Abstract
2,3,7,8-Tetrachlordibenzo-p-dioxin (TCDD) is a potent suppressor of humoral immunity, disrupting antibody production in response to both T cell–dependent and T cell–independent antigens. Among the cell types required for humoral responses, the B cell is highly, and directly, sensitive to TCDD. B cells become antibody-secreting cells via plasmacytic differentiation, a process regulated by several transcription factors, including activator protein-1, B-cell CLL/lymphoma 6 (BCL-6), and B lymphocyte–induced maturation protein 1 (Blimp-1). The overarching conceptual framework guiding experimentation is that TCDD disrupts plasmacytic differentiation by altering the expression or activity for upstream regulators of Blimp-1. Multiparametric flow cytometry was used to investigate TCDD-induced alterations in both activation marker and transcription factor expression following lipopolysaccharide (LPS) activation of purified B cells. TCDD significantly impaired LPS-activated expression of major histocompatibility complex class II, cluster of differentiation (CD)69, CD80, and CD86. Immunosuppressive concentrations of TCDD also suppressed LPS-activated Blimp-1 and phosphorylated c-Jun expression, whereas elevating BCL-6 expression. Because BCL-6 and c-Jun are directly and indirectly regulated by the kinases AKT, extracellular signal–regulated kinase (ERK), and Jun N-terminal kinase (JNK), it was hypothesized that TCDD alters toll-like receptor–activated kinase phosphorylation. TCDD at 0.03 and 0.3nM significantly impaired phosphorylation of AKT, ERK, and JNK in CH12.LX B cells activated with LPS, CpG oligonucleotides, or resiquimod (R848). In primary B cells, R848-activated phosphorylation of AKT, ERK, and JNK was also impaired by TCDD at 30nM. These results suggest that impairment of plasmacytic differentiation by TCDD involves altered transcription factor expression, in part, by suppressed kinase phosphorylation.
Keywords: TCDD, B cell, TLR, kinase, antibody suppression, differentiation
The aryl hydrocarbon receptor (AhR) ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), is a persistent environmental pollutant that bioaccumulates in the food web (Kelly et al., 2007) and hence has remained a chemical of public concern. TCDD causes a diverse range of biological effects including immunosuppression (Vos et al., 1974) in experimental animal models. The primary immunoglobulin response (immunoglobulin M [IgM]) is highly sensitive to disruption by TCDD, with an in vivo effective dose 50 of 0.7 μg/kg for suppression of the IgM antibody–forming cell response in mice (Smialowicz et al., 1994). Although the immunosuppressive effects of TCDD are well established, the mechanistic underpinnings responsible for humoral immune suppression by TCDD are not fully understood.
B cells are known to be highly, and directly, sensitive to TCDD (Dooley and Holsapple, 1988; Sulentic et al., 1998). Exposure to TCDD suppresses the lipopolysaccharide (LPS)-activated IgM response an average of 46% when present at the time of B-cell activation, but delaying TCDD addition as little as 3 h following LPS activation prevents, at least in part, suppression of the IgM response (Holsapple et al., 1986). The relatively narrow window of sensitivity by which TCDD can exert a suppressive influence on the IgM response suggests that a critical event to the immune-suppressive actions likely involves interference with an event shortly following B-cell activation.
The plasma cell, a specialized B cell capable of vigorous antibody secretion, arises through a highly regulated differentiation process. At the intracellular level, B cell to plasma cell differentiation is controlled by activation, repression, and induction of transcription factors controlling the gene expression program in B cells. Activator protein-1 (AP-1), B-cell CLL/lymphoma 6 (BCL-6), B lymphocyte–induced maturation protein 1 (Blimp-1), and paired box gene 5 (Pax5) are key transcription factors regulating B-cell fate (reviewed in Igarashi et al., 2007). The regulatory interactions controlling the “plasmacytic differentiation control circuit” are depicted in Figure 1. In resting B cells, BCL-6 and Pax5 suppress expression of Blimp-1 to maintain the gene expression program of a quiescent, but vigilant, B cell. Additionally, BCL-6 directly interacts with a keystone initiator of plasmacytic differentiation, AP-1, by reducing the transcriptional activity of AP-1 through a specific interaction with c-Jun, one of the Jun family members that comprises the AP-1 complex (Vasanwala et al., 2002). AP-1 binding within the Blimp-1 promoter drives gene expression (Ohkubo et al., 2005), and once translated into protein, Blimp-1 silences gene expression of BCL-6 and Pax5 (Shaffer et al., 2002), creating a positive feedback loop in which Blimp-1 expression increases during differentiation. In vivo profiling of the prdm1 locus, from which Blimp-1 is transcribed, demonstrates that Blimp-1 expression is quantitatively related to the plasma cell phenotype, as cells with the highest prdm1 locus activation are plasma cells (Kallies et al., 2004).
FIG. 1.
Schematic representation of transcription factor and kinase relationships regulating plasmacytic differentiation. Positive regulation is depicted by an arrow and negative regulation is depicted by a bracket.
The signaling cascade feeding into plasmacytic differentiation begins with engagement of receptors, such as the B-cell antigen receptor or toll-like receptor (TLR), causing activation of protein kinases leading to mitogen-activated kinases and transcription factor networks. B-cell signaling initiated at either the B-cell receptor or the immunoglobulin-inducing TLRs converges on several kinases, including Jun N-terminal kinase (JNK) and extracellular signal–regulated kinase (ERK). JNK phosphorylates c-Jun, one component of AP-1, increasing the transcriptional activity of AP-1 (Smeal et al., 1991). ERK plays an important role in regulation of the plasmacytic differentiation control circuit by controlling BCL-6 abundance. Following phosphorylation by ERK, BCL-6 is ubiquitinated and targeted for proteasomal degradation (Niu et al., 1998), in effect releasing a brake controlling expression of Blimp-1.
Recent in vitro studies demonstrated that TCDD suppressed LPS-activated AP-1 DNA binding and Blimp-1 gene expression (Schneider et al., 2009), which was further extended in vivo to TCDD impairment of Blimp-1 protein expression and major histocompatibility complex (MHC) class II upregulation (North et al., 2009). The overarching conceptual framework guiding experimentation is that TCDD disrupts plasmacytic differentiation by altering the expression or activity for upstream regulators of Blimp-1. Because TCDD-mediated suppression of plasmacytic differentiation is well established (Holsapple et al., 1991; Kerkvliet, 2002), the ability of TCDD to impair B-cell activation and intercellular signaling preceding initiation of the B-cell differentiation program was investigated. On that basis, it was hypothesized that TCDD suppresses B-cell activation and Blimp-1 expression by altering expression of BCL-6 and AP-1. Because BCL-6 and AP-1 are directly and indirectly regulated by AKT, ERK, and JNK, it was further hypothesized that TCDD interferes with TLR-activated kinase phosphorylation. Our data indicate that TCDD impairs TLR-activated increases in cluster of differentiation (CD)80 and CD86, likely in part due to alteration in c-Jun phosphorylation as well as BCL-6 and Blimp-1 expression. Disrupted expression of BCL-6 and c-Jun phosphorylation was attributable, at least in part, to TCDD suppression of AKT, ERK, and JNK phosphorylation.
MATERIALS AND METHODS
Animals.
Female 6- to 8-week-old C57BL6 mice were purchased from the National Cancer Institute, and all experiments as described were approved by the Michigan State University Institutional Animal Care & Use Committee. Mice were euthanized by cervical dislocation and spleens removed for processing into single-cell suspensions by mechanical disruption.
Chemicals.
TCDD was purchased from Accustandard (New Haven, CT) and prepared in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St Louis, MO). Stocks of Salmonella typhosa LPS (Sigma-Aldrich), resiquimod (R848; Alexis Biochemicals, Plymouth Meeting, PA), and CpG ODN 1826 (CpG, high performance liquid chromatography-purified thioester modified with a sequence of 5′-TCCATGACGTTCCTGACGTT-3′; Eurofins MWG Operon, Huntsville, AL) were prepared in individual aliquots and stored at −20°C until use.
