairs of identical twins with CD, 75% were concordant for disease.14 Nonetheless, it appears that there are instances of discordance for CD among monozygotic twins. If it is assumed that twins ingest similar dietary grains, such discordance suggests the importance of environmental factors in disease expression. Class II HLA genotypes DQ2 and DQ8 play a major predisposing role, being found in almost 100% of patients. HLA class II genes are highly polymorphic, with the majority of polymorphisms encoding the regions of the molecule that constitute the sides and floor of the peptide binding groove. Different combinations of HLA-DQ haplotypes influence the risk of disease, which is much higher in subjects showing a double copy of the DQB * 02 genes (DQ2/DQ2 and DQ2/DR7) than the DQ8 (DR4)/X genotype.15,16 Inheritance of specific HLA-DQ genotypes explains 40% of the genetic predisposition to CD, while the remaining 60% is related to a complex, still-undefined mosaic of non-HLA genes, each of which adds a small contribution to the risk of CD development.
For instance, recent reports have described non-HLA genes encoding for proteins involved in the regulation of intestinal permeability to be associated with susceptibility for CD. Polymorphisms in the MYO9B gene were shown to be associated with increased susceptibility for gluten-sensitive enteropathy (GSE) in a Dutch cohort of GSE patients,17 and more recently, the TJ-related genes PARD3 and MAGI2 were also shown to be genetically associated with CD.18
Type 1 Diabetes
The first diabetes susceptibility genes to be identified were the HLA genes, located on chromosome 6p21.19 Subsequent studies demonstrated an association between the disease and the insulin gene region on chromosome 11p. In the mid- to late 1990s, high throughput screening of the entire human genome in families with two or more affected siblings was used to identify additional chromosomal regions that may contain susceptibility genes for T1D.19 Over 20 loci showed evidence for linkage with the disease in different data sets. All of the studies consistently reported linkage to the HLA gene region (designated IDDM1). Several genome screens, in combination with family-based association studies, also supported a role for the insulin gene region (designated IDDM2) in disease susceptibility.19 Linkage to eight additional loci was replicated in independent data sets: IDDM4 (chromosome 11q13), IDDM5 (chromosome 6q25), IDDM7 (chromosome 2q31), IDDM8 (chromosome 6q27), IDDM10 (chromosome 10p11–q11), IDDM12 (chromosome 2q33), IDDM13 (chromosome 2q35), and IDDM15 (chromosome 6q21). In addition, the locus-designated IDDM6 (chromosome 18q21) showed consistent evidence for association with the disease in family studies.19 Although the chromosomal locations of these loci are known, the precise identity of the susceptibility genes in these regions remains to be determined. The genome screens confirmed that, as for CD, the HLA gene region (IDDM1 locus) is the major genetic determinant of disease risk, accounting for 42% of the familial inheritance of T1D.19 The IDDM2 locus (the insulin gene region) contributes an additional 10% of genetic susceptibility.
Interestingly, also polymorphisms in the MYO9B gene were shown to be associated with susceptibility for T1D.20 This observation indicates that the susceptibility for the development of both CD and T1D is related to genes involved with intestinal permeability.
The role of the intestinal barrier in the development of CD and T1D will be discussed below.
