Abstract
We investigated differences in the pulmonary and systemic clearance of Stachybotrys chartarum spores in two strains of mice, BALB/c and C57BL/6J. To evaluate clearance, mice were intratracheally instilled with a suspension of radiolabeled S. chartarum spores or with unlabeled spores. The lungs of C57BL/6J mice showed more rapid spore clearance than the lungs of BALB/c mice, which correlated with increased levels of spore-associated radioactivity in the GI tracts of C57BL/6J as compared with BALB/c mice. To identify mechanisms responsible for mouse strain differences in spore clearance and previously described lung inflammatory responses, we exposed alveolar macrophages (AMs) lavaged from BALB/c and C57BL/6J mice to S. chartarum spores, S. chartarum spore toxin (SST), and satratoxin G (SG) in vitro. The S. chartarum spores were found to be highly toxic with most cells from either mouse strain being killed within 24 h when exposed to a spore:cell ratio of 1:75. The spores were more lethal to AMs from C57BL/6J than those from BALB/c mice. In mice, the SST elicited many of the same inflammatory responses as the spores in vivo, including AM recruitment, pulmonary hemorrhage, and cytokine production. Our data suggest that differences in pulmonary spore clearance may contribute to the differences in pulmonary responses to S. chartarum between BALB/c and C57BL/6J mice. Enhanced AM survival and subsequent macrophage-mediated inflammation may also contribute to the higher susceptibility of BALB/c mice to S. chartarum pulmonary effects. Analogous genetic differences among humans may contribute to reported variable sensitivity to S. chartarum.
Keywords: Stachybotrys chartarum, rodent, toxicity, acute, safety evaluation, macrophage, immunotoxicology, lung, pulmonary or respiratory system, respiratory toxicology, dose-response, risk assessment
Inhalation of fungal spores and their mycotoxins initiates inflammatory and allergic responses as well as pulmonary infections (Hendry and Cole, 1993; Larsen et al., 1996). Inhalation of Stachybotrys chartarum has been associated with multiple symptoms, including muscle aches, headaches, cough, pulmonary hemorrhage, dermatitis, and interstitial lung disease (Cooley et al., 1998). Infant deaths from acute idiopathic pulmonary hemorrhage have been hypothesized to be caused in part by this fungus (Dearborn et al., 1999; Etzel et al., 1998). Responsiveness to S. chartarum is idiosyncratic; some humans respond, others do not. Because separating environmental from genetic effects is difficult in humans, we used a mouse model (Rosenblum Lichtenstein et al., 2006) and an in vitro cell assay to elucidate mechanisms of responses to S. chartarum and to clarify the importance of strain differences that reflect genetic components of host responses.
Stachybotrys chartarum grows well at room temperature on wet surfaces composed of cellulose-containing materials such as paper, ceiling tiles, and cardboard (Murtoniemi et al., 2003). With appropriate temperature, light, and relative humidity, it produces mycotoxins, including macrocyclic trichothecenes such as satratoxin G (SG), which inhibit protein and DNA synthesis, induce protein degradation, disrupt cellular function, and cause cellular injury and inflammation (Bae et al., 2009; Hastings et al., 2005; Hudson et al., 2005; Kankkunen et al., 2009; McCrae et al., 2007; Shi et al., 2009; Yike et al., 2007). The fungus also releases stachylysin that can lead to pulmonary hemorrhage (Vesper SJ and Vesper MJ, 2002).
Previous experiments used exposures of rats and mice to S. chartarum spores intranasally and intratracheally; lung tissue was examined for histological changes and bronchoalveolar lavage for evidence of injury and inflammation (Nikulin et al., 1996, 1997; Rao et al., 2000; Rosenblum Lichtenstein et al., 2006). Stachybotrys chartarum has been consistently reported to cause pulmonary hemorrhage and extensive inflammation as well as apoptosis, cytokine release, DNA damage, and changes in gene expression (Bae et al., 2009; Chung et al., 2003b; Dearborn et al., 1999; Islam et al., 2006, 2008; Penttinen et al., 2007; Rand et al., 2002, 2003; Rosenblum Lichtenstein et al., 2006; Wang and Yadav, 2006, 2007). In addition to mycotoxins, S. chartarum releases SchS34, a 34-kD antigen (Rand and Miller, 2008).
We have previously shown that BALB/c mice respond more to pulmonary exposure to S. chartarum spores than do C57BL/6J or C3H/HeJ mice (Rosenblum Lichtenstein et al., 2006). Lungs from exposed BALB/c mice showed the most hemorrhage, the highest permeability of the air-blood barrier, the most white blood cell recruitment, and the highest inflammatory cytokine and chemokine levels in response to intratracheally instilled spores of S. chartarum. Our data also show that mice have dose-dependent pulmonary responses to S. chartarum and that their genetic background affects the slope of these dose-response curves.
We wanted to know whether strain differences influence clearance kinetics of spores. BALB/c mice clear both Bacillus anthracis and Cryptococcus neoformans from the lungs more efficiently than do C57BL/6J mice (Decken et al., 1998; Lyons et al., 2004). The mucociliary escalator and macrophage activity play important roles in the pulmonary clearance of pathogens in the lungs; multiple environmental factors modulate this role.
In this study, we sought to clarify the roles of pulmonary clearance and macrophage cytotoxicity in modulating responses to S. chartarum. We hypothesized that the observed strain differences in S. chartarum responsiveness are mediated in part by different rates of pulmonary spore clearance. The two mouse strains we employed here, C57BL/6J and BALB/c, are inbred strains developed independently and differ from each other at over 50% of characterized genetic loci (Taconic, 1998a,b). Both strains are TLR2+ and TLR4+ (Matsuguchi et al., 2003; Poltorak et al., 1998). BALB/c mice have been shown to be Th2 dominant, whereas C57BL/6 mice are Th1 dominant as summarized by Rosenblum Lichtenstein et al. (2006). Because alveolar macrophages (AMs) from C57BL/6 mice have been shown to have a higher phagocytic capacity than AMs from BALB/c mice (Su et al., 2001), we hypothesized that C57BL/6J mice might clear spores more efficiently than BALB/c mice, leading to differences in integrated spore dose over time. This may contribute to the increased pulmonary inflammation seen in BALB/c as compared with C57BL/6J mice. We also hypothesized that differences in AM sensitivity to S. chartarum-induced cytotoxicity influence both inflammation and spore clearance.
MATERIALS AND METHODS
Fungal strains and spore suspensions.
A trichothecene-producing strain of S. chartarum was obtained from Harriet Burge (Environmental Microbiology Lab, San Bruno, CA) (Rao et al., 2000) and grown on potato dextrose agar (PDA) plates at 15°C. Spores were vacuumed from the surface of 21-day agar cultures using a modified filter cassette with a 37-mm, 0.4-μm polycarbonate membrane filter (Poretics Corp., Livermore, CA). After the spores were removed from the filter, they were suspended in D-PBS (Mediatech Inc., Herndon, VA) at the desired concentration, as measured under light microscopy at ×200 in a hemocytometer chamber. Minor hyphal fragment content and negligible spore clumping were observed; most spores were ovoid in shape with a mean size of 6 × 9 μm. Two Stachybotrys chemotypes have been described—an atranone-producing chemotype and a trichothecenes-producing chemotype (Andersen et al., 2002, 2003; Rand et al., 2006). The S. chartarum strain described in this study is a trichothecene-producing chemotype.