B cell isolation.
Purified mouse B cells were isolated using Miltenyi Biotec B cell Isolation kits (Auburn, CA) according to manufacturer instructions. Purity of isolated B cells was verified by flow cytometry (FCM) analysis of CD19 expression and were routinely found to be 95–98% CD19+.
Cell culture.
CH12.LX cells, obtained from Dr Gregory Haughton (University of North Carolina, Chapel Hill, NC), were maintained in RPMI-1640 supplemented with 10% bovine calf serum, 100 U/ml penicillin/streptomycin, 1mM sodium pyruvate, nonessential amino acids, and 50μM 2-mercaptoethanol. Cells were passed every 2–3 days and maintained at a density between 1 × 104 and 1 × 106 cells/ml. Cell viability was assessed by trypan blue exclusion and found to exceed 95% for all experiments with CH12.LX cells. The CH12.LX cell line is a mouse lymphoblast capable of increasing IgM secretion in response to LPS or sheep erythrocyte treatment. Furthermore, the CH12.LX cell line is highly sensitive to TCDD and is therefore an excellent model for investigating signal transduction events involved in TCDD-mediated suppression of the IgM response.
Primary splenocytes and purified B cells were cultured in RPMI-1640 supplemented with 10% bovine calf serum, 100 U/ml penicillin/streptomycin, and 50μM 2-mercaptoethanol. Cell viability was assessed by trypan blue exclusion and found to exceed 90% for all experiments with primary B cells.
FCM analysis.
Activation marker and transcription factor expression in purified B cells was assessed by FCM at 1, 8, 24, 48, or 72 h following treatment. To identify viable populations, LIVE/DEAD Near-IR dye (Invitrogen, Carlsbad, CA) was used according to manufacturer’s instructions. FcγIII/II receptors were blocked with Fc Block (BD Biosciences, San Jose, CA). For activation marker determinations, a cocktail of antibodies for detection of MHC class II, CD69, CD80, and CD86 (Biolegend, San Diego, CA) were added to samples after blocking. For enumeration of plasma cells, cells were stained with anti-CD138 (BD Biosciences). After staining and washing, cells were fixed with Cytofix (BD Biosciences) according to manufacturer’s instructions. A minimum of 20,000 events were collected per sample on a BD FACSCanto II using FACSDiva software (BD Biosciences) and then analyzed using FlowJo 8.8.6 (Treestar Software, Ashland, OR) or Kaluza (Beckman Coulter Inc., Brea, CA). Cell viability was highest at 24 h, ranging from 80 to 95% viable, and declining over the time course. In the case of LPS-activated B cells, the viability at 72 h was 60–70%, whereas nonactivated cells ranged from 3 to 5% viable. The only statistically significant differences between populations were attributable to LPS activation. Cells were gated to exclude doublets from analysis by plotting forward scatter pulse height compared with forward scatter pulse area.
For transcription factor expression measurements, B cells were first labeled with LIVE/DEAD Near-IR to identify viable cells and then fixed with Cytofix according to manufacturer’s instructions. Samples were stored in 90% serum/10% DMSO at −80°C until the day of analysis. For staining and analysis, B cells were thawed at room temperature and washed twice with FCM buffer containing 0.5% saponin (Calbiochem, San Diego, CA), blocked using FcBlock, and then stained with antibodies specific for c-Jun, phosphorylated c-Jun, BCL-6, and Blimp-1. BCL-6 was detected using anti-rabbit IgG antibodies conjugated to PE-Cy5.5 (Invitrogen). A three step staining method was used to allow detection of BCL-6 with anti-rabbit IgG antibodies while avoiding misidentification of c-Jun expression. Until the final resuspension in FCM buffer prior to analysis, all buffers and washes contained 0.5% saponin to maintain permeabilization. A minimum of 20,000 events were collected per sample on a BD FACSCanto II using FACSDiva software and then analyzed using FlowJo 8.8.6. Cells were gated to exclude doublets from analysis by plotting forward scatter pulse height compared with forward scatter pulse area.
Studies measuring kinase phosphorylation utilized either CH12.LX or primary splenocytes. CH12.LX cells were grown in log phase until reaching a density of 6 × 105 cells/ml and then 1 ml of cells were transferred to 12 × 75–mm FCM tubes and placed in a 37°C water bath to equilibrate for 2–3 h. Primary splenocytes were diluted to a density of 1 × 106 cells/ml and then 1 ml of cells were transferred to 12 × 75–mm FCM tubes and placed in a 37°C water bath to equilibrate for 2–3 h. Hundred times concentrated treatments were prepared immediately prior to their addition. For CH12.LX cells, TCDD (0, 0.003, 0.03, or 0.3nM) and/or DMSO vehicle (0.1% final concentration) coupled with LPS (30 μg/ml), CpG (12 μg/ml), or R848 (0.2 μg/ml) for activation. Primary splenocytes were treated with DMSO vehicle (0.1%), TCDD (30nM), R848 (0.2 μg/ml), or R848 + TCDD. After treatment addition to individual tubes, cells were vortex mixed briefly and returned to the water bath for the remainder of the time course. At 15-, 30-, and 60-min posttreatment, cells were fixed by direct addition of electron microscopy grade 32% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for a final concentration of 10% paraformaldehyde, briefly vortex mixed, and then returned to the water bath for 30 min. At the completion of fixation, cells were pelleted by centrifugation, resuspended in 500 μl of cold ≥ 99.9% methanol, and stored at 4°C until all time points were completed and then transferred to −80°C for storage. For primary splenocytes, FcγIII/II receptors were blocked with Fc Block and then B cells were identified by staining B220 and TCRβ (Biolegend) after fixation and permeabilization. B cells were identified as B220+ TCRβ− cells following acquisition. Phosphorylated ERK1/2-specific (threonine 202/tyrosine 204) and phosphorylated JNK1/2-specific (threonine 183/tyrosine 185) antibodies were purchased from Cell Signaling (Danvers, MA). Phosphorylated AKT (serine 473) specific antibodies were purchased from BD Biosciences. A minimum of 20,000 events were collected per sample on a BD FACSCanto II using FACSDiva software for acquisition and then analyzed using FlowJo 8.8.6. For analysis, cells were gated to exclude doublets from analysis by plotting forward scatter pulse height compared with forward scatter pulse area. To calculate % change, the following formula was used: (Geometric Mean Fluorescence of stimulated samples − Geometric Mean Fluorescence of untreated samples)/(Geometric Mean Fluorescence of untreated samples) × 100 = % change.
Mouse IgM sandwich ELISA.
A detailed description of the procedure for determination of mouse IgM concentration can be found in Sulentic et al. (1998). In brief, cell culture supernatants were collected 48-h postactivation and analyzed by a kinetic colorimetric sandwich ELISA specific for mouse IgM. Total culture IgM concentration was calculated by comparison to a standard curve generated using mouse IgM (Sigma). IgM concentration was normalized to total cell count, as determined by Z1 Dual Threshold Coulter Counter, 48-h postactivation.
Statistical analysis.
Statistical analysis of IgM secretion by CH12.LX cells was performed using ANOVA followed by Dunnett’s post hoc test, comparing all groups with their respective vehicle-treated TLR ligand–activated treatment group. A p value threshold of 0.05 was used for determining statistical significance. GraphPad Prism 4.05 (La Jolla, CA) was used for ANOVA calculation.
For multivariate analysis of FCM data, the probability binning approach was used (Roederer et al., 2001). Probability binning is a nonparametric test designed specifically for analysis of FCM, particularly in cases where multiple parameters are measured simultaneously. As applied in this manuscript, each control group is divided into an equal number of multidimensional bins (four dimensions with each bin containing at least 10 events) that contain equal numbers of events such that a randomly identified event has an equal probability of belonging in any given bin. A treated group is then compared, via chi-square analysis, with the control group to determine the likelihood that the distribution of events within the treated group occurring by chance.