Gliadin as an Environmental Component of Autoimmunity
Celiac Disease
Gluten is the environmental factor that triggers CD. It is the gliadin fraction of wheat gluten and similar alcohol-soluble proteins in other grains (collectively known as prolamins) that are associated with the development of intestinal damage. A common feature of the prolamins of wheat, rye, and barley is a high content of glutamine (>30%) and proline (>15%), whereas the nontoxic prolamins of rice and corn have lower glutamine and proline content. However, the environmental component influencing CD development is complex and still unclear. Some aspects of gluten intake may influence the risk of CD occurrence, particularly (1) the amount of ingested gluten (the higher the amount, the higher the risk); (2) the quality of ingested gluten (some grains contain more toxic epitopes than others); and (3) the pattern/timing of infant feeding. Recent studies suggest that the pattern of infant nutrition may have a critical role on the development of CD and other autoimmune disorders. Breastfeeding is thought to delay or reduce the risk of developing CD.21 The positive effects of breast milk can be attributed, at least in part, to its influence on the microbial colonization process of the newborn intestine. The genus Bifidobacterium is predominant in feces of breast-fed infants, while a larger variety of bacterial groups (Bacteroides, Streptococcus, Clostridium, etc.) integrate the fecal microbiota of formula-fed infants. Changes in the composition of the intestinal microbiota also occur as a consequence of the subsequent shifts from breastfeeding or formula feeding to weaning and the introduction of solid food. Alterations in the intestinal balance between beneficial and potentially harmful bacteria have also been associated with allergy, type 1 diabetes40 and inflammatory bowel diseases.22-24
Type 1 Diabetes
It is believed that genetically predisposed individuals develop T1D after encountering one or more environmental triggers of the disease.25 Rapid advances could be made in disease prevention and treatment if these environmental triggers were identified. Among others, gliadin has recently been the object of a series of studies that aim at establishing its role in the pathogenesis of T1D. Early introduction of gliadin-containing cereals was recently reported to increase the risk of islet cell autoimmunity in humans.26,27 Gliadin-specific, lamina propria–derived T cells play an important role in the pathogenesis of CD.28 The same HLA class II haplotype, DQ (α 1 * 0501, ß1 * 0201), that is associated with gliadin peptides in CD is also one of two HLA class II haplotypes inherited most frequently by people with T1D.29 There is also evidence of immunological activity in the small intestine of T1D patients: jejunal specimens from T1D patients have been found to contain significantly greater concentrations of interferon gamma (IFNγ)- and tumor necrosis factor-alpha (TNF-α) positive cells than those of healthy controls, suggesting an inflammatory response.30 A second study found significantly greater expression of HLA-DR and HLA-DP molecules on intestinal villi of jejunal specimens from T1D patients than in specimens from healthy controls.31 A more recent report confirmed these findings by studying the mucosal immune response to gliadin in the jejunum of patients with T1D.32 Small intestinal biopsies from children with T1D were cultured with gliadin and examined for epithelial infiltration and lamina propria T-cell activation. The density of intraepithelial CD3+ cells and of lamina propria CD25+ mononuclear cells was higher in jejunal biopsies from T1D patients versus control subjects. In the patients' biopsies cultured with enzymatically treated gliadin, there was epithelial infiltration by CD3 cells, a significant increase in lamina propria CD25+ and CD80+ cells, enhanced expression of lamina propria cells positive for ligand and receptor molecules α4/β7 and ICAM 1, and increased expression of CD54 and crypt HLA-DR.33 Also, α4 positive T cells were recovered from the pancreatic islets of a T1D patient,34 providing a direct link between gliadin-activated T cells and destruction of pancreatic islet cells.
Findings from studies using non obese diabetic (NOD) mice and the BioBreeding diabetes-prone (BBDP) rats have implicated wheat gliadin as a dietary diabetogen (Visser et al., unpublished results).35,36 In BBDP rats, gliadin exposure is accompanied by increased intestinal permeability,37 and changes in gut microflora composition (Visser, unpublished data) (Fig. 2) presumably allow food antigens to come in contact with the underlying lamina propria. Feeding NOD mice and BBDP rats a gluten-free hydrolysed casein diet resulted in a delay and reduction of T1D development.35,38-40 Interestingly, these animal models of T1D also showed that the moment of exposure to wheat proteins is important for the development of T1D. Delaying the exposure to diabetogenic wheat proteins by prolonging the breastfeeding period reduced T1D development in the BBDP rats.41 Furthermore, exposing neonatal rats or mice to diabetogenic wheat components or bacterial antigens reduced T1D incidence, which is probably due to the induction of immunological tolerance.36,39,42
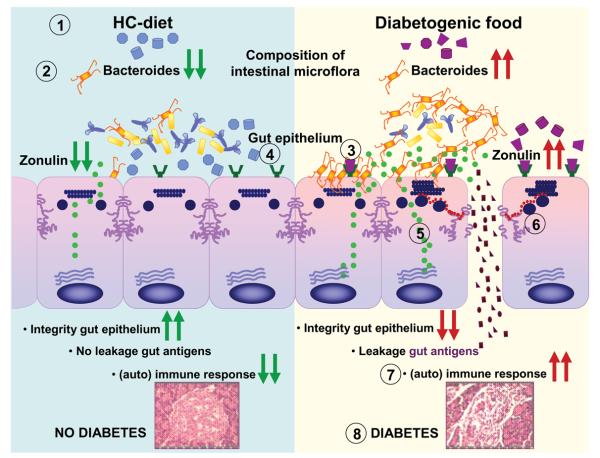
Figure 2.
