Abstract
Studies in mice and guinea pigs have shown that albumin is a new biomarker of organophosphorus toxicant (OP) and nerve agent exposure. Our goal was to determine whether OP-labeled albumin could be detected in the blood of humans exposed to OP. Blood from four OP-exposed patients was prepared for mass spectrometry analysis by digesting 0.010 ml of serum with pepsin and purifying the labeled albumin peptide by offline high performance liquid chromatography. Dimethoxyphosphate-labeled tyrosine 411 was identified in albumin peptides VRY411TKKVPQVSTPTL and LVRY411TKKVPQVSTPTL from two patients who had attempted suicide with dichlorvos. The butyrylcholinesterase activity in these serum samples was inhibited 80%. A third patient whose serum BChE activity was inhibited 8% by accidental inhalation of dichlorvos had undetectable levels of adduct on albumin. A fourth patient whose BChE activity was inhibited 60% by exposure to chlorpyrifos had no detectable adduct on albumin. This is the first report to demonstrate the presence of OP-labeled albumin in human patients. It is concluded that tyrosine 411 of human albumin is covalently modified in the serum of humans poisoned by dichlorvos and that the modification is detectable by mass spectrometry. The special reactivity of tyrosine 411 with OP suggests that other proteins may also be modified on tyrosine. Identification of other OP-modified proteins may lead to an understanding of neurotoxic symptoms that appear long after the initial OP exposure.
Keywords: organophosphorus toxicant, albumin, butyrylcholinesterase, mass spectrometry
Organophosphorus toxicants (OP) are toxic chemicals used in agriculture, medicine, and warfare. Their acute toxicity is due to inhibition of acetylcholinesterase in the cholinergic nervous system by covalent modification of the active site serine. OP are also highly effective inhibitors of butyrylcholinesterase (BChE, accession number gi:116353), though inhibition of BChE has no known clinical sequelae. Carbamates also inhibit cholinesterases by covalently binding to the active site serine. Acylpeptide hydrolase in red blood cells is 10 times more reactive with dichlorvos, chlorpyrifos oxon, and diisopropylfluorophosphate than acetylcholinesterase and has the potential to serve as a biomarker of OP exposure (Quistad et al., 2005; Richards et al., 2000).
We have successfully used mass spectrometry to identify covalently labeled BChE peptides from patients poisoned with the OP, dichlorvos and chlorpyrifos, and with the carbamates, carbofuran and Aldicarb (Li et al., 2009, 2010). To find these peptides, we had to develop methods for the purification of carbamate-labeled and OP-labeled BChE from 2 ml plasma or serum. We were then able to use mass spectrometry to demonstrate covalent binding of these poisons to serine 198 of human BChE, to identify the mass of the adduct on serine 198, and to deduce the type of pesticide to which the patient was exposed.
The purpose of the present work was to determine whether albumin is labeled in people who have been exposed to OP. Mice (Peeples et al., 2005) and guinea pigs (Read et al., 2010; Williams et al., 2007) treated with OP in vivo have covalently bound OP on albumin. Mass spectrometry has identified tyrosine 411 of human albumin (accession number gi:122920512) as the residue that is covalently modified by dichlorvos, chlorpyrifos oxon, diisopropylfluorophosphate, soman, sarin, and fluorophosphinate-biotin when human albumin or plasma is treated ex vivo with these OP (Li et al., 2007).
The rate of reaction of albumin with OP is slow compared with the rate of reaction of BChE with OP (Li et al., 2008). However, there is a 10,000-fold higher concentration of albumin (0.6mM; 40,000 mg/l) compared with BChE (50nM; 4 mg/l); in human serum. This difference in concentration compensates for the slow reactivity, resulting in OP labeling of albumin in vivo. The most reactive residue in human albumin is tyrosine 411, though other tyrosines are also labeled when conditions are maximized in vitro (Ding et al., 2008; Means and Wu, 1979). Tyrosine 411 can be found in peptic peptides VRY411TKKVPQVSTPTL and LVRY411TKKVPQVSTPTL, which are related by a missed cleavage. The most reactive residue in BChE is serine 198 in the tryptic peptide SVTLFGES198AGAASVSLHLLSPGSHSLFTR.
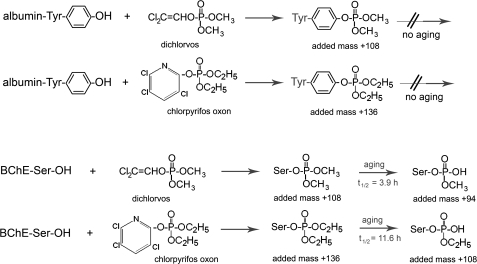
The adducts on albumin and BChE after reaction with dichlorvos and chlorpyrifos oxon are shown in Figure 1. OP-albumin adducts are stable, whereas OP-BChE adducts lose an alkyl group in a process called “aging.” The present report is the first to identify OP-albumin adducts in humans exposed to OP.