There are several important distinctions that make probability binning exceptionally useful. First is the multidimensional nature of the test, allowing for a level of population resolution unachievable with most statistical methods. Second, it provides a quantitative measure, T(χ), that describes how much a sample differs from the control, not simply the likelihood of an observation occurring by chance as with p values. A T(χ) = 0 indicates that two populations are indistinguishable statistically, whereas a T(χ) = 6, the threshold for significance used in these investigations, approximates a p value of 0.0039. Third, probability binning is a nonparametric test and is thus more suited to analysis of the non-Gaussian populations typically encountered in lymphocytes. Fourth, probability binning allows an investigator to determine which parameters form the basis for significant differences between populations. For statistical analysis, all biological replicates from individual treatment groups and times were concatenated. Flowjo 8.8.6 was used for all probability binning.
RESULTS
Time and Concentration Response Profiling for TCDD Disruption of Transcription Factor Expression
Although impairment of Blimp-1 messenger RNA (mRNA) expression has been demonstrated using both primary splenocytes and CH12.LX cells in vitro (Schneider et al., 2009), Blimp-1 protein expression in B cells was not directly assessed. Using multiparametric FCM, the time- and concentration-dependent effects of TCDD on LPS-activated expression of Blimp-1, BCL-6, c-Jun, and phosphorylated c-Jun in purified splenic B cells were assessed.
It is noteworthy that the LPS-activated IgM response is not as sensitive to the suppressive effects of TCDD as the sheep erythrocyte elicited response. Therefore, 30nM TCDD was selected as a starting concentration for primary B cells to cause significant impairment of the primary IgM response that approaches the suppression observed in vivo. To provide a broad coverage for the dose response, 10-fold decreasing concentrations of TCDD were then selected for investigation. Figure 2 shows that LPS caused a time-related increase in the expression of c-Jun, phosphorylated c-Jun, and Blimp-1 when compared with resting (i.e., no LPS treatment) B cells. BCL-6 initially increased with LPS treatment over the first 48 h relative to resting B cells but declined to expression levels below resting B cells by 72-h posttreatment. Peak expression of c-Jun and Blimp-1 occurred 72-h post-LPS, whereas abundance of phosphorylated c-Jun and BCL-6 peaked 48-h post-LPS. TCDD caused a concentration-related change in the normal pattern of LPS-activated transcription factor expression. Examined at their individual peak times of expression, TCDD increased BCL-6 and c-Jun abundance, while suppressing phosphorylated c-Jun and Blimp-1 expression. Probability binning analysis of the examined transcription factors showed statistically significant alteration in the LPS + TCDD population relative to LPS + DMSO-treated samples as early as 24-h posttreatment (T(χ) greater than 36 for all LPS + TCDD-treated groups) but is maximally different 72-h posttreatment (T(χ) greater than 336 for all LPS + TCDD-treated groups). T(χ) values can be used as a metric to rank how different samples are from a control population, and based on T(χ) values of LPS + TCDD-treated groups, the 0.03 and 0.3nM TCDD (T(χ) of 457.3 and 336.3, respectively) treatments cluster nearer to LPS + DMSO treatment than 3 and 30nM TCDD-treated B cells (T(χ) values of 981.0 and 811.2, respectively).
FIG. 2.
In vitro TCDD treatment impaired LPS-activated transcription factor expression. Purified splenic B cells cultured at a density of 3 × 106 cells/ml were treated with 0, 0.03, 0.3, 3, or 30nM TCDD in DMSO vehicle and activated with LPS (30 μg/ml). At 24, 48, and 72 h after treatment, B cells were analyzed by multivariate FCM for expression of c-Jun, phosphorylated c-Jun, BCL-6, and Blimp-1. Values depicted are geometric mean fluorescence ± SEM of quadruplicate biological replicates for viable B cells. Results are representative of two separate experiments.
LPS treatment caused an increase in phosphorylated c-Jun over resting cells at all time points evaluated, as much as 269% over resting values at 48 h. Thirty nanomolar TCDD treatment caused a significant impairment of LPS-activated c-Jun phosphorylation when compared with LPS + DMSO-treated B cells at all time points evaluated (T(χ) greater than 6.9 at all time points) but caused the most significant suppression at 24-h post-LPS (T(χ) of 86.7), a decrease of 14.3% from the LPS + DMSO-treated B cells. Three nanomolar TCDD caused a statistically significant suppression at 24- and 48-h post-LPS (T(χ) greater than 28.3) but not at 72 h. TCDD at 0.03 and 0.3nM significantly impaired c-Jun phosphorylation at 24 h (T(χ) greater than 33.3) but not at 48 or 72 h. Total c-Jun expression increased over the 72-h time course for all LPS-treated samples. LPS + DMSO B cells peaked at 72 h with a 24.5% increase over untreated B cells. All concentrations of TCDD caused a significant increase in the total amount of c-Jun at 24- and 48-h post-LPS (T(χ) greater than 16.9) but did not persist for all TCDD concentrations at 72 h.
An inverse relationship for protein expression was expected for BCL-6 and Blimp-1, as depicted in Figure 1. Within plasma cells, BCL-6 expression is silenced and Blimp-1 increased in populations assessed using bulk lysis techniques (Shaffer et al., 2002). Simultaneous analysis of both transcription factors at 48-h post-LPS, the peak time for BCL-6 expression and intermediate time for Blimp-1 expression, showed that LPS treatment induced increase both transcription factors (Fig. 3A). Thirty nanomolar TCDD treatment shifted the B-cell population to a higher expression of BCL-6, as demonstrated by 54.8% frequency of LPS + DMSO-treated B cells expressing elevated levels of BCL-6 compared with 69.4% frequency of LPS + 30nM TCDD-treated B cells elevating BCL-6 expression. TCDD treatment did not affect Blimp-1 expression at 48 h as much as BCL-6, as a 44.3% frequency of LPS + DMSO-treated B cells had elevated Blimp-1 expression compared with a 42.1% frequency of LPS + 30nM TCDD-treated B cells, a difference of 2.2%.
FIG. 3.
Relationship of Blimp-1 to BCL-6 expression at 48 h (A) and 72 h (B). Purified B cells were analyzed by FCM simultaneously for Blimp-1 and BCL-6 expression 48- and 72-h posttreatment. Results depicted are concatenated analysis of at least four biological replicates. Plots depict immunofluorescence for viable cells, with each level of contour corresponding to 5% of the population. Numbers in the corner of each quadrant depict the percentage of viable cells within the quadrant. Results are representative of two separate experiments.
At 72-h posttreatment, the effect of 30nM TCDD on the percentage of B cells expressing elevated levels of Blimp-1 is modestly greater, 43.8% in LPS + DMSO-treated B cells compared with 40.3% in LPS + 30 nMTCDD, a difference of 3.5% (Fig. 3B). In contrast, the percentage of Blimp-1High BCL-6Low B cells was markedly different between LPS + DMSO and LPS + 30nM TCDD treatments, 25.2 versus 12.6%, respectively. Furthermore, the percentage of Blimp-1Low BCL-6High B cells was also affected by TCDD treatment, going from 9.2% of viable cells in the LPS + DMSO-treated group to 25.0% of cells in the LPS + 30nM TCDD-treated group.
Time- and Concentration-Dependent Effects by TCDD on Cell Surface Activation Marker Expression
We have previously reported that TCDD impaired LPS-induced MHC class II upregulation on the surface of B cells following in vivo treatment in a dose-dependent manner (North et al., 2009). In experimental models of cell-mediated immunity, 15 μg/kg TCDD reduced P815-elicited increases in CD86 on the cell surface of CD45R+ B cells (Prell and Kerkvliet, 1997). Based on in vivo observations that TCDD treatment impaired both MHC class II and CD86, it was hypothesized that TCDD impairs upregulation of activation markers following in vitro immune stimulation. An examination of both the time- and the concentration-dependent changes in surface expression for activation markers MHC class II, CD69, CD80, and CD86 following in vitro LPS stimulation of B cells was performed.