Postulated mechanism of action of gluten in T1D pathogenesis. Diet affects the composition of the intestinal microflora. A hydrolyzed casein (gluten-free) diet reduces the number of Bacteroides species within the microflora, while the diabetogenic (gluten-containing) diet favors a high titer of Bacteroides [1]. Colonization of the gut with an imbalanced microflora in which the Bacteroides species is favored over other species, such as Bifidobacterium and Lactobacillus, activates the zonulin pathway [2]. In parallel, gliadin, a component of the grain protein gluten, binds to the chemokine receptor CXCR3 (expressed on intestinal epithelial cells) and induces a MyD88-dependent activation of the zonulin pathway [3]. A hydrolyzed casein diet prevents activation of the CXCR3-zonulin pathway [4]. Activation of the zonulin pathway leads to increased zonulin release [5]. The released zonulin binds to zonulin receptors on the surface of the intestinal epithelium and causes disassembly of TJs by causing changes in TJ dynamics, including phosphorylation of occludin and ZO-1, changes in occludin-ZO-1 and ZO-1-myosin IB protein–protein interaction, and actin polymerization [6]. The disassembly of TJs leads to the impairment of the barrier function, leading to increased passage of luminal antigens in the lamina propria where they are taken up and processed by mucosal antigen-presenting cells and presented to T cells [7]. The cascade of immune events eventually leads to autoimmune disease [8].
Rats that were fed wheat protein–based diets develop T1D and display a mild celiac-like enteropathy.34 Mesenteric lymph nodes (MLNs), which drain the gut, are the major inductive site where dietary antigens are recognized in the gut-associated lymphoid tissue. The authors described an increase in the expression ratio of T-bet:Gata3, master transcription factors for Th1 and Th2 cytokines, respectively, in the MLN from wheat-fed BBDP rats compared with that from BBDR rats, mainly due to decreased Gata3 expression.34 CD3+CD4+IFNγ+ T cells were more prevalent in the MLN of wheat-fed BBDP rats, but remained at control levels in BBDP rats fed a diabetes-retardant wheat-free diet. BBDP MLN cells proliferated in response to wheat protein antigens in a specific, dose-dependent manner, and >93% of cells were CD3+CD4+ T cells. This proliferation was associated with a low proportion of CD4+CD25+ T cells and a high proportion of dendritic cells in the MLN of BBDP rats.34 These results suggest that, before insulitis is established, the MLNs of wheat-fed BBDP rats contain an unusually high proportion of Th1 cells that proliferate specifically in response to wheat protein antigens. Collectively, these studies suggest a deranged mucosal immune response to gliadin in T1D and a direct link between gliadin-induced activation of gut mucosal T cells and insult of pancreatic islet cells (Fig. 2).