SG ELISA.
SG levels in the spore suspension were measured by a modified competitive direct ELISA (Chung et al., 2003a). Briefly, SG antibody was diluted in PBS (1:10,000), coated on microtiter plates (100 μl/well), and incubated overnight at 4°C. Plates were washed and blocked with 1% (w/v) bovine serum albumin (BSA) in PBS at 25°C for 1 h. SG standards (0–500 ng/ml) were made, and SG horseradish peroxidase conjugate was diluted (1:4000 [v/v]) in 1% (w/v) BSA in PBS. Equal volumes of diluted SG horseradish peroxidase conjugate (50 μl) and standards diluted similarly were then mixed and incubated in microtiter plates at 25°C for 1 h. Plates were washed, and bound peroxidase was determined by 100 μl/well incubation with 3,3',5,5'-tetramethylbenzidine (Neogen, Lansing, MI) at 25°C for 0.5 h. Color reaction was stopped with 2N sulfuric acid (100 μl/well), and plates were read at 450 nm wavelength on ELISA plate reader (Molecular Devices, Menlo Park, CA). SG concentrations in samples were determined from the standard curve using Softmax software (Molecular Devices).
Dual radiolabeled spores.
Stachybotrys chartarum spores were streaked onto PDA plates supplemented with 1 ml of D-[U-14C] glucose (287 mCi/mmol, 200 μCi/ml; GE Healthcare Life Sciences, Piscataway, NJ) and 1 ml of [6-3H] thymidine (23 Ci/mmol, 1 mCi/ml; GE Healthcare Life Sciences). Growth conditions and harvesting were as described above. The dual label enabled tracing of both spore DNA and carbohydrate components such as the cell wall and glycosylated proteins. Spores were washed with saline four times prior to use to remove any unincorporated radiolabel. After the fourth wash, no radiolabel was detectable in the wash fluid.
Animal care.
Animal use protocols for these experiments were approved by the committee on animal experiments, and all institutional and federal guidelines on animal care and handling were followed. Male BALB/c (Taconic, Germantown, NY) and C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) were housed at the Harvard Animal Facilities for a minimum 1-week acclimation period, fed Purina Mouse Chow and water ad libitum, and kept on a 12-h light-dark cycle. All mice in these experiments were age matched (7–10 weeks). All experiments described below were performed on both mouse strains concurrently.
In Vivo spore clearance.
Spores suspensions were delivered into the lungs of both mouse strains by intratracheal instillation (IT) at 7.5 ± 1 weeks. Each mouse received 6.25 × 105 (a concentration of 107 spores/ml saline in 62.5 μl saline) spores per 25-g body weight for the quantitative clearance experiments (visual counting) and 6.25 × 104 spores per mouse (not adjusted for body weight) for the radiolabeled clearance experiments. We used the same volume for all the animals in the experiments to standardize the administered radioactive dose from day to day.
Between six and twelve mice per day were administered spores. On each day, the order of the strains was randomly alternated. At least three animals for each strain and time were sacrificed, and organs were harvested. The amount of radioactivity in the delivered dose was quantified by delivering an equivalent quantity of spore suspension to a vial (n = 4) for digestion (see below) and subsequent counting of radioactivity.
Immediately after the spore instillation and 24 h later, the mice were humanely killed by an intraperitoneal lethal dose of sodium pentobarbital (Anthony Products Co., Arcadia, CA) (10 mg/mouse). Mice were then exsanguinated by cutting the abdominal aorta and by puncturing the hemidiaphragm to create a bilateral pneumothorax. The lungs and trachea were removed en bloc, suspended in 4-ml distilled water, and homogenized through a stainless steel sieve with 100-μm holes. We counted intact spores on a hemocytometer under a light microscope at ×200 magnification. To calculate the number of spores in the lungs (SL), we multiplied the total volume (VH) of homogenized lungs (4000 μl) by the number of visible spores counted (SC) in the hemocytometer grids then divided by the volume of one hemocytometer grid (VG) (0.9 μl) multiplied by the total number (N) of grids counted (between 4 and 10 per mouse). We used the equation
For radioactivity measurements, mice were humanely killed as described above at 5 min, 8 h, and 1, 2, and 7 days after spore instillation. The lungs and trachea, stomach, cecum, and large intestine were removed and placed into preweighed scintillation vials. The small intestines were removed and cut into pieces and distributed among scintillation vials. Vials were reweighed and tissue weights calculated.
Tissues were dissolved in 1.0 ml Solvable (Perkin Elmer, Boston, MA) and 0.1 ml of 100 mM EDTA, followed by 0.3 ml 30% hydrogen peroxide. The caps were kept loose for 15 min to allow gas to escape. Caps were tightened, and the vials were incubated in a 60°C water bath for 1 h. The tubes were cooled to room temperature, and 15 ml of Ultima Gold (Perkin Elmer) was added. Samples were read in a Wallac Oy WinSpectral Scintillation Counter (Turku, Finland) using the 3H/14C dual label program. The radioactivity detected in the stomach, small intestine, large intestine, and cecum was totaled for each mouse to determine the total radioactivity in the GI tract.
In Vitro spore cytotoxicity.
Four BALB/c and C57BL/6J mice were lavaged for each of the three data points, as described previously (Rosenblum Lichtenstein et al., 2006). We did a 1-ml wash, followed by eleven 0.75-ml washes with D-PBS. The bronchoalveolar lavage fluid was pooled, resulting in one tube for each mouse strain. Seven different experiments were performed to achieve a sample size of ≥3 per time point. BSA (30%) (Calbiochem, La Jolla, CA) was preadded to the ice-chilled Falcon tube at 30 μl/mouse (∼9 ml of lavage fluid was recovered per mouse) to enhance cell survival. The cell suspensions were centrifuged at 200 × g for 15 min and the supernatants discarded. The cells were resuspended in 1-ml red blood cell lysis solution (Metcalf et al., 1986) and held at room temperature for 10 min. Supplemented RPMI-1640 was prepared with the following: 1× Cell-Gro RPMI-1640 with L-glutamine (Mediatech Inc.), 1% penicillin 100 U/ml and streptomycin 100 μg/ml (100×) (Sigma, St Louis, MO), and 10% fetal bovine serum, standard (HyClone, Logan, UT). Five microliters of supplemented RPMI-1640 was added to the cells which were then centrifuged at 200 × g for 10 min. The supernatant was discarded, and the cells were resuspended in 2 ml of supplemented RPMI-1640. The cells were counted on a hemocytometer and diluted to 750,000 cells/ml. One hundred microliters of the cell suspension was pipetted into each well of a 96-well plate (Greiner bio-one μclear plates), and the strains were alternated from well to well. Plates were incubated at 37°C with 5% CO2 overnight to allow the AMs to attach. Washes in subsequent steps are intended to remove nonadherent cell populations (such as neutrophils) that we have previously shown to be less than 3% of the white cell population in lung lavage fluid in healthy mice (Rosenblum Lichtenstein et al., 2006). Two to three replicates of each pool were used for each spore dose and time point in each of seven experiments, including three dose-response and four time course experiments. Every data point shown is based on two to three replicates from three to seven different experiments for a total of 6–15 wells per data point.