LPS produced a time-dependent increase in all activation markers examined during the first 24 h after treatment, during which time 89.3% of LPS + DMSO-treated B cells upregulated the expression of at least one cell surface activation marker compared with 14.7% of the resting B cells (data not shown). Most of the suppressive effect of TCDD on LPS-elicited cell surface activation marker upregulation during the first 24 h is attributable to effects on MHC class II and CD69, as probability binning considering only CD80 and CD86 expression showed LPS + TCDD-treated B cells are indistinguishable from LPS + DMSO-treated B cells (T(χ) less than 1.5 for all concentrations of TCDD). Figure 4A shows MHC class II expression declined in untreated cells following purification and culture in vitro, whereas LPS treatment sustained or increased MHC class II expression, depending on the time point examined. TCDD treatment caused a concentration-related decrease in LPS-activated MHC class II expression at 8- and 24-h posttreatment (T(χ) from 9.1 for LPS + 0.03nM TCDD to 90.7 for LPS + 30nM TCDD). LPS-activated CD69 expression was significantly attenuated by TCDD 8-h posttreatment but recovered by 24 h and significantly exceeded the level observed in LPS + DMSO-treated B cells (T(χ) from 12.5 for LPS + 0.03nM TCDD to 30.4 for LPS + 30nM TCDD).
FIG. 4.
Simultaneous time course and concentration response for TCDD alterations of LPS-stimulated activation marker MHC class II and CD69 expression. Primary splenic B cells were isolated by negative selection and cultured at 3 × 106 cells/ml. Cells were treated with 0, 0.03, 0.3, 3, or 30nM TCDD in DMSO vehicle (0.025%) and activated with LPS (30 μg/ml). At 1-, 8-, 24-, 48-, and 72-h postactivation cells were collected for FCM analysis of MHC class II, CD69, CD80, and CD86 expression. Figure 4A depicts MHC class II and CD69 expression during the first 24 h. Figure 4B depicts CD80 and CD86 expression from 24 to 72 h. Results depicted are geometric mean fluorescence ± SEM for at least four biological replicates. Results are representative of two experiments.
Twenty-four hours was sufficient to observe significant upregulation of all activation markers after LPS treatment and decreases caused by TCDD treatment, but the primary IgM response develops over the course of 72 h. Although previous in vivo results showed that TCDD suppressed LPS-activated MHC class II expression as early as 24-h posttreatment, an effect confirmed above, purified B cells cultured in vitro may respond to LPS differently from their in vivo counterparts. Additionally, CD80 and CD86 expression increased relatively little during the initial 24 h following activation relative to 72 h. The time course of activation marker examination was therefore extended to 24-, 48-, and 72-h postactivation for CD80 and CD86. LPS treatment caused a time-related increase in total surface expression of CD80 and CD86 (Fig. 4B), with peak expression occurring 72-h post-LPS. Multivariate probability binning analysis of activation marker expression in LPS + DMSO-treated B cells compared with all other treatment groups showed that 30nM TCDD impaired expression of activation marker expression as early as 24-h post-LPS (T(χ) = 69.4), but as activation marker expression increased in LPS + DMSO-treated groups over time, the impairment caused by TCDD became more apparent. By 72 h, TCDD significantly impaired activation marker expression from 0.03nM (T(χ) = 73.5) to 30nM TCDD (T(χ) = 415.6).
Single parameter examination of costimulatory molecules CD80 and CD86 showed TCDD suppressed LPS-activated expression in a concentration-related manner (Fig. 4B). CD80 and CD86 stimulate T cells during the primary humoral response, and deletion of either CD80 or CD86 results in significant impairment of humoral immunity. However, even in partial knockouts either allele can partially compensate for the loss of the counterpart (Borriello et al., 1997). LPS treatment increased expression of both markers simultaneously, causing 24.7% of B cells to express high levels of both CD80 and CD86. TCDD treatment impaired the generation of CD80High CD86High B cells, reducing the frequency of double-positive cells to 11.5%, a level similar to that observed in resting B cells at 72 h (Fig. 5).
FIG. 5.
TCDD impaired LPS-activated CD80 and CD86 coexpression. Purified B cells were treated with LPS (30 μg/ml) + DMSO vehicle or LPS + 30nM TCDD. Cell surface expression of CD80 and CD86 was measured 72-h posttreatment. Results depict immunofluorescence from concatenated samples consisting of at least four biological replicates. Numbers in the corner of each quadrant depict the percentage of viable cells within the quadrant. Results are representative of two separate experiments.
Although MHC class II and CD69 were also reduced by TCDD treatment at 72 h, they decreased by 9.0 and 12.7% in abundance, respectively, in LPS + 30nM TCDD-B cells compared with LPS + DMSO-treated B cells (data not shown). In contrast, 30nM TCDD caused a 23.5% decrease in CD80 expression relative to LPS + DMSO-treated B cells at 72 h. TCDD treatment suppressed LPS-activated CD86 expression 12.6%.
Simultaneous Evaluation of Time- and Concentration-Dependent Effects by TCDD on TLR Stimulation of the In Vitro Primary IgM Response
Although results of in vivo and in vitro studies described above indicate TCDD-mediated disruption of LPS-activated Blimp-1 expression are consistent, a pivotal, direct mechanism for TCDD-mediated disruption of the primary IgM response could not be concluded. By utilizing different TLR ligands to activate plasmacytic differentiation, we sought to identify discrete differences between responses that provide insight into TCDD-mediated disruption of the primary IgM response. LPS drives plasmacytic differentiation of B cells via TLR4 and CD180 activation. Other TLR ligands are also known to drive plasmacytic differentiation, in particular the small molecule, R848 and CpG oligonucleotides, through TLR7/8 and TLR9, respectively (reviewed in Lanzavecchia and Sallusto, 2007) as well as increase Blimp-1 expression (Genestier et al., 2007).
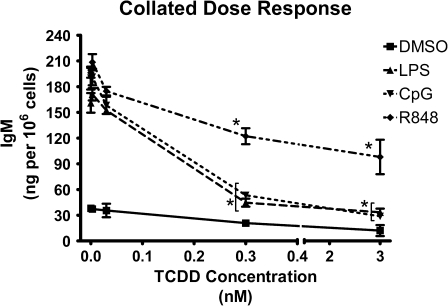
Although previous studies have demonstrated that the LPS-induced IgM antibody response is markedly suppressed by TCDD, sensitivity of IgM responses induced by other TLR ligands has not been investigated. Direct addition of R848 or CpG to purified splenic B cells induced increased IgM secretion, fivefold and fourfold, higher than LPS treatment, as measured by ELISA 3 days following activation (Fig. 6A). Likewise, TCDD suppressed the magnitude of secreted IgM induced by R848 and CpG. The cultured B cells were also harvested and assayed by FCM for CD138 (syndecan-1) expression to assess whether under the culture conditions employed to assess IgM secretion, TLR ligand activation was capable of driving B cells to fully differentiated plasma cells. All three TLR ligands (i.e., LPS, R848, and CpG) were capable of inducing a measurable percentage of splenic B cells to express CD138, which was almost completely blocked by TCDD treatment (Fig. 6B). These studies were extended further to CH12.LX cells, a cell line we have previously found to exhibit marked sensitivity to suppression by TCDD of the LPS-induced IgM response, as well as a useful model for investigations of B cell signaling (Sulentic et al., 1998). As observed in primary B cells and in CH12.LX cells, the TLR ligands LPS, CpG, and R848 all induced increased secretion of IgM. In contrast to splenic B cells, both CpG and R848 induced increases in IgM secretion comparable with that observed with LPS treatment. Moreover, TCDD produced a concentration-dependent suppression of the TLR ligand-induced IgM response for all stimuli examined (Fig. 7). Significant suppression occurred for all stimuli at concentrations of 0.3nM TCDD and higher. LPS- and CpG-activated IgM responses had similar profiles of suppression caused by TCDD, with 3nM TCDD producing a 72% decrease in LPS-induced IgM secretion and 83% decrease in CpG-induced IgM secretion. Three nanomolar TCDD suppressed R848-induced IgM secretion more moderately when compared with other TLR ligands, declining 53%.