Intestinal Barrier Component of Autoimmunity
The Zonulin System
In recent years, much has been discovered about the structure, function and regulation of TJ (Fig. 1). However, the precise mechanism(s) by which they operate is still not completely understood. The discovery of zonula occludens toxin (Zot), an enterotoxin elaborated by Vibrio cholerae that reversibly opens the TJ, increased our understanding of the intricate mechanisms that regulate the intestinal epithelial paracellular pathway. Zot action is mediated through a cascade of intracellular events that lead to PKCα-dependent polymerization of actin microfilaments that are strategically localized to regulate the paracellular pathway.43 By using immunofluorescence binding studies, we found that Zot binding varies within the intestine, and is detectable in the jejunum and distal ileum, but not in the colon, and decreases along the villous-crypt axis.44 This binding distribution coincides with the differential intestinal epithelial paracellular permeability along the villous axis45 and with the regional effect of Zot on intestinal permeability44 and on F-actin redistribution in the mature cells of the villi.43,46 These combined data demonstrate that Zot regulates the TJ in a rapid, reversible, and reproducible fashion. Based on this observation, we postulated that Zot may mimic the effect of a functionally and immunologically related, physiologically relevant endogenous modulator of epithelial TJs. By using affinity-purified anti-Zot antibodies and the Ussing chamber assay, we were able to identify a human intestinal Zot homolog that we designated as zonulin.47 Affinity-purified zonulin reduced transepithelial electrical resistance (TEER) compared to the media control in both monkey jejunum (35.3% decrement) and ileum (25.6% decrement), but not in the colon.47 V. cholerae–derived Zot and human zonulin both act on intestinal TJs43,44,47-49 (Fig. 3) and display the same regional activity44 coincident with Zot receptor distribution in the intestine,43,44 suggesting that these two molecules interact with the same receptor. We compared the primary amino acid structures of Zot and zonulin to provide insights into the structural requirements for ligand engagement of the receptor coupled to intestinal TJ regulation. The NH2-termini of zonulin and the Zot active fragment share a common octapeptide motif (GGVLVQPG) that is critical for intestinal receptor binding.47,50 The synthetic octapeptide motif, named AT1001, has since been demonstrated to be an efficient inhibitor of both Zot and zonulin.47,50 The physiological role(s) of the zonulin system remains to be established. This pathway appears to be involved in several functions, including TJ regulation responsible for the movement of fluid, macromolecules and leukocytes between the bloodstream and the intestinal lumen and vice versa. Another possible physiological role of intestinal zonulin is the protection against microorganism colonization of the proximal intestine (innate immunity).51
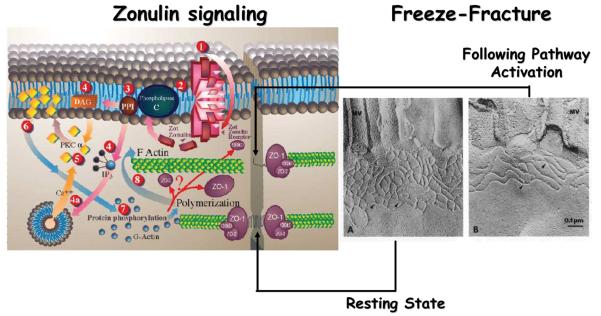
Figure 3.
Proposed zonulin intracellular signaling leading to the opening of the intestinal TJs. Zonulin interacts with a specific surface receptor [1] whose distribution within the intestine varies. The protein then activates phospholipase C [2] that hydrolyzes phosphatidyl inositol [3] to release inositol 1,4,5-tris phosphate (PPI-3) and diacylglycerol (DAG) [4]. PKCα is then activated [5], either directly (via DAG) [4] or through the release of intracellular Ca2+ (via PPI-3) [4a]. Membrane-associated, activated PKCα [6] catalyzes the phosphorylation of target protein(s), with subsequent polymerization of soluble G-actin in F-actin [7]. This polymerization causes the rearrangement of the filaments of actin and the subsequent displacement of proteins (including ZO-1) from the junctional complex [8]. As a result, intestinal TJs become looser (see freeze-fracture electron microscopy). Once the zonulin signaling is over, the TJs resume their baseline steady state.