Spore suspensions were prepared in supplemented RPMI-1640 for each experiment. The spores were diluted to 250,000 spores/ml using a hemocytometer. Spores were serially diluted 1:5 in supplemented RPMI-1640, resulting in concentrations of 5000, 1000, 200, and 40 spores per 75,000 macrophages for the dose-response experiments. A dose of 1000 spores per 75,000 macrophages was used for the time course protocols. In all cases, a negative control sample was prepared that contained media without spores.
After overnight recovery from plating, the media was removed and 100 μl of spore preparations or control media were added to each well. Plates were incubated at 37°C in air with 5% CO2 for 24 h for the dose response and for 2, 5, or 24 h for the time course experiments.
At each designated time, the cells were washed twice with 300 μl of D-PBS with calcium and magnesium (Cellgro, Herndon, VA). A 20-ml solution with 5 μl of ethidium homodimer 1 (EthD1, Invitrogen LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells, Eugene, OR) and 5 μl of calcein AM (Invitrogen LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells) was prepared and 200 μl was added to each well. The cells were stained for 30–45 min at room temperature before examination by microscopic imaging.
Cell treatments with Stachybotrys chartarum spore toxin and SG.
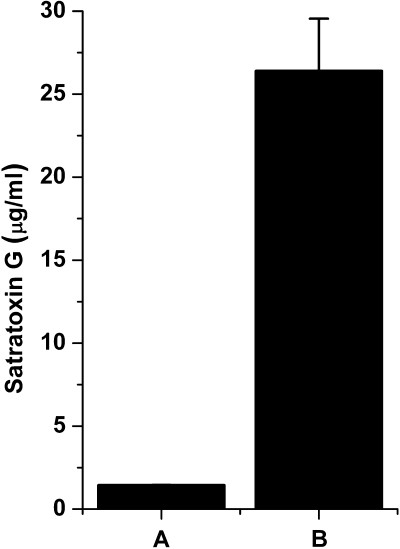
We assessed whether a water soluble extract from S. chartarum spores would demonstrate comparable cytotoxicity differences as intact spores. Stachybotrys chartarum spore toxin (SST) was prepared by adding ∼1.5 × 107 spores grown as described earlier to 15 ml of D-PBS for 1 h at room temperature. Spores were centrifuged, and the resulting supernatant was filtered through a 0.1-μm low-protein binding filter. Serial dilutions of SST (solution B, 26 ± 3 μg SG/ml; Fig. 1B) at 1:50 (528 ng SG/ml), 1:100 (264 ng SG/ml), 1:500 (53 ng SG/ml), 1:2500 (10 ng SG/ml), 1:12,500 (2 ng SG/ml), and saline controls in supplemented RPMI-1640 were added to 75,000 cells per well, as described above. Cells were also treated with SG isolated and purified from S. chartarum cultures (Islam, 2009). SG (100,000 ng) was reconstituted in 7 ml of saline and then diluted into supplemented RPMI-1640 at dilutions of 1:10 (1428 ng/ml), 1:20 (714 ng/ml), 1:100 (143 ng/ml), 1:500 (29 ng/ml), 1:2500 (5.7 ng/ml), 1:12,500 (1.1 ng/ml), media only, and saline controls. All toxin treatments were for 24 h, and cells were stained as described above.
FIG. 1.
SST per spore characterization. SG equivalents as measured by ELISA in (A) 106 Stachybotrys chartarum spores/ml and (B) full strength SST preparation used in Figure 6. Data are mean ± SD.
Cell viability analysis.
The cells were imaged on a BD Pathway 855 Bioimager at 200× magnification. Cells were illuminated, and fluorescence emission was collected from the bottom of the plate using a field size of ∼300 μm2 and three fields per well. All images were collected using flat field correction and 2 × 2 binning of pixels to increase the signal-to-noise ratio. Sequential confocal images of calcein AM and EthD1 fluorescence were collected every 1.7 μm.
Segmentation was used to draw regions around each cell as defined by a fluorescent signal. The regions of interest (ROIs) for both calcein AM and EthD1 were analyzed using BD Attovision software. We optimized the settings to maximize the number of cells counted whereas minimizing the numbers of cells counted twice. The ROI numbers for each of three fields for each well were summed. The percent live cells were calculated by taking the number of calcein positive cells in each well and dividing by the mean of the sums of the number of calcein AM positive and EthD1 positive cells in the zero spores control wells.
Statistical analyses.
Statistical analyses were performed using SAS statistical software (Version 8.2; SAS Institute, Cary, NC). All data displayed on a logarithmic scale were log transformed for statistical analyses. For the visual counting of spores in lung homogenates, ANOVA was performed. For the experiments measuring radiolabeled spore clearance from the lung, we used a repeated measures analysis that accounts for both the 3H and 14C labels for each mouse. For the experiments measuring radioactivity in the GI tract, multivariate analysis of variance (MANOVA) was used to analyze the data to account for the use of two different isotopes and our examining multiple tissues. For in vitro cytotoxicity experiments, we used a repeated measures analysis that allows for residual correlation among observations derived from the same BAL pool to account for differences among pools and spore preparations. In the dose-response model, we utilized a broken stick analysis to describe the change in the slopes of both lines at the 200 spores per well dose. A broken stick analysis allows us to describe two of the expected linear regions in a typical dose-response S-curve (Ruppert et al., 2003). In this instance, we have a relatively flat region at doses too low to cause significant cell death and a steeper region where increasing dose causes an approximately linear increase in cell death when the data are graphed on a log-log scale.
RESULTS
Stachybotrys chartarum Spore Suspension Contained SG
Because SG is water soluble, AMs and pulmonary epithelium would likely be exposed to soluble SG when exposed to S. chartarum suspensions. We therefore measured the SG concentration in this spore preparation. A 1-ml D-PBS suspension with 106 spores had an SG concentration of 1.4 ug/ml or 1.4 pg/spore (Fig. 1), which is comparable with previous estimates of 1 pg/spore for trichothecenes and S. chartarum by Yike and Dearborn (2004).
Mouse Strain Differences Influence Pulmonary Clearance of Stachybotrys chartarum Spores
C57BL/6J mice cleared S. chartarum spores from the lungs significantly faster than BALB/c mice (Figs. 2 and 3). Comparable spore numbers were delivered to the lungs of BALB/c and C57BL/6J mice. In C57BL/6J mice, 96.3 ± 11.1% (mean ± SE) were recovered, and in BALB/c mice, 81.4 ± 9.9% were recovered (time 0, Fig. 2). After 1 day, C57BL/6J mice had one-third as many spores remaining in the lungs as the BALB/c mice (Fig. 2; p = 0.02, ANOVA). Because S. chartarum spores have limited germination potential in the lung (Pestka et al., 2008), the spore numbers in the lung homogenates are unlikely to reflect spore growth.
FIG. 2.