FIG. 6.
TCDD-mediated suppression of TLR-activated IgM responses in purified splenic B cells. (A) Purified B cells (1 × 106 cells/ml) were activated with LPS (30 μg/ml), CpG (1.2 μg/ml), or R848 (0.2 μg/ml) and treated with 10nM TCDD in DMSO vehicle (0.05%). 72-h postactivation, culture supernatant IgM was determined by ELISA and normalized to total cell count. Results from triplicate determinations are represented as mean IgM per 106 cells ± SEM. *Values that are significantly different from activated cells treated with vehicle at p < 0.05. Results from triplicate determinations are represented as mean IgM per 106 cells ± SEM. (B) Purified B cells from the same cultures as used for ELISA above were also analyzed by FCM for CD138 expression. Results are representative of two separate experiments.
FIG. 7.
TCDD-mediated suppression of TLR-activated IgM responses in CH12.LX cells. CH12.LX cells (2 × 104 cells/ml) were activated with LPS (30 μg/ml), CpG (1.2 μg/ml), or R848 (0.2 μg/ml) and treated with 0, 0.003, 0.03, 0.3, or 3nM TCDD in DMSO vehicle (0.035%). At 48 h after, activation culture supernatant IgM was determined by ELISA and normalized to total cell count. Results from triplicate determinations are represented as mean IgM per 106 cells ± SEM. *Values that are significantly different from activated cells treated with vehicle at p < 0.05. Results are representative of more than four separate experiments.
TCDD-Mediated Alteration of TLR-Activated Kinase Phosphorylation
Previous studies in CH12.LX cells established TCDD-mediated alteration in the LPS-activated DNA binding and transcriptional activity of AP-1 (Schneider et al., 2009; Suh et al., 2002). In those investigations, Jun, but not Fos, was identified as one component of the AP-1 DNA–binding complex induced by LPS treatment of CH12.LX B cells. Because c-Jun is directly regulated by JNK and BCL-6 is directly regulated by ERK, it was hypothesized that TCDD impairs LPS-activated plasmacytic differentiation by altering the profile of kinase phosphorylation resulting from TLR activation. AKT, or protein kinase B, is phosphorylated within minutes of B cell receptor (BCR) activation (Donahue and Fruman, 2007), is known to be phosphorylated in B cells after LPS treatment (Hebeis et al., 2005) and indirectly regulates BCL-6 expression through control of FoxO phosphorylation (Omori et al., 2006). Because TCDD suppresses both LPS-activated and BCR-activated primary IgM responses, it was further hypothesized that TCDD impairs TLR-activated AKT phosphorylation.
Preliminary studies in primary B cells and CH12.LX cells established that LPS treatment stimulated modest phosphorylation of AKT, ERK, and JNK (data not shown), consistent with the observations of others (Krutzik et al., 2005). In the absence of B-cell activation, it is difficult to discriminate between normal response variations versus suppression; therefore, alternative B-cell activation stimuli were sought. Because it had already been established that CpG- and R848-activated IgM responses are suppressed by TCDD in CH12.LX cells (Fig. 7), their relative effectiveness for JNK phosphorylation was examined. Both the TLR9 ligand, CpG, and the TLR7/8 ligand, R848, induced JNK phosphorylation on a level equivalent to phorbol 12-myristate 13-acetate (PMA)/ionomycin 15-min postactivation (Fig. 8). Although PMA/ionomycin is not a TLR activator, it is a robust pharmacologic mimic of BCR activation. Therefore, treatments that meet or exceed the stimulation induced by PMA/ionomycin were considered robust inducers of kinase phosphorylation.
FIG. 8.
Relative TLR ligand efficacy for JNK phosphorylation. CH12.LX cells were activated by addition of LPS (30 μg/ml), PMA/ionomycin (40nM/0.5μM), CpG (12 μg/ml), or R848 (0.2 μg/ml). JNK phosphorylation status was fixed 15 min following activation by direct addition of concentrated paraformaldehyde to each group. After methanol permeabilization, CH12.LX cells were stained for phosphorylated JNK and analyzed by FCM. Histograms show the population distribution for JNK phosphorylation. Geometric mean fluorescence values are shown directly below the histograms for comparison of treatments.
To assess whether TCDD alters JNK, ERK, or AKT phosphorylation, CH12.LX cells were activated with single TLR ligands in combination with 0, 0.003, 0.03, or 0.3nM TCDD in DMSO vehicle. Literature reports showing TLR ligand–induced kinase phosphorylation were used to select concentrations for stimulation of AKT, ERK, and JNK (Bishop et al., 2000; Yi et al., 1996). TCDD concentrations were selected on the basis of causing, respectively, no significant effect (0.003nM TCDD), significant but submaximal (0.03nM TCDD), or maximal (0.3nM TCDD) suppression of LPS-activated IgM secretion in CH12.LX cells.
The kinetic and dynamic pattern of kinase phosphorylation resulting from TLR activation differs depending on the kinase examined and the TLR ligand applied. LPS treatment, as observed in preliminary studies, caused modest alterations in AKT, ERK, and JNK geometric mean phosphorylation abundance that peaked at 60 min following treatment (data not shown). CpG treatment caused the largest increases in geometric mean kinase phosphorylation abundance, increasing as much as 132% for CpG + vehicle treatment at 30 min and declining by 60-min postactivation (data not shown). Mean kinase phosphorylation abundance induced by R848 peaked earlier than other TLR ligands, increasing as much as 75% by 15-min postactivation and declining at 30- and 60-min postactivation (data not shown).
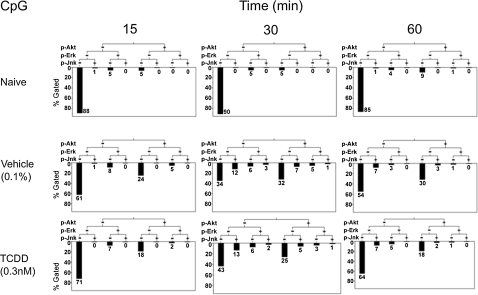
Probability binning analysis comparing TLR ligand + DMSO with TLR ligand + TCDD for AKT, ERK, and JNK phosphorylation suggested higher TCDD concentrations significantly decreased kinase phosphorylation. TLR-activated CH12.LX cells treated with 0.03 or 0.3nM TCDD shifted at least 8 standard deviations (T(χ) greater than 8) away from the TLR ligand + DMSO-treated groups. LPS treatment (Fig. 9) caused a minority of the population to phosphorylate kinases; however, TCDD was particularly effective in impairing the generation of pAKTHigh pERKHigh pJNKLow populations at 30-min postactivation (7% in LPS + DMSO compared with 1% in LPS + 0.3nM TCDD-treated cells, illustrated by shorter bars under given hierarchical clusters). As illustrated in Figure 10, CpG treatment caused 8% of CH12.LX cells to shift into a pAKTHigh status at 30- and 60-min postactivation, in contrast to untreated CH12.LX cells in which less than 1% of the population expressed high levels of phosphorylated AKT. TCDD at 0.3nM significantly impaired CpG-induced AKT phosphorylation, which was most marked at 60 min (30% in CpG + DMSO compared with 18% in CpG + 0.3nM TCDD-treated cells). In addition, there were more pAKTLow pERKLow pJNKLow cells in the TCDD (CpG + 0.3nM TCDD) through out the entire time course when compare with the time match vehicle control groups. Figure 12 demonstrates again that the kinetic and dynamic profile of kinase phosphorylation differs with each of the TLR ligands. Specifically, R848 induced a very rapid and dramatic induction of kinase phosphorylation with 25% of the control cells (R848 + DMSO) exhibiting a population that was pAKTHigh pERKHigh pJNKHigh at 15 min. Equally dramatic was that TCDD treatment almost completely eliminated the triple-positive population responding to R848 at 15-min postactivation.