Zonulin and Autoimmune Diseases
Intestinal TJ dysfunction occurs in a variety of clinical conditions, including food allergies, infections of the gastrointestinal tract, autoimmune diseases, and inflammatory bowel diseases.1 Several autoimmune diseases are characterized by loss of intestinal barrier function.1 To explore the possibility that zonulin is involved in the pathogenesis of these diseases, we focused our studies on CD and T1D, two autoimmune conditions in which the finely tuned regulation of intestinal TJ permeability is lost.52-54 To determine whether zonulin production is perturbed during the acute phase of CD, intestinal tissues from patients with active CD and non-CD controls were probed for zonulin expression.52 Quantitative immunoblotting of intestinal tissue lysates from active CD patients confirmed the increase in zonulin protein compared to control tissues.52 The zonulin upregulation during the acute phase of CD was confirmed by measuring zonulin concentration in sera of 189 CD patients using a sandwich ELISA. Compared to healthy controls, CD subjects showed significantly higher zonulin serum concentrations (P < 0.000001) during the acute phase of the disease that decreased following a gluten-free diet.52 Similar results were obtained from T1D subjects.53,54 In the BBDP rat model of T1D, we reported that zonulin-dependent increases in intestinal permeability precede the onset of T1D by 2–3 weeks.53 Oral administration of the zonulin inhibitor AT1001 to BBDP rats blocked autoantibody formation and zonulin-mediated intestinal permeability increase, reducing the incidence of diabetes.53 Moreover, feeding BBDP rats a gluten-free diet reduced the serum zonulin levels (Visser et al., unpublished results) (Fig. 2). Taken together, these studies suggest that the zonulin-dependent loss of intestinal barrier function is one of the initial steps in the pathogenesis of T1D in the BBDP animal model of the disease.
The involvement of zonulin in T1D pathogenesis was corroborated by our studies in humans, showing that a large subgroup of T1D patients has high serum zonulin levels that correlated with increased intestinal permeability and changes in claudin-1, claudin-2, and myosin IXB genes expression.54 Moreover, we also provided preliminary evidence suggesting that, like in the BBDP rat model of the disease, zonulin upregulation precedes the diagnosis of the disease in T1D patients.54
Link between Gliadin, Zonulin, and Increased Intestinal Permeability in Autoimmunity
Our group has generated evidence that gliadin induces increased intestinal permeability by releasing preformed zonulin.55,56 Intestinal cell lines exposed to gliadin released zonulin in the cell medium with subsequent zonulin binding to the cell surface, rearrangement of the cell cytoskeleton, loss of occludin-ZO1 protein–protein interaction, and increased monolayer permeability.56 Pre-treatment with the zonulin antagonist AT1001 blocked these changes without affecting zonulin release. When exposed to luminal gliadin, intestinal biopsies from celiac patients in remission expressed a sustained luminal zonulin release and increase in intestinal permeability. Conversely, biopsies from non-CD patients demonstrated a limited, transient zonulin release, which was paralleled by a reduction in intestinal permeability that never reached the level of permeability seen in CD tissues. Interestingly, when gliadin was added to the basolateral side of either cell lines55 or intestinal biopsies,56 no zonulin release was detected. The latter finding suggests that gliadin interacts with an intestinal luminal receptor and prompted us to identify this moiety. In vitro experiments showed specific colocalization of gliadin with the chemokine receptor CXCR3 expressed in human and mouse intestinal epithelium and lamina propria.57 Gliadin exposure led to a physical association of CXCR3 and MyD88. Ex vivo experiments revealed that gliadin exposure to intestinal segments from wild-type mice increased zonulin release and intestinal permeability, while CXCR3−/− intestinal segments failed to respond to gliadin.57 The increased intestinal permeability appeared to be a specific effect of gliadin, since another CXCR3 ligand, IP-10, did not affect intestinal barrier function. Based on these data, we postulate that gliadin binds to CXCR3 and leads to activation of the zonulin pathway and increased intestinal permeability in a MyD88-dependent fashion.57
Conclusive Remarks
The classical paradigm of autoimmune pathogenesis involving specific gene makeup and exposure to environmental triggers has been recently challenged by the addition of a third element, the loss of intestinal barrier function. Genetic predisposition, miscommunication between innate and adaptive immunity, exposure to environmental triggers, and loss of intestinal barrier function secondary to dysfunction of intercellular TJ all seem to be key components in the pathogenesis of autoimmune diseases. Both in CD and T1D gliadin may play a role in causing loss of intestinal barrier function and/or inducing the autoimmune response in genetically predisposed individuals.58 This new theory implies that once the autoimmune process is activated, it is not auto-perpetuating, but rather can be modulated or even reversed by preventing the continuous interplay between genes and environment. Since TJ dysfunction allows this interaction, new therapeutic strategies aimed at re-establishing the intestinal barrier function offer innovative, unexplored approaches for the treatment of these devastating diseases.