Spores in lung homogenates. C57BL/6J mice clear more spores by 24 h than do BALB/c mice. Mean visible spores at 200× magnification in homogenized lungs ± SE versus time (hours). N = 5 mice/strain measured in two different experiments (ANOVA, *p = 0.02 for C57BL/6J vs. BALB/c).
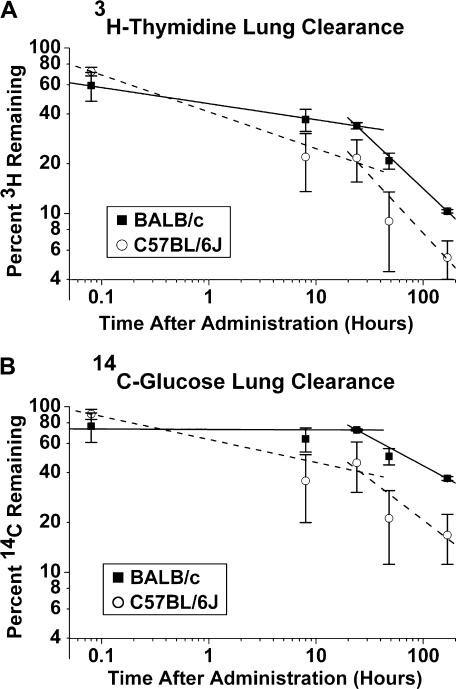
FIG. 3.
Radiolabeled spore clearance in lungs. Both BALB/c and C57BL/6J mice clear radiolabeled spores from the lungs over 7 days (p = 0.04, repeated measures analysis). BALB/c mice appear to retain more spores and/or spore components in the lung, although this difference is not statistically significant (p = 0.08, repeated measures analysis). 3H is cleared faster than 14C (p = 0.0001, repeated measures analysis). N = 3–6 mice per time point and strain. (A) Mean percent of administered 3H-thymidine (log scale) detected in whole digested lung and trachea ± SE versus time (log scale) after IT administration (hours). The equation that best describes the clearance of 3H in BALB/c mice is log(y) = 1.64 − 0.20 log(x) (data are linear, p = 0.05) and for C57BL/6J mice it is log(y) = 1.57 – 0.32 log(x) (data are linear, p = 0.01). (B) Mean percent of administered 14C-glucose (log scale) detected in whole digested lung and trachea ± SE versus time (log scale) after administration (hours). For 14C, the equation that best describes the clearance in BALB/c mice is log(y) = 1.84 − 0.08 log(x) (error in data is too large to determine linearity, p = 0.14) and for C57BL/6J mice it is log(y) = 1.76 – 0.21 log(x) (data are linear, p = 0.03).
One limitation using this methodology is that fibrous material in the homogenates makes the spores difficult to count accurately. In addition, we do not count spore components, only whole spores. We therefore estimated spore retention in the lungs using radiolabeled spores. Both strains cleared a significant percentage of the instilled spores and spore components (p < 0.001) over the 1-week time course. Consistent with the visual counts, the radiolabel data also showed that C57BL/6J mice cleared spores or spore components from the lung more rapidly than BALB/c mice during the first 24 h after instillation (p = 0.04; Figs. 3A and B). (For 3H-thymidine clearance, the best fit lines are log(y1C57BL/6J) = 1.54 − 0.29 (log(hours)), log(y1BALB/c) = 1.64 − 0.10 (log(hours)), log(y2C57BL/6J) = 1.14 − 0.88 (log(hours)), and log(yBALB/c) = 1.50 − 0.68 (log(hours)). For 14C-glucose clearance, the best fit lines are log(y1C57BL/6J) = 1.70 – 0.21 (log(hours)), log(y1BALB/c) = 1.82 − 0.02 (log(hours)), log(y2C57BL/6J) = 1.41 – 0.55 (log(hours)), and log(yBALB/c) = 1.79 − 0.30 (log(hours)).) The methodology used in this set of experiments to detect spores has the advantage of detecting both degraded spore components as well as whole spores. For both mouse strains, the 3H-thymidine (DNA) was cleared more rapidly than the 14C-glucose component (most likely carbohydrates, cell wall, and glycosylated proteins in the membrane, cytoplasm, and nucleus; p = 0.0001, repeated measures analysis; Figs. 3A and B). Radiolabel detected within the first 24 h is more likely to reflect whole spores, while radiolabel detected at later times is more likely to reflect spore components. Some radioactive spore constituents might thus persist in the lungs following spore degradation. Taken together, these data suggest that C57BL/6J mice are able to clear S. chartarum spores more rapidly than BALB/c mice.
Spores Are Cleared via the GI Tract
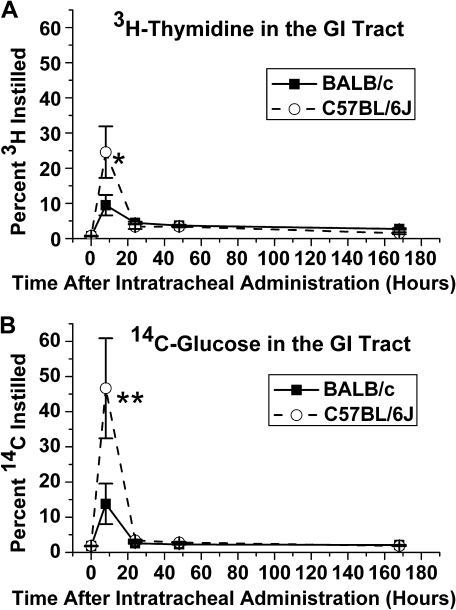
The appearance of radioactivity in the GI tract after IT into the lungs demonstrates clearance via the mucociliary escalator. Significantly, more spore-associated radioactivity was present in the GI tract of C57BL/6J mice 8 h after IT than BALB/c mice (p < 0.05, MANOVA; Figs 4A and B). Both strains showed significantly more radioactivity in the GI tract at 8 h than at other times, suggesting that this clearance pathway operates early for both strains (p = 0.0001, MANOVA). The C57BL/6J mice were thus more efficient at clearing the spores from the lungs via the mucociliary escalator than the BALB/c mice.
FIG. 4.
Radiolabeled spore detection in the GI tract. C57BL/6J mice clear significantly more spores from the lungs to the GI tract at 8 h. (A) Mean percent of intratracheal administered 3H-thymidine-labeled spores detected in digested esophagus, stomach, small intestines, cecum, and large intestines ± SE versus time after administration (hours). Strains significantly different at 8 h (*p = 0.05, MANOVA). (B) Mean percent of intratracheally administered spores labeled with 14C-glucose detected in digested esophagus, stomach, small intestines, cecum, and large intestines ± SE versus time after administration (hours). Strains significantly different at 8 h (**p = 0.04, MANOVA).