FIG. 9.
Hierarchial clustering analysis of simultaneous time response profiling for TCDD alteration of LPS TLR ligand–activated AKT, ERK, and JNK phosphorylation. CH12.LX cells (6 × 105 cells/ml) were treated by simultaneous addition of vehicle (DMSO 0.1% final concentration), LPS alone or in combination with 0.3nM TCDD. Phosphorylation status was fixed by direct addition of concentrated paraformaldehyde at 15-, 30-, or 60-min postactivation, followed by permeabilization with methanol. CH12.LX cells were stained for phosphorylated forms of AKT, ERK, and JNK and then analyzed by FCM. Results depict phosphorylation positive or negative for AKT, ERK, and JNK. Longer bars indicate more cells in a given phosphorylation status. Results are representative of four separate experiments.
FIG. 10.
Hierarchial clustering analysis of simultaneous time response profiling for TCDD alteration of CpG TLR ligand–activated AKT, ERK, and JNK phosphorylation. CH12.LX cells (6 × 105 cells/ml) were treated by simultaneous addition of vehicle (DMSO 0.1% final concentration), CpG alone or in combination with 0.3nM TCDD. Phosphorylation status was fixed by direct addition of concentrated paraformaldehyde at 15-, 30-, or 60-min postactivation, followed by permeabilization with methanol. CH12.LX cells were stained for phosphorylated forms of AKT, ERK, and JNK and then analyzed by FCM. Results depict phosphorylation positive or negative for AKT, ERK, and JNK. Longer bars indicate more cells in a given phosphorylation status. Results are representative of four separate experiments.
FIG. 12.
TCDD suppression of kinase phosphorylation in primary B cells. Isolated splenocytes were treated by simultaneous addition of DMSO vehicle (0.1%), TCDD (30nM), R848 (0.2 μg/ml), or a combined treatment of R848 + TCDD. At 15 min after treatment, splenocytes were fixed by direct addition of concentrated formaldehyde. After permeabilization, cells were blocked and then stained for B220, TCRβ, pAKT, pERK, and pJNK. B cells were identified as a B220+ TCR− population and then analyzed for pAKT, pERK, and pJNK expression. Results depicted are immunofluorescence for pAKT and pERK. Percentages of viable cells within each quadrant are shown in the corner of each individual plot. Data are representative of two experiments.
To further extend the biological relevance of TCDD disruption for kinase phosphorylation, in vitro experiments using splenocytes were performed. Based on results obtained in the CH12.LX cell line, R848 was chosen as the B-cell activation stimulus because the kinase phosphorylation elicited by R848 was intermediate in magnitude when compared with LPS and CpG. At 15-min postactivation, primary B cells were assessed for AKT, ERK, and JNK phosphorylation. As depicted in Figure 12, R848 caused phosphorylation of both AKT and ERK for the majority of B cells. Probability binning analysis comparing DMSO- and 30nM TCDD-treated B cells in the absence of R848 activation showed no distinguishable difference for phosphorylation of AKT, ERK, or JNK. Conversely, TCDD at 30nM significantly suppressed R848-activated phosphorylation of AKT, ERK, and JNK (T(χ) of 11.5). pAKTHigh pERKHigh comprised 51.3% of R848-treated B cells, whereas 38.1% of R848 + TCDD-cotreated B cells were pAKTHigh pERKHigh. The reciprocal pAKTLow pERKLow populations were 26.3% of R848-treated B cells and 32% of R848 + TCDD-treated B cells. Relative to mean expression for pAKT and pERK in all B cells, which increased 230.4% and 383.5%, respectively, as calculated based on geometric mean fluorescence values, pJNK increased 94.8% relative to DMSO-treated B cells. Although R848 induced a more modest relative increase in pJNK, TCDD caused a measurable decrease in pJNK. R848 + TCDD-cotreated B cells increased pJNK by 79.5% over DMSO, a difference of 15.3% from single treatment with R848 for geometric mean immunofluorescence.
DISCUSSION
Previous studies have demonstrated attenuation of Blimp-1 protein expression in vivo as a potentially critical component of the mechanism responsible for TCDD-mediated suppression of the primary IgM response (North et al., 2009). Here, we show using LPS-activated B cells that concomitant with TCDD-mediated impairment of Blimp-1 upregulation, there is augmentation of the Blimp-1 repressor, BCL-6, marked attenuation in the expression of B-cell activation markers, CD80 and CD86, and ultimately fewer B cells differentiating to a plasmacytic phenotype as assessed by CD138 expression. These findings suggested that in addition to the direct regulation by the AhR of genes critically involved in controlling the B-cell differentiation program and immunoglobulin production, TCDD may also affect important signaling event during B-cell activation, which influence Blimp-1 regulation. Concordant with this notion, results described here also show that TCDD treatment impaired early kinase phosphorylation, irrespective of whether B cells were activated through TLR4 using LPS, TLR7/8 using R848, or TLR9 using CpG.
During the activation phase of plasmacytic differentiation, cell surface activation markers such as MHC class II, CD69, CD80, and CD86 initially increase in expression. TCDD suppression of MHC class II upregulation in response to LPS treatment was observed in vivo (North et al., 2009). In vitro examination of a more comprehensive panel of activation markers following LPS activation demonstrated that TCDD suppressed more than just surface expression of MHC class II. The significant impairment of CD80 and CD86 may be particularly relevant in understanding the suppression of primary T cell–dependent humoral immune responses produced by TCDD. Single-gene knockout studies in mice have demonstrated that CD80 and CD86 can partially compensate for each other, but CD80/86 double-knockout mice exhibit a profound impairment in humoral immunity, reminiscent of the profound IgM response suppression caused by TCDD in vivo (Borriello et al., 1997). One role of CD80 and CD86 is to provide costimulatory signals to T cells, which in turn stimulate B cells via cytokines. Because the primary IgM response typically requires interaction between B and T cells, a failure of B cells to provide sufficient stimulation to T cells would result in a spiraling failure of costimulation, eventually resulting in considerable suppression of the humoral response. It is important to emphasize that in the present investigation, CD80 and CD86 are not required for antibody production or plasmacytic differentiation because TLR ligands were employed as polyclonal B-cell activators and do require T cell help; however, regardless of the activation stimulus, induction of CD80 and CD86 is a hallmark of B-cell activation.
In vitro studies of purified B cells replicated in vivo suppression of Blimp-1 expression (North et al., 2009) and extended the effects of TCDD to an increase in BCL-6 expression. As shown in Figure 1, sustained expression of BCL-6 will exert a negative influence on Blimp-1 upregulation. BCL-6 is also known to directly repress CD80 expression (Niu et al., 2003). Hence, the elevated levels of BCL-6 observed 48 and 72 h following TCDD treatment in vitro provide a partial explanation for the significant impairment of CD80 expression at 48 and 72 h in LPS + TCDD-treated B cells.
AP-1 plays a fundamental role in initiating plasmacytic differentiation by activating Blimp-1 transcription (Ohkubo et al., 2005). In vitro treatment of B cells with TCDD significantly impaired LPS-activated phosphorylation of c-Jun, one component of the AP-1 transcription factor. We have previously shown that AP-1 DNA binding in LPS-activated CH12.LX is almost completely abrogated by 10nM TCDD 48-h posttreatment (Schneider et al., 2009), an effect more pronounced than expected in light of the decreased levels of phosphorylated c-Jun caused by 30nM TCDD in primary B cells. Although this may be partially attributed to differences in model systems (i.e., splenic B cells vs. CH12.LX cells), the influence played by BCL-6 may be another important factor. Simple abundance of AP-1 components likely underestimates effects on AP-1–regulated transcription because BCL-6 can directly bind and impair AP-1 activity (Vasanwala et al., 2002). In fact, we have recently demonstrated in CH12.LX cells decreased AP-1 DNA–binding activity to TRE sites within the Blimp-1 promoter (Schneider et al., 2009). It is therefore likely that AP-1 transcriptional activity may be significantly impaired given that TCDD both decreased levels of phosphorylated c-Jun, while increasing the abundance of BCL-6.