Acknowledgments
Funding: Work presented in this review was supported in parts by grants from the National Institutes of Health Grants DK-48373 and DK-078699 to AF and by the innovative pilot grant, Dutch Diabetes Foundation, grant number 2006.11.019 to JV.
Footnotes
Conflicts of Interest
A. Fasano has financial interests in Alba Therapeutics.
References
- 1.Fasano A. Tight Junctions. CRC Press, Inc.; Boca Raton, FL: 2001. Pathological and therapeutic implications of macromolecule passage through the tight junction; pp. 697–722. [Google Scholar]
- 2.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 3.Fasano A. Intestinal zonulin: open sesame! Gut. 2001;49:159–162. doi: 10.1136/gut.49.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Halstensen TS, Kett K, et al. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterol. 1989;97:1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P. Overview of the mucosal immune system. Curr. Top. Microbiol. Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkman PJ, Saper MA, Samraoui B, et al. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkman PJ, Saper MA, Samraoui B, et al. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987;329:512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- 8.Cuvelier C, Mielants H, De Vos M, et al. Major histocompatibility complex class II antigen (HLA-DR) expression by ileal epithelial cells in patients with seronegative spondylarthropathy. Gut. 1990;31:545–549. doi: 10.1136/gut.31.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wendling D. Role of the intestine in the physiopathology of inflammatory rheumatism. Rev. Rhum. Mal. Osteoartic. 1992;59:389–392. [PubMed] [Google Scholar]
- 10.Bjarnson I, Williams P, Smethurst P, et al. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut. 1986;27:1292–1297. doi: 10.1136/gut.27.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjarnason I, Peters TJ, Levi AJ. Intestinal permeability: clinical correlates. Dig. Dis. 1986;4:83–92. doi: 10.1159/000171140. [DOI] [PubMed] [Google Scholar]
- 12.Pratesi R, Gandolfi L, Garcia SG, et al. Prevalence of coeliac disease: unexplained age-related variation in the same population. Scand. J. Gastroenterol. 2003;38:747–50. doi: 10.1080/00365520310003255. [DOI] [PubMed] [Google Scholar]
- 13.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch. Int. Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 14.Nistico L, Fagnani C, Coto I, et al. Concordance, disease progression, and heritability of coeliac disease in Italian twins. Gut. 2006;55:803–808. doi: 10.1136/gut.2005.083964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louka AS, Sollid LM. HLA in coeliac disease: unravelling the complex genetics of a complex disorder. Tissue Antigens. 2003;61:105–117. doi: 10.1034/j.1399-0039.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 16.Vader W, Stepniak D, Kooy Y, et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc. Natl. Acad. Sci. USA. 2003;100:12390–12395. doi: 10.1073/pnas.2135229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monsuur AJ, Bakker PI, Alidazeh BZ, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat. Gen. 2005;37:1341–1344. doi: 10.1038/ng1680. [DOI] [PubMed] [Google Scholar]
- 18.Wapenaar MC, Monsuur AJ, van Bodegraven AA, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57:463–467. doi: 10.1136/gut.2007.133132. [DOI] [PubMed] [Google Scholar]