Stachybotrys chartarum Kills More AMs from C57BL/6J than BALB/c
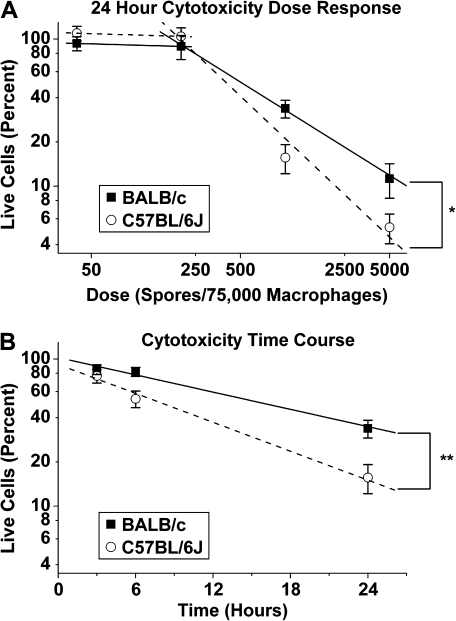
C57BL/6J AMs died at lower spore doses (p = 0.007) and sooner (p = 0.04) than those from BALB/c mice (Figs. 5A and B). Although both strains exhibited significant cell death at both the 1000 and 5000 spore doses, significantly more C57BL/6J AMs died at spore doses as low as 1000 spores per well with 75,000 cells (p = 0.0001; Fig. 5A). Many of the dead cells showed a punctate nuclear morphology in their staining with EthD1 consistent with apoptosis. At 24 h after spore addition, for doses less than 200 spores per 75,000 macrophages, the log(yC57BL/6J) = 2.29 – 0.20 (log(dose)) and log(yBALB/c) = 2.04 – 0.06 (log(dose)), where y represents the percent of live cells and dose represents the number of spores per well. For doses greater than 200 spores per 75,000 macrophages, the log(yC57BL/6J) = 4.04 – 0.97 (log(dose)) and log(yBALB/c) = 3.4731 – 0.68 (log(dose)), where y represents the percent of live cells and dose represents the number of spores per 75,000 macrophages. At doses higher than 200 spores/well, the slopes are significantly different (p = 0.007) between the two mouse strains.
FIG. 5.
Spore cytotoxicity in vitro. Spores are significantly more cytotoxic to C57BL/6J AMs than to BALB/c AMs. A total of 75,000 cells were plated per well for all wells. Data are shown as mean ± SE. (A) Dose (spores per macrophage) verses live cells as a percent of baseline (baselines are calculated as the total number of live and dead cells in matched wells with no spores). Graph is on a log-log scale. Slopes from 0.0027 to 0.06667 spores per macrophage for C57BL/6J (0.97) are significantly different from BALB/c (0.68) (*p = 0.007, repeated measures analysis, broken stick model). (B) Time course of cytotoxicity at 0.0133 spores per macrophage dose. Time (hours) versus live cells as a percent of baseline (baselines are calculated as the total number of live and dead cells in matched wells with no spores). Percent is graphed on a log scale. Slope of C57BL/6J (0.098) is significantly different from BALB/c (0.086) (**p = 0.04, repeated measures analysis).
C57BL/6J AMs died more rapidly in response to S. chartarum than do BALB/c AMs. The equations describing their cell death after addition of 1000 spores are log(yC57BL/6J) = 1.87 – 0.098 (time) and log(yBALB/c) = 1.84 – 0.086 (time), where y is the percent of live cells and time is the amount of time after spore addition in hours. The slopes of these two lines are significantly different (p = 0.04). This strain difference is consistent with C57BL/6J mice producing significantly lower levels of inflammatory cytokines in vivo (Rosenblum Lichtenstein et al., 2006).
SST and SG Kill More AMs from C57BL/6J than BALB/c
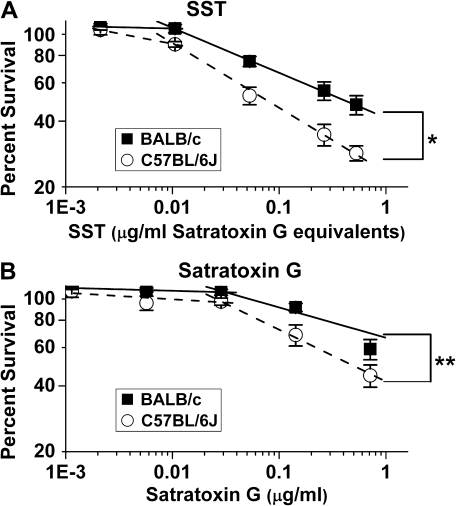
Increasing doses of SST cause increased cytotoxicity in AMs from both mouse strains (p = 0.0001; Fig. 6A). This is also true for SG only (p = 0.0001), (Fig. 6B). However, we observed a range of low concentrations where little change is seen, but at higher concentrations there is a linear relationship when percent survival is plotted against concentration. Importantly, we show that SG, although having significant cytotoxicity, does not account for all the cytotoxicity of SST. Thus, SG is a significant component, but there are also others. The concentration that is lethal for 25% of the cells (LC25) for SST for C57BL/6J is 0.017 μg/ml SG equivalents and for BALB/c is 0.052 μg/ml SG equivalents. The LC25 for SG for C57BL/6J is 0.075 μg/ml and for BALB/c is 0.243 μg/ml. We used LC25 rather than LC50 because LC50 is outside the known linear range of our data for SG in BALB/c macrophages. Based on these numbers, SG accounts for ∼20 to 25% of the toxicity of the SST.
FIG. 6.
SST and SG cytotoxicity in vitro. SST and SG both cause a significant dose response in both strains of mice (p = 0.0001). SST and SG are significantly more cytotoxic to C57BL/6J AMs than to BALB/c AMs (*p = 0.02 and **p = 0.059 respectively)—75,000 live cells plated per well for all wells. Data are shown as mean ± SE. Graphs are on a log-log scale. (A) Dose (SST [μg/ml SG equivalents]) verses live cells as a percent of baseline (baselines are calculated as the total number of cells in matched wells with RPMI-1640). Slopes from 0.01–0.5 μg/ml SG equivalents of SST for C57BL/6J (–0.41) are significantly different from BALB/c (–0.23). (B) Dose (μg SG/ml RPMI 1640) verses live cells as a percent of baseline (baselines are calculated as the total number of live and dead cells in matched wells with no SST). Slopes from 0.03–0.7 μg/ml SG for C57BL/6J (–0.27) are significantly different from BALB/c (–0.18).
After 1 day of treatment with SST, more C57BL/6J AMs die than BALB/c AMs at doses between 0.01 and 0.5 μg SG equivalents/ml (p = 0.02). The slopes between those doses are –0.41 and –0.23 for C57BL/6J and BALB/c, respectively (Fig. 6A). Similarly, after 1 day of treatment with SG, more C57BL/6J AMs die than BALB/c AMs at doses between 0.03 and 0.7 μg SG/ml (p = 0.059). The slopes between those doses are –0.27 and –0.18 for C57BL/6J and BALB/c, respectively (Fig. 6B). The two mouse strains showed differences in sensitivity to both SST and SG, with SG accounting for 15–30% of the cytotoxicity of SST at the calculated LD50.