FIG. 1.
Covalent binding of dichlorvos and chlorpyrifos oxon to albumin and BChE. Tyrosine 411 of human albumin makes a covalent bond with dichlorvos, adding a mass of 108 amu, and, with chlorpyrifos oxon, adding a mass of 136 amu. Adducts on tyrosine do not age. Serine 198 of human BChE makes covalent bonds with dichlorvos and chlorpyrifos oxon. The BChE adducts dealkylate during the aging process so that the added masses characteristically observed in the mass spectrometer are 94 and 108 amu. The dimethoxyphosphate adduct on human BChE ages with a half-time of 3.9 h (Worek et al., 1999), whereas the diethoxyphosphate adduct ages with a half-time of 11.6 h (Masson et al., 1997). The amino acid sequence of human BChE is in accession number gi:116353 in the National Center for Biotechnology Information nonredundant database and that of human albumin in accession number gi:122920512.
MATERIAL AND METHODS
Materials
Pepsin (Sigma, St Louis, MO; catalog number P6887 from porcine gastric mucosa) 1 mg/ml in 10mM HCl was stored at −80°C. Alpha-cyano 4-hydroxycinnamic acid (CHCA; Sigma; catalog number 70990) was recrystallized before use. A 10 mg/ml solution in 50% acetonitrile, 0.1% trifluoroacetic acid was stored at room temperature in the dark. Purified human serum albumin, essentially fatty acid free (Fluka via Sigma; catalog number 05418), butyrylthiocholine (catalog number B3253), and dithiobisnitrobenzoic acid (catalog number D8130) were from Sigma. Modified trypsin (Promega, Madison, WI; catalog number V5113) 0.4 μg/μl in 50mM acetic acid was stored at −80°C. Chlorpyrifos oxon 98% pure and dichlorvos 98% pure were from ChemService Inc. (West Chester, PA; catalog numbers MET-674B and PS-89, respectively).
Human serum.
Serum samples from three attempted suicides and one accidentally poisoned individual were provided by Dr Ivan Ricordel, Paris Police in a protocol approved by the Institutional Review Board of the University of Nebraska Medical Center. The samples were shipped on dry ice and stored at −80°C. The name of the poison in three cases was established from the reports of family members who found bottles of dichlorvos pesticide in the home. The name of the poison in one case was unknown but was identified as chlorpyrifos (Li et al., 2010).
The two patients who attempted suicide with dichlorvos had severe cholinergic symptoms, including bilateral myosis and hypersalivation. One patient was unconscious when admitted to the hospital. The other was drowsy without coma. The patient who accidentally inhaled dichlorvos had a brief period of respiratory difficulty and paresthesis in four limbs but no other symptoms. Nothing is known about the patient who attempted suicide with chlorpyrifos.
BChE activity assay.
BChE activity was measured with 1mM butyrylthiocholine in 0.1M potassium phosphate pH 7.0 in the presence of 0.5mM dithiobisnitrobenzoic acid by measuring the increase in absorbance at 412 nm at 25°C. The reaction rate in delta absorbance per minute was converted to micromoles per minute using the extinction coefficient E = 13600/M·cm (Ellman et al., 1961). One unit of activity is defined as 1 μmol of substrate hydrolyzed per minute.
Pepsin digestion to release labeled albumin peptide.
From previous work, it was known that tyrosine 411 of human albumin is labeled by OP and that the labeled peptide is easily released by digestion with pepsin (Li et al., 2007, 2008). It was also known that the labeled albumin peptide could be detected in the mass spectrometer if the peptide was isolated by offline high performance liquid chromatography (HPLC) before liquid chromatography tandem mass spectrometry (LCMSMS). A 0.01 ml aliquot of human serum was adjusted to pH 2.5 by addition of 0.01 ml of 1% trifluoroacetic and digested with 2.5 μg of pepsin for 2 h at 37°C.
Offline HPLC purification of the peptic albumin peptides from patient samples.
Peptides in the digested serum were purified by HPLC (Waters LC 625 system) on a Phenomenex Prodigy, 5 μ C18 column 100 × 4.6mm eluted with a 60-min gradient starting at 0.1% trifluoroacetic acid in water and ending at 60% acetonitrile, 0.1% trifluoroacetic acid, at a flow rate of 1 ml/min. One-milliliter fractions were collected. A 1 μl aliquot from each 1 ml fraction was analyzed by matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry to identify fractions containing the unlabeled albumin tyrosine 411 peptides VRY411TKKVPQVSTPTL m/z 1717 and LVRY411TKKVPQVSTPTL m/z 1830. When starting with 10 μl of serum from a patient sample, the unlabeled peptides were detectable by MALDI-TOF but the labeled peptides were present at too low a level to be detectable. However, the elution position of the labeled peptides could be estimated from previous studies. The unlabeled albumin tyrosine 411 peptides eluted between 19–24% acetonitrile, whereas labeled peptides eluted 1–8 min later. HPLC fractions predicted to contain the labeled peptides were dried in a vacuum centrifuge and dissolved in 50 μl of 5% of acetonitrile, 0.1% formic acid in preparation for analysis on the QTRAP 4000 mass spectrometer.