- 19.Kelly MA, Rayner ML, Mijovic CH, et al. Molecular aspects of type 1 diabetes. Mol. Pathol. 2003;56:1–10. doi: 10.1136/mp.56.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago JL, Martinez A, Nunez C, et al. Association of MYO9B haplotype with type 1 diabetes. Hum. Immunol. 2008;69:112–115. doi: 10.1016/j.humimm.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Sollid LM. Breast milk against celiac disease. Gut. 2002;51:767–768. doi: 10.1136/gut.51.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grönlund M-M, Arvilommi H, Kero P, et al. Importance of intestinal colonization in the maturation of humoral immunity in early infancy: a prospective follow up study of healthy infants aged 0–6 months. Arch. Dis. Child. Fetal. Neon. 2000;83:F186–F192. doi: 10.1136/fn.83.3.F186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirjavainen PV, Arvola T, Salminen SJ, et al. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut. 2002;51:51–55. doi: 10.1136/gut.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterol. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Lefebvre DE, Powell KL, Strom A, et al. Dietary proteins as environmental modifiers of type 1 diabetes mellitus. Annu. Rev. Nutr. 2006;26:175–202. doi: 10.1146/annurev.nutr.26.061505.111206. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler AG, Schmid S, Huber D, et al. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 27.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 28.Lundin KEA, Scott H, Hansen T, et al. Gliadin-specific, HLA-DQ (α180501,ß1 * 0201) restricted T cells isolated from the small intestinal mucosa of celiac patients. J. Exp. Med. 1993;178:187–196. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agardh D, Nilsson A, Tuomi T, et al. Prediction of silent celiac disease at diagnosis of childhood type 1 diabetes by tissue transglutaminase autoantibodies and HLA. Pediatric Diab. 2001;2:58–65. doi: 10.1034/j.1399-5448.2001.002002058.x. [DOI] [PubMed] [Google Scholar]
- 30.Westerholm-Ormio M, Vaarala O, Pihkala P, et al. Imunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes. 2003;52:2287–2295. doi: 10.2337/diabetes.52.9.2287. [DOI] [PubMed] [Google Scholar]
- 31.Savilahti E, Ormala T, Saukkonen U, et al. Jejuna of patients with insulin-dependent diabetes mellitus (IDDM) show signs of immune activation. Clin. Exp. Immunol. 1999;116:70–77. doi: 10.1046/j.1365-2249.1999.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auricchio R, Paparo F, Maglio M, et al. In vitro deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes. 2004;53:1680–1683. doi: 10.2337/diabetes.53.7.1680. [DOI] [PubMed] [Google Scholar]
- 33.Hanninen A, Salmi M, Simell O, et al. Endothelial cell-binding properties of lymphocytes infiltrated into human diabetic pancreas: Implications for pathogenesis in IDDM. Diabetes. 2003;42:1656–1662. doi: 10.2337/diab.42.11.1656. [DOI] [PubMed] [Google Scholar]
- 34.Chakir H, Lefebvre DE, Wang H, et al. Wheat protein-induced proinflammatory T helper 1 bias in mesenteric lymph nodes of young diabetes-prone rats. Diabetologia. 2005;48:1576–1584. doi: 10.1007/s00125-005-1842-z. [DOI] [PubMed] [Google Scholar]
- 35.Scott FW, Cloutier HE, Kleeman R, et al. Potential mechanisms by which certain foods promote or inhibit the development of spontaneous diabetes in BB rats. Dose, timing, early effect on islet area, and switch in infiltrate from Th1 to Th2 cells. Diabetes. 1997;46:589–598. doi: 10.2337/diab.46.4.589. [DOI] [PubMed] [Google Scholar]
- 36.Funda DP, Kaas A, Taskalova-Hogenova H, et al. Gluten-free but also gluten-enriched (gluten+) diet prevent diabetes in NOD mice; the gluten enigma in type 1 diabetes. Diab. Metab. Res. Rev. 2008;24:59–63. doi: 10.1002/dmrr.748. [DOI] [PubMed] [Google Scholar]
- 37.Meddings JB, Jarand J, Urbanski SJ, et al. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am. J. Physiol. 1999;276:G951–957. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- 38.Visser J, Brugman S, Klatter F, et al. Short-term dietary adjustment with a hydrolyzed casein-based diet postpones diabetes development in the diabetes-prone BB rat. Metabolism. 2003;52:333–337. doi: 10.1053/meta.2003.50052. [DOI] [PubMed] [Google Scholar]