DISCUSSION
C57BL/6J mice cleared trichothecene-producing S. chartarum spores from their lungs at a faster rate than did BALB/c mice. When spores are delivered to the lungs of C57BL/6J mice, more of the spores were cleared via the mucociliary escalator and then swallowed and excreted through the GI tract than in BALB/c mice. Others may be degraded by macrophages within the lungs. This more efficient clearance in C57BL/6J mice via innate mechanisms may partially explain the diminished pulmonary injury and inflammation that have been reported in this strain (Rosenblum Lichtenstein et al., 2006). In contrast, spores or spore components in the lungs of BALB/c mice persisted longer and thus may have elicited a greater response. For example, 37 ± 1% of the carbohydrate component of the spores persisted in the lungs of BALB/c mice a week after instillation, whereas only 17 ± 5% remained in the lungs of C57BL/6J mice. This persistence might also explain how an extract of S. chartarum can also induce allergic asthma in BALB/c mice after repeated pulmonary exposures (Viana et al., 2002). The carbohydrate component of the labeled spores most likely includes mycotoxins and glycosylated proteins that may be causing some of the inflammatory responses. Some of the remaining 14C label may also reflect metabolites of spores, mycotoxins, or proteins. C57BL/6J mice may metabolize spores or their mycotoxins more rapidly, and this may in part mediate the increased clearance rate in the C57/BL6J mice. Whichever spore components the 14C label represents, the additional spore-derived material remaining in the BALB/c mouse lungs over time is most likely mediating the increased inflammation observed in those lungs.
We reported that strain differences in response to S. chartarum are distinct from the strain differences seen in response to lipopolysaccharide or to other fungi (Rosenblum Lichtenstein et al., 2006). C57BL/6J is the least responsive strain to S. chartarum and BALB/c is the most responsive, as indicated by both cellular and cytokine changes in lung lavage fluid (Rosenblum Lichtenstein et al., 2006). The nature of these strain differences seems to be unique to S. chartarum. Pulmonary exposure to Coccidioides immitis, C. neoformans, and Aspergillus fumigatus show that C57BL/6 mice are more sensitive to infection and inflammation and that BALB/c are more resistant (Cox et al., 1988; Hoag et al., 1995; Stephens-Romero et al., 2005; Zaragoza et al., 2007). Moreover, murine endotoxin susceptibility also shows a different pattern of strain responsiveness (Wells et al., 2003). Stachybotrys chartarum is the only fungus that we know of to which BALB/c mice are more sensitive than C57BL/6J mice.
It has been reported that C57BL/6J are generally poor at clearing other types of mold, such as Histoplasma capsulatum, B. anthracis, Coccidioides posadasii, and A. fumigatus, which in part explains their increased susceptibility to these molds (Ehrchen et al., 2008; Lyons et al., 2004; Stephens-Romero et al., 2005; Wu-Hsieh, 1989). In contrast, we showed that C57BL/6J mice may use bulk transport out of the lungs via the mucociliary escalator as a first step toward efficient clearance of the spores and their constituents from the lungs. BALB/c mice, on the other hand, may retain more spores in the lungs, which more effectively induces cytokine release, cascades of cytokine effects, and infiltration of neutrophils.
C57BL/6J AMs also have significantly increased cell death compared with BALB/c. Increased AM death may lead to less cytokine release because dead cells cannot release inflammatory mediators. This results in less pulmonary injury and inflammation as measured in bronchoalveolar lavage parameters.
Similarly, macrophage apoptosis, which appears to be a contributing mechanism for cell death, has been reported to aid in microbial killing, to downregulate the inflammatory response, and even to aid in pulmonary clearance in pneumococcal and streptococcal models (Dockrell et al., 2003; Marriott and Dockrell, 2006; Marriott et al., 2006). If the greater rate of cell death in AMs from C57BL/6J mice is attributable to apoptosis, then the C57BL/6J mice might be expected to have less pulmonary inflammation and better clearance, a scenario compatible with our results. In addition, in a C. neoformans model, depletion of AMs was shown to be protective against infection (Kechichian et al., 2007).
These two mechanisms in which cell death leads to less inflammation may interact. For example, we showed that BALB/c mice have focal inflammation consisting primarily of AMs after administration of S. chartarum into the lungs (Rosenblum Lichtenstein et al., 2006). This focal inflammation may have blocked some of the mouse's airways and might physically impede clearance of the spores that caused the initial inflammation.
SG accounts for 15–30% of the toxicity of SST. Other trichothecenes, proteins, and mycotoxins may account for the remainder of the cytotoxicity. In addition, various mycotoxins may act synergistically to account for the toxicity of SST. The significant dose response to SG and SST in AMs from both mouse strains suggests that SG accounts for a significant amount of the cytotoxicity of S. chartarum.
Genetic variation in the human population that leads to decreased rates of AM apoptosis in the presence of S. chartarum could lead to increased spore-induced cytokine release, increased inflammation, decreased spore clearance, and ultimately to a higher pulmonary spore exposure over time, exacerbating the effects of spore exposure. Small differences in rates of apoptosis could be magnified by downstream effects leading to greater differences in response to S. chartarum exposure. In addition, it has been hypothesized that exposure to cigarette smoke might make infants more susceptible to acute idiopathic pulmonary hemorrhage (Etzel et al., 1998). Interestingly, it has been reported that cigarette smoke exposure inhibits the natural activity of the mucociliary escalator (Cohen et al., 1979). Such an environmental cause of reduced clearance might further exacerbate pulmonary hemorrhage in an already susceptible infant.
Through this and previous work, we have demonstrated that different mouse strains respond differently to S. chartarum. We interpret the data as showing that both reduced clearance of and higher macrophage cytotoxicity from the bioactive components of S. chartarum in humans might cause similar differences in susceptibility among the human population. The results from this study are the first evidence that differences in spore clearance and macrophage susceptibility to spore-induced death may contribute to strain differences in susceptibility to S. chartarum seen in vivo. That these differences have a genetic basis is not unlikely.
A Centers for Disease Control (CDC) report concluded that S. chartarum was responsible for acute idiopathic pulmonary hemorrhage in a cluster of infants (Dearborn et al., 1999; Etzel et al., 1998), although there may have been other contributing factors such as environmental tobacco smoke or consequences of water damage including bacterial exposures (Dearborn et al., 1999). The original CDC report and a later case study suggested that African-American babies were more likely than Caucasian babies to die of acute idiopathic pulmonary hemorrhage after exposure to S. chartarum (Dearborn et al., 1999, 2002; Etzel et al., 1998). It is unclear if the observed ethnic differences were because of choice of controls or to economic or other environmental factors that are correlates of race, genetic differences, or a combination of both.
Genetic variability in human populations may account for some of the wide variation among individuals responding to mold exposure in contaminated occupational and domestic settings. In addition, other concomitant conditions, such as cigarette smoke exposure or bacterial infection, might further exacerbate poor spore clearance in susceptible populations. Future directions include determination of the genetic loci responsible for differential clearance of S. chartarum and their interactions with environmental factors. It would be of further interest to test the effects of cigarette smoke or concomitant exposure to other pathogens on spore clearance.
FUNDING
National Institutes of Health (NIH HL007118).
Acknowledgments
We thank Barbara Burleigh, Lester Kobzik, Joseph Mizgerd, Amy Imrich, and Lauren Oleson for their helpful comments about this work. We thank Lynnelle McNamee and Melissa Curran for their critical reading of the manuscript.