Comparing the in vitro LPS + TCDD with LPS + DMSO-treated cells by probability binning showed that T(χ) values for both transcription factor and activation marker expression correlate with the magnitude to which the IgM response was suppressed. TCDD at 0.03 and 0.3nM caused less suppression of the LPS-activated IgM response than 3 and 30nM, which was similarly reflected in the T(χ) values showing 0.03 and 0.3nM TCDD clustering nearer to LPS + DMSO treatment than 3 and 30nM TCDD. Application of more sophisticated statistical analysis tools for multiparametric FCM allowed better discrimination of TCDD effects by correlating changes for multiple proteins within a single cell, which was previously unachievable using bulk lysis techniques or single parameter analysis.
TCDD treatment consistently suppressed TLR-activated IgM responses in CH12.LX cells, and a similar pattern for TCDD suppression of kinase phosphorylation was observed regardless of the activating TLR ligand. Although TCDD was less effective at suppressing the R848-activated IgM responses compared with that induced by LPS and CpG, TCDD at 0.3nM consistently suppressed kinase phosphorylation regardless of the TLR ligand.
Kinases are key regulators of AP-1 activation preceding plasmacytic differentiation. Because kinases act as amplifiers in cell signaling cascades (Asthagiri and Lauffenburger, 2001), small perturbations can profoundly alter phenotypic outcomes. It is also noteworthy that bistable switches, such as the BCL-6/Blimp-1/Pax5 axis, can be ultrasensitive to activation and resistant to reversal (Palani and Sarkar, 2008). Increases or decreases in the level of kinase phosphorylation, and therefore activation, can decrease both LPS-activated Blimp-1 mRNA expression and plasmacytic differentiation (Lin et al., 2006). Immunosuppressive concentrations of TCDD decreased the abundance of phosphorylated AKT and to a lesser extent ERK and JNK in TLR-activated CH12.LX cells (Figs. 10–12). Primary B cells were also found to possess decreased levels in TLR-induced phosphorylated kinases with TCDD treatment (Figure 12 and Supplementary tables 1–3). In fact, the most striking effects within the first 60-min post-TLR activation by TCDD were observed on the abundance of pAKT. This finding is particularly interesting in light of new evidence showing that AKT phosphorylation results in nuclear exclusion and degradation of FOXO, which has recently been proposed to function as a critical repressor of Blimp-1 (Greer and Brunet, 2005; Omori and Rickert, 2007). Likewise, TCDD impairment of LPS-activated levels for phosphorylated ERK and JNK provide novel mechanistic insight into possible signaling events leading to alterations in BCL-6 and phosphorylated c-Jun expression observed in vitro. The observed increase in BCL-6 abundance caused by TCDD, as shown in Figure 2, is consistent with impaired ERK activation, as active ERK phosphorylates BCL-6 leading to ubiquitination and degradation (Niu et al., 1998). Given the role of BCL-6 as a negative regulator for Blimp-1 expression (Shaffer et al., 2000), it is tempting to speculate that impaired ERK phosphorylation indirectly leads to reduced Blimp-1 expression via prevention of BCL-6 downregulation. JNK derives its name from one of its best-characterized activities, the phosphorylation of c-Jun. Because TCDD impairs the abundance of phosphorylated JNK after TLR activation, reduced levels of phosphorylated c-Jun is a natural consequence.
FIG. 11.
Hierarchial clustering analysis of simultaneous time response profiling for TCDD alteration of R848 TLR ligand–activated AKT, ERK, and JNK phosphorylation. CH12.LX cells (6 × 105 cells/ml) were treated by simultaneous addition of vehicle (DMSO 0.1% final concentration), R848 alone or in combination with 0.3nM TCDD. Phosphorylation status was fixed by direct addition of concentrated paraformaldehyde at 15-, 30-, or 60-min postactivation, followed by permeabilization with methanol. CH12.LX cells were stained for phosphorylated forms of AKT, ERK, and JNK and then analyzed by FCM. Results depict phosphorylation positive or negative for AKT, ERK, and JNK. Longer bars indicate more cells in a given phosphorylation status. Results are representative of four separate experiments.
Single treatment with TCDD or LPS typically altered kinase phosphorylation less than 20% relative to untreated CH12.LX cells. Serum treatment is known to cause greater phosphorylation of ERK and JNK than TCDD (Tan et al., 2002), consistent with the modest induction observed above. As a result, many studies of TCDD-activated kinase phosphorylation have been performed under reduced serum conditions. Although not optimum for assessing changes in kinase phosphorylation status, studies presented here were performed in 5 or 10% serum in an attempt to maintain the conditions identical to those used for assessing B-cell differentiation and antibody secretion. Although the relative effects of LPS on kinase phosphorylation are modest, LPS stimulated a significant increase in IgM secretion at 48 h, illustrative of the point that kinases act as signal amplifiers. Indeed, TCDD treatment alone, even with modest changes in kinase phosphorylation observed in this study, can activate plasmacytic differentiation of B cells, albeit at very modest levels relative to classic humoral response activators (Kramer et al., 1987).
TCDD-mediated suppression of kinase phosphorylation may provide a partial explanation for the window of sensitivity that exists for in vitro disruption of the primary IgM response. Kinases in B cells respond rapidly to immunological stimuli, but the peak signal typically decays rapidly back to background level within minutes to hours of the initial activation (Irish et al., 2006). Respectively, if the initial signaling cascade induced via TLR or BCR engagement has cycled from peak activation back to basal levels and triggered downstream transcription factors, such as AP-1 and NFκB, the window of sensitivity may have passed rendering the cell refractory to TCDD. The reported window of sensitivity for the LPS-activated IgM response is 3 h (Holsapple et al., 1986).
Observations described above, including TCDD suppression of kinase phosphorylation, disruption of transcription factor expression controlling plasmacytic differentiation, and impaired expression of activation markers, could all individually impair humoral immunity, yet TCDD impairs normal TLR-activated expression for all. Furthermore, TCDD-activated AhR directly regulates the Igμ 3′ α enhancer (Sulentic et al., 2004b), an event in the IgM response distal to Blimp-1 upregulation during plasmacytic differentiation. Interference in the initiation of plasmacytic differentiation, maintenance of costimulation necessary for humoral immunity, and Ig expression provides partial explanations for the profound immunosuppression caused by TCDD in vivo. Taken together, the cumulative mechanism for TCDD-mediated suppression of the IgM response is multifaceted. Collectively, these results are mechanistically consistent with in vitro studies establishing TCDD-induced deregulation of the Igμ 3′ α enhancer (Sulentic et al., 2004a,b), Pax5 (Schneider et al., 2008), and Blimp-1 (Schneider et al., 2009) and proceed to expand potential mechanisms for TCDD suppression of humoral immunity.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (P42 ES04911, R01 ES02520, and T32 ES07255).
Supplementary Material
Acknowledgments
Conflict of interest: N.E.K. has grant support from the Dow Chemical Company for research on dioxin immunotoxicity. These funds were not used in support of research described in this manuscript.