- 39.Brugman S, Klatter F, Visser J, et al. Neonatal oral administration of DiaPep277, combined with hydrolysed casein diet, protects against Type 1 diabetes in BB-DP rats. An experimental study. Diabetologia. 2004;47:1331–1333. doi: 10.1007/s00125-004-1452-1. [DOI] [PubMed] [Google Scholar]
- 40.Brugman S, Klatter F, Visser J, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 41.Visser J, Groen H, Klatter F, et al. The diabetes prone BB rat model of IDDM shows duration of breastfeeding to influence Type 1 diabetes development later in life. Diabetologia. 2003;46:1711–1713. doi: 10.1007/s00125-003-1239-9. [DOI] [PubMed] [Google Scholar]
- 42.Scott FW, Rowsell P, Wang GS, et al. Oral exposure to diabetes-promoting food or immunomodulators in neonates alters gut cytokines and diabetes. Diabetes. 2002;51:73–78. doi: 10.2337/diabetes.51.1.73. [DOI] [PubMed] [Google Scholar]
- 43.Fasano A, Fiorentini C, Donelli G, et al. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J. Clin. Invest. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fasano A, Uzzau S, Fiore C, et al. The enterotoxic effect of zonula occludens toxin (Zot) on rabbit small intestine involves the paracellular pathway. Gastroenterol. 1997;112:839–846. doi: 10.1053/gast.1997.v112.pm9041245. [DOI] [PubMed] [Google Scholar]
- 45.Marcial MA, Carlson SL, Madara JL. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J. Membr. Biol. 1984;80:59–70. doi: 10.1007/BF01868690. [DOI] [PubMed] [Google Scholar]
- 46.Uzzau S, Lu R, Wang W, et al. Purification and preliminary characterization of the zonula occludens toxin receptor from human (CaCo2) and murine (IEC6) intestinal cell lines. FEMS Microbiol. Lett. 2001;194:1–5. doi: 10.1111/j.1574-6968.2001.tb09437.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Uzzau S, Goldblum SE, et al. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000;113:4435–4440. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- 48.Fasano A, Baudry B, Pumplin DW, et al. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baudry B, Fasano A, Ketley JM, et al. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect. Immun. 1992;60:428–434. doi: 10.1128/iai.60.2.428-434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Pierro M, Lu R, Uzzau S, et al. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001;276:19160–19165. doi: 10.1074/jbc.M009674200. [DOI] [PubMed] [Google Scholar]
- 51.El Asmar R, Panigrahi P, Bamford P, et al. Host-dependent activation of the zonulin system is involved in the impairment of the gut barrier function following bacterial colonization. Gastroenterol. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 52.Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;358:1518–1519. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- 53.Watts T, Berti I, Sapone A, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type-I diabetes in BB diabetic prone rats. Proc. Natl. Acad. Sci. USA. 2005;102:2916–2921. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 55.Clemente MG, Virgiliis S, Kang JS, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drago S, El Asmar R, De Pierro M, et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006;41:408–419. doi: 10.1080/00365520500235334. [DOI] [PubMed] [Google Scholar]
- 57.Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterol. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbeau WE, Bassaganya-Riera J, Hontecillas R. Putting the pieces of the puzzle together – a series of hypotheses on the etiology and pathogenesis of type 1 diabetes. Med. Hypotheses. 2007;68:607–619. doi: 10.1016/j.mehy.2006.07.052. [DOI] [PubMed] [Google Scholar]