References
- Andersen B, Nielsen KF, Jarvis BB. Characterization of Stachybotrys from water-damaged buildings based on morphology, growth, and metabolite production. Mycologia. 2002;94:392–403. [PubMed] [Google Scholar]
- Andersen B, Nielsen KF, Thrane U, Szaro T, Taylor JW, Jarvis BB. Molecular and phenotypic descriptions of Stachybotrys chlorohalonata sp. nov. and two chemotypes of Stachybotrys chartarum found in water-damaged buildings. Mycologia. 2003;95:1227–1238. doi: 10.1080/15572536.2004.11833031. [DOI] [PubMed] [Google Scholar]
- Bae HK, Shinozuka J, Islam Z, Pestka JJ. Satratoxin G interaction with 40S and 60S ribosomal subunits precedes apoptosis in the macrophage. Toxicol. Appl. Pharmacol. 2009;237:137–145. doi: 10.1016/j.taap.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YJ, Jarvis BB, Tak H, Pestka JJ. Immunochemical assay for satratoxin G and other macrocyclic trichothecenes associated with indoor air contamination by Stachybotrys chartarum. Toxicol. Mech. Meth. 2003a;13:247–252. doi: 10.1080/713857196. [DOI] [PubMed] [Google Scholar]
- Chung YJ, Yang GH, Islam Z, Pestka JJ. Up-regulation of macrophage inflammatory protein-2 and complement 3A receptor by the trichothecenes deoxynivalenol and satratoxin G. Toxicology. 2003b;186:51–65. doi: 10.1016/s0300-483x(02)00605-4. [DOI] [PubMed] [Google Scholar]
- Cohen D, Arai SF, Brain JD. Smoking impairs long-term dust clearance from the lung. Science. 1979;204:514–7. doi: 10.1126/science.432655. [DOI] [PubMed] [Google Scholar]
- Cooley JD, Wong WC, Jumper CA, Straus DC. Correlation between the prevalence of certain fungi and sick building syndrome. Occup. Environ. Med. 1998;55:579–84. doi: 10.1136/oem.55.9.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RA, Kennell W, Boncyk L, Murphy JW. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect. Immun. 1988;56:13–7. doi: 10.1128/iai.56.1.13-17.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn DG, Smith PG, Dahms BB, Allan TM, Sorenson WG, Montana E, Etzel RA. Clinical profile of 30 infants with acute pulmonary hemorrhage in Cleveland. Pediatrics. 2002;110:627–637. doi: 10.1542/peds.110.3.627. [DOI] [PubMed] [Google Scholar]
- Dearborn DG, Yike I, Sorenson WG, Miller MJ, Etzel RA. Overview of investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environ. Health Perspect. 1999;107(Suppl. 3):495–499. doi: 10.1289/ehp.99107s3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MK. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 2003;171:5380–5388. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]
- Ehrchen JM, Roth J, Roebrock K, Varga G, Domschke W, Newberry R, Sorg C, Muller-Tidow C, Sunderkotter C, Kucharzik T, et al. The absence of cutaneous lymph nodes results in a Th2 response and increased susceptibility to Leishmania major infection in mice. Infect. Immun. 2008;76:4241–4250. doi: 10.1128/IAI.01714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel RA, Montana E, Sorenson WG, Kullman GJ, Allan TM, Dearborn DG, Olson DR, Jarvis BB, Miller JD. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch. Pediatr. Adolesc. Med. 1998;152:757–762. doi: 10.1001/archpedi.152.8.757. [DOI] [PubMed] [Google Scholar]
- Hastings C, Rand T, Bergen HT, Thliveris JA, Shaw AR, Lombaert GA, Mantsch HH, Giles BL, Dakshinamurti S, Scott JE. Stachybotrys chartarum alters surfactant-related phospholipid synthesis and CTP:cholinephosphate cytidylyltransferase activity in isolated fetal rat type II cells. Toxicol. Sci. 2005;84:186–194. doi: 10.1093/toxsci/kfi045. [DOI] [PubMed] [Google Scholar]
- Hendry KM, Cole EC. A review of mycotoxins in indoor air. J. Toxicol. Environ. Health. 1993;38:183–198. doi: 10.1080/15287399309531711. [DOI] [PubMed] [Google Scholar]
- Hoag KA, Street NE, Huffnagle GB, Lipscomb MF. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 1995;13:487–495. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- Hudson B, Flemming J, Sun G, Rand TG. Comparison of immunomodulator mRNA and protein expression in the lungs of Stachybotrys chartarum spore-exposed mice. J. Toxicol. Environ. Health. 2005;68:1321–1335. doi: 10.1080/15287390590953572. [DOI] [PubMed] [Google Scholar]
- Islam Z, Harkema JR, Pestka JJ. Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ. Health Perspect. 2006;114:1099–1107. doi: 10.1289/ehp.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Hegg CC, Bae HK, Pestka JJ. Satratoxin G-induced apoptosis in PC-12 neuronal cells is mediated by PKR and caspase independent. Toxicol. Sci. 2008;105:142–152. doi: 10.1093/toxsci/kfn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Shinozuka J, Harkema J, Pestka JJ. Purification and comparative neurotoxicity of the trichothecenes satratoxin G and roridin L2 from Stachybotrys chartarum. J. Toxicol. Environ. Health A. 2009;72:1242–1251. doi: 10.1080/15287390903129234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankkunen P, Rintahaka J, Aalto A, Leino M, Majuri ML, Alenius H, Wolff H, Matikainen S. Trichothecene mycotoxins activate inflammatory response in human macrophages. J. Immunol. 2009;182:6418–6425. doi: 10.4049/jimmunol.0803309. [DOI] [PubMed] [Google Scholar]
- Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect. Immun. 2007;75:4792–8. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen FO, Christensen LH, Clementsen P, Gravesen S, Stahl Skov P, Norn S. Microfungi in indoor air are able to trigger histamine release by non-IgE-mediated mechanisms. Inflamm. Res. 1996;45(Suppl. 1):S23–S24. doi: 10.1007/BF03354071. [DOI] [PubMed] [Google Scholar]
- Lyons CR, Lovchik J, Hutt J, Lipscomb MF, Wang E, Heninger S, Berliba L, Garrison K. Murine model of pulmonary anthrax: kinetics of dissemination, histopathology, and mouse strain susceptibility. Infect. Immun. 2004;72:4801–4809. doi: 10.1128/IAI.72.8.4801-4809.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott HM, Dockrell DH. Streptococcus pneumoniae: the role of apoptosis in host defense and pathogenesis. Int. J. Biochem. Cell Biol. 2006;38:1848–1854. doi: 10.1016/j.biocel.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Marriott HM, Hellewell PG, Cross SS, Ince PG, Whyte MK, Dockrell DH. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J. Immunol. 2006;177:6480–6488. doi: 10.4049/jimmunol.177.9.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, Yoshikai Y. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Diagn. Lab. Immunol. 2003;10:259–266. doi: 10.1128/CDLI.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae KC, Rand TG, Shaw RA, Mantsch HH, Sowa MG, Thliveris JA, Scott JE. DNA fragmentation in developing lung fibroblasts exposed to Stachybotrys chartarum (atra) toxins. Pediatr. Pulmonol. 2007;42:592–599. doi: 10.1002/ppul.20608. [DOI] [PubMed] [Google Scholar]