References
- Asthagiri AR, Lauffenburger DA. A computational study of feedback effects on signal dynamics in a mitogen-activated protein kinase (MAPK) pathway model. Biotechnol. Prog. 2001;17:227–239. doi: 10.1021/bp010009k. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Hsing Y, Hostager BS, Jalukar SV, Ramirez LM, Tomai MA. Molecular mechanisms of B lymphocyte activation by the immune response modifier R-848. J. Immunol. 2000;165:5552–5557. doi: 10.4049/jimmunol.165.10.5552. [DOI] [PubMed] [Google Scholar]
- Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur. J. Immunol. 2007;37:2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- Dooley RK, Holsapple MP. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: I. The role of the B lymphocyte. Immunopharmacology. 1988;16:167–180. doi: 10.1016/0162-3109(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J. Immunol. 2007;178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Hebeis B, Vigorito E, Kovesdi D, Turner M. Vav proteins are required for B-lymphocyte responses to LPS. Blood. 2005;106:635–640. doi: 10.1182/blood-2004-10-3919. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Dooley RK, McNerney PJ, McCay JA. Direct suppression of antibody responses by chlorinated dibenzodioxins in cultured spleen cells from (C57BL/6 x C3H)F1 and DBA/2 mice. Immunopharmacology. 1986;12:175–186. doi: 10.1016/0162-3109(86)90001-9. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Snyder NK, Wood SC, Morris DL. A review of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: 1991 update. Toxicology. 1991;69:219–255. doi: 10.1016/0300-483x(91)90184-3. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Ochiai K, Muto A. Architecture and dynamics of the transcription factor network that regulates B-to-plasma cell differentiation. J. Biochem. 2007;141:783–789. doi: 10.1093/jb/mvm106. [DOI] [PubMed] [Google Scholar]
- Irish JM, Czerwinski DK, Nolan GP, Levy R. Kinetics of B cell receptor signaling in human B cell subsets mapped by phosphospecific flow cytometry. J. Immunol. 2006;177:1581–1589. doi: 10.4049/jimmunol.177.3.1581. [DOI] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in Blimp-1 expression. J. Exp. Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. Food web-specific biomagnification of persistent organic pollutants. Science. 2007;317:236–239. doi: 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int. Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Kramer CM, Johnson KW, Dooley RK, Holsapple MP. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) enhances antibody production and protein kinase activity in murine B cells. Biochem. Biophys. Res. Commun. 1987;145:25–33. doi: 10.1016/0006-291x(87)91282-4. [DOI] [PubMed] [Google Scholar]
- Krutzik PO, Hale MB, Nolan GP. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J. Immunol. 2005;175:2366–2373. doi: 10.4049/jimmunol.175.4.2366. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr. Opin. Immunol. 2007;19:268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Lin KI, Kao YY, Kuo HK, Yang WB, Chou A, Lin HH, Yu AL, Wong CH. Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2-mediated induction of transcription factor Blimp-1. J. Biol. Chem. 2006;281:24111–24123. doi: 10.1074/jbc.M601106200. [DOI] [PubMed] [Google Scholar]
- Niu H, Cattoretti G, Dalla-Favera R. BCL6 controls the expression of the B7-1/CD80 costimulatory receptor in germinal center B cells. J. Exp. Med. 2003;198:211–221. doi: 10.1084/jem.20021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Ye BH, Dalla-Favera R. Antigen receptor signaling induces MAP kinase-mediated phosphorylation and degradation of the BCL-6 transcription factor. Genes Dev. 1998;12:1953–1961. doi: 10.1101/gad.12.13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North CM, Crawford RB, Lu H, Kaminski NE. Simultaneous in vivo time course and dose response evaluation for TCDD-induced impairment of the LPS-stimulated primary IgM response. Toxicol. Sci. 2009;112:123–132. doi: 10.1093/toxsci/kfp187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo Y, Arima M, Arguni E, Okada S, Yamashita K, Asari S, Obata S, Sakamoto A, Hatano MJ, et al. A role for c-fos/activator protein 1 in B lymphocyte terminal differentiation. J. Immunol. 2005;174:7703–7710. doi: 10.4049/jimmunol.174.12.7703. [DOI] [PubMed] [Google Scholar]
- Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Omori SA, Rickert RC. Phosphatidylinositol 3-kinase (PI3K) signaling and regulation of the antibody response. Cell Cycle. 2007;6:397–402. doi: 10.4161/cc.6.4.3837. [DOI] [PubMed] [Google Scholar]
- Palani S, Sarkar CA. Positive receptor feedback during lineage commitment can generate ultrasensitivity to ligand and confer robustness to a bistable switch. Biophys. J. 2008;95:1575–1589. doi: 10.1529/biophysj.107.120600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prell RA, Kerkvliet NI. Involvement of altered B7 expression in dioxin immunotoxicity: B7 transfection restores the CTL but not the autoantibody response to the P815 mastocytoma. J. Immunol. 1997;158:2695–2703. [PubMed] [Google Scholar]
- Roederer M, Moore W, Treister A, Hardy RR, Herzenberg LA. Probability binning comparison: a metric for quantitating multivariate distribution differences. Cytometry. 2001;45:47–55. doi: 10.1002/1097-0320(20010901)45:1<47::aid-cyto1143>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Schneider D, Manzan MA, Crawford RB, Chen W, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated impairment of B cell differentiation involves dysregulation of paired box 5 (Pax5) isoform, Pax5a. J. Pharmacol. Exp. Ther. 2008;326:463–474. doi: 10.1124/jpet.108.139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Manzan MA, Yoo BS, Crawford RB, Kaminski N. Involvement of Blimp-1 and AP-1 dysregulation in the 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated suppression of the IgM response by B cells. Toxicol. Sci. 2009;108:377–388. doi: 10.1093/toxsci/kfp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Lin K-I, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Smeal T, Binetruy B, Mercola DA, Birrer M, Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Smialowicz RJ, Riddle MM, Williams WC, Diliberto JJ. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on humoral immunity and lymphocyte subpopulations: differences between mice and rats. Toxicol. Appl. Pharmacol. 1994;124:248–256. doi: 10.1006/taap.1994.1029. [DOI] [PubMed] [Google Scholar]
- Suh J, Jeon YJ, Kim HM, Kang JS, Kaminski NE, Yang K-H. Aryl hydrocarbon receptor-dependent inhibition of AP-1 activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin in activated B cells. Toxicol. Appl. Pharmacol. 2002;181:116–123. doi: 10.1006/taap.2002.9403. [DOI] [PubMed] [Google Scholar]
- Sulentic CE, Holsapple MP, Kaminski NE. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol. Pharmacol. 1998;53:623–629. [PubMed] [Google Scholar]
- Sulentic CEW, Kang JS, Na YJ, Kaminski NE. Interactions at a dioxin responsive element (DRE) and an overlapping kappaB site within the hs4 domain of the 3'alpha immunoglobulin heavy chain enhancer. Toxicology. 2004a;200:235–246. doi: 10.1016/j.tox.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Sulentic CEW, Zhang W, Na YJ, Kaminski NE. 2,3,7,8-tetrachlorodibenzo-p-dioxin, an exogenous modulator of the 3'alpha immunoglobulin heavy chain enhancer in the CH12.LX mouse cell line. J. Pharmacol. Exp. Ther. 2004b;309:71–78. doi: 10.1124/jpet.103.059493. [DOI] [PubMed] [Google Scholar]
- Tan Z, Chang X, Puga A, Xia Y. Activation of mitogen-activated protein kinases (MAPKs) by aromatic hydrocarbons: role in the regulation of aryl hydrocarbon receptor (AHR) function. Biochem. Pharmacol. 2002;64:771–780. doi: 10.1016/s0006-2952(02)01138-3. [DOI] [PubMed] [Google Scholar]
- Vasanwala FH, Kusam S, Toney LM, Dent AL. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J. Immunol. 2002;169:1922–1929. doi: 10.4049/jimmunol.169.4.1922. [DOI] [PubMed] [Google Scholar]
- Vos JG, Moore JA, Zinkl JG. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57B1/6 mice. Toxicol. Appl. Pharmacol. 1974;29:229–241. doi: 10.1016/0041-008x(74)90060-x. [DOI] [PubMed] [Google Scholar]
- Yi AK, Chace JH, Cowdery JS, Krieg AM. IFN-gamma promotes IL-6 and IgM secretion in response to CpG motifs in bacterial DNA and oligodeoxynucleotides. J. Immunol. 1996;156:558–564. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.