- Metcalf JA, Gallin JI, Nauseef WM, Root RK. Reagents—Isotonic Lysing Medium. 1986. pp. 93. Raven Press, NY. [Google Scholar]
- Murtoniemi T, Nevalainen A, Hirvonen MR. Effect of plasterboard composition on Stachybotrys chartarum growth and biological activity of spores. Appl. Environ. Microbiol. 2003;69:3751–3757. doi: 10.1128/AEM.69.7.3751-3757.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulin M, Reijula K, Jarvis BB, Hintikka EL. Experimental lung mycotoxicosis in mice induced by Stachybotrys atra. Int. J. Exp. Pathol. 1996;77:213–218. doi: 10.1046/j.1365-2613.1996.9250323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulin M, Reijula K, Jarvis BB, Veijalainen P, Hintikka EL. Effects of intranasal exposure to spores of Stachybotrys atra in mice. Fundam. Appl. Toxicol. 1997;35:182–188. [PubMed] [Google Scholar]
- Penttinen P, Tampio M, Maki-Paakkanen J, Vahakangas K, Pelkonen J, Hirvonen MR. DNA damage and p53 in RAW264.7 cells induced by the spores of co-cultivated Streptomyces californicus and Stachybotrys chartarum. Toxicology. 2007;235:92–102. doi: 10.1016/j.tox.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Yike I, Dearborn DG, Ward MD, Harkema JR. Stachybotrys chartarum, trichothecene mycotoxins, and damp building-related illness: new insights into a public health enigma. Toxicol. Sci. 2008;104:4–26. doi: 10.1093/toxsci/kfm284. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Rand TG, Flemming J, David Miller J, Womiloju TO. Comparison of inflammatory responses in mouse lungs exposed to atranones A and C from Stachybotrys chartarum. J. Toxicol. Environ. Health. 2006;69:1239–1251. doi: 10.1080/15287390500360307. [DOI] [PubMed] [Google Scholar]
- Rand TG, Mahoney M, White K, Oulton M. Microanatomical changes in alveolar type II cells in juvenile mice intratracheally exposed to Stachybotrys chartarum spores and toxin. Toxicol. Sci. 2002;65:239–245. doi: 10.1093/toxsci/65.2.239. [DOI] [PubMed] [Google Scholar]
- Rand TG, Miller JD. Immunohistochemical and immunocytochemical detection of SchS34 antigen in Stachybotrys chartarum spores and spore impacted mouse lungs. Mycopathologia. 2008;165:73–80. doi: 10.1007/s11046-007-9080-1. [DOI] [PubMed] [Google Scholar]
- Rand TG, White K, Logan A, Gregory L. Histological, immunohistochemical and morphometric changes in lung tissue in juvenile mice experimentally exposed to Stachybotrys chartarum spores. Mycopathologia. 2003;156:119–131. doi: 10.1023/a:1022920205355. [DOI] [PubMed] [Google Scholar]
- Rao CY, Burge HA, Brain JD. The time course of responses to intratracheally instilled toxic Stachybotrys chartarum spores in rats. Mycopathologia. 2000;149:27–34. doi: 10.1023/a:1007239017018. [DOI] [PubMed] [Google Scholar]
- Rosenblum Lichtenstein JH, Molina RM, Donaghey TC, Brain JD. Strain differences influence murine pulmonary responses to Stachybotrys chartarum. Am. J. Respir. Cell Mol. Biol. 2006;35:415–423. doi: 10.1165/rcmb.2005-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert D, Wand MP, Carroll RJ. Semiparametric Regression. 2003. pp. 59–60. Cambridge University Press, Cambridge, UK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Porter K, Parameswaran N, Bae HK, Pestka JJ. Role of GRP78/BiP degradation and ER stress in deoxynivalenol-induced interleukin-6 upregulation in the macrophage. Toxicol. Sci. 2009;109:247–255. doi: 10.1093/toxsci/kfp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens-Romero SD, Mednick AJ, Feldmesser M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect. Immun. 2005;73:114–125. doi: 10.1128/IAI.73.1.114-125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SH, Chen H, Jen CJ. C57BL/6 and BALB/c bronchoalveolar macrophages respond differently to exercise. J. Immunol. 2001;167:5084–5091. doi: 10.4049/jimmunol.167.9.5084. [DOI] [PubMed] [Google Scholar]
- Taconic. 2010a. Available at: http://www.taconic.com/wmspage.cfm?parm1=768. Accessed April 19, 2010. [Google Scholar]
- Taconic. 2010b. Available at: http://www.taconic.com/wmspage.cfm?parm1=760. Accessed April 19, 2010. [Google Scholar]
- Vesper SJ, Vesper MJ. Stachylysin may be a cause of hemorrhaging in humans exposed to Stachybotrys chartarum. Infect. Immun. 2002;70:2065–2069. doi: 10.1128/IAI.70.4.2065-2069.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana ME, Coates NH, Gavett SH, Selgrade MK, Vesper SJ, Ward MD. An extract of Stachybotrys chartarum causes allergic asthma-like responses in a BALB/c mouse model. Toxicol. Sci. 2002;70:98–109. doi: 10.1093/toxsci/70.1.98. [DOI] [PubMed] [Google Scholar]
- Wang H, Yadav JS. DNA damage, redox changes, and associated stress-inducible signaling events underlying the apoptosis and cytotoxicity in murine alveolar macrophage cell line MH-S by methanol-extracted Stachybotrys chartarum toxins. Toxicol. Appl. Pharmacol. 2006;214:297–308. doi: 10.1016/j.taap.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Wang H, Yadav JS. Global gene expression changes underlying Stachybotrys chartarum toxin-induced apoptosis in murine alveolar macrophages: evidence of multiple signal transduction pathways. Apoptosis. 2007;12:535–548. doi: 10.1007/s10495-006-0008-x. [DOI] [PubMed] [Google Scholar]
- Wells CA, Ravasi T, Faulkner GJ, Carninci P, Okazaki Y, Hayashizaki Y, Sweet M, Wainwright BJ, Hume DA. Genetic control of the innate immune response. BMC Immunol. 2003;4:5. doi: 10.1186/1471-2172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B. Relative susceptibilities of inbred mouse strains C57BL/6 and A/J to infection with Histoplasma capsulatum. Infect. Immun. 1989;57:3788–3792. doi: 10.1128/iai.57.12.3788-3792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yike I, Dearborn DG. Pulmonary effects of Stachybotrys chartarum in animal studies. Adv. Appl. Microbiol. 2004;55:241–273. doi: 10.1016/S0065-2164(04)55009-8. [DOI] [PubMed] [Google Scholar]
- Yike I, Rand T, Dearborn DG. The role of fungal proteinases in pathophysiology of Stachybotrys chartarum. Mycopathologia. 2007;164:171–181. doi: 10.1007/s11046-007-9037-4. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect. Immun. 2007;75:2729–2739. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]