Reaction of human plasma with chlorpyrifos oxon and dichlorvos.
Human plasma was treated with 0.1–1.5mM chlorpyrifos oxon or dichlorvos for 16 h at 37°C. A 10 μl aliquot was mixed with 10 μl of 1% trifluoroacetic acid before digestion with 1 μl of 1 mg/ml pepsin. After 2-h incubation at 37°C, the digest was diluted 400-fold with 0.1% trifluoroacetic acid and 1 μl was spotted on a matrix assisted laser desorption ionization (MALDI) plate.
The labeled and unlabeled albumin tyrosine 411 peptides were identified in the same MALDI-TOF mass spectrum. Unlabeled peptides had masses of 1717 and 1830 amu. Chlorpyrifos oxon–labeled peptides had masses of 1853 and 1966 amu. Dichlorvos-labeled peptides had masses of 1825 and 1938 amu. Relative quantities of labeled and unlabeled peptides were calculated by comparison of cluster areas using the Data Explorer software. This method of quantitation assumes that the labeled and unlabeled peptides ionize with similar efficiencies. Each sample serves as its own internal control because the peptides are in the same MALDI spot in the same MALDI spectrum.
MALDI-TOF mass spectrometry.
A 1 μl aliquot of essentially salt-free sample was spotted onto a 384 well Opti-TOF sample plate (#1016491; Applied Biosystems, Foster City, CA). After the spot was dry, it was overlaid with 1 μl of CHCA matrix. Mass spectrometry spectra were acquired with a matrix assisted laser desorption ionization tandem time of flight 4800 mass spectrometer (Applied Biosystems), in positive reflector mode, with laser intensity at 4000 V, using delayed extraction, and default calibration. Spectra were saved to Data Explorer V4.9 software for analysis. Each spectrum was the sum of 500 laser shots. The mass spectrometer was calibrated with Cal Mix 5 (bradykinin, 2–9 clip; angiotensin I; Glu-fibrinopeptide B; adrenocorticotropic hormone [ACTH], 1–17 clip; ACTH, 18–39 clip; and ACTH, 7–38 clip from Applied Biosystems Inc., Framingham, MA).
Multiple reaction monitoring on the QTRAP 4000 mass spectrometer (Applied Biosystems).
Five microliter aliquots from selected fractions of HPLC-purified peptic peptides from patient sera were injected onto a Vydac C18 polymeric reverse-phase nanocolumn for a second phase of HPLC separation. Peptides were separated on a HPLC nanocolumn (#218MS3.07515 Vydac C18 polymeric rev-phase, 75 μm i.d. × 150 mm long; P.J. Cobert Assoc, St Louis, MO) with a 90 min linear gradient from 0 to 60% acetonitrile, 0.1% formic acid, at 300 nl/min, and electrosprayed through a fused silica emitter (360 μm o.d., 75 μm i.d., 15 μm taper, New Objective) directly into the QTRAP 4000, a hybrid quadrupole linear ion trap mass spectrometer. The mass spectrometer was calibrated on selected fragments from the tandem mass spectrometry (MSMS) spectrum of Glu-Fibrinopeptide B. The MSMS data were collected and processed using Analyst 1.4.1 software (Applied Biosystems).
The QTRAP 4000 was operated in multiple reaction monitoring (MRM) mode. The MRM algorithm screens ions entering the mass spectrometer for selected parent ion masses, fragments the selected parent ions when they appear, and examines the fragments for selected product ions. A signal is recorded when both the correct parent and the product ions are observed. Preliminary experiments showed that triply charged parent ions gave better signals in the QTRAP mass spectrometer than did doubly charged parent ions. For dichlorvos-labeled samples, a strong product ion fragment was observed at 748.8 amu ( from parent peptide VRY411TKKVPQVSTPTL, [M + 3H]+3 = 609.4 amu. A strong product ion was observed at 805.3 amu from parent peptide LVRY411TKKVPQVSTPTL, [M + 3H]+3 = 647.4 amu. These parent/product ion pairs were used in the MRM experiments on dichlorvos-labeled samples. The dwell time for collecting the MRM signals was 40 ms, collision energy was 30 V, and collision gas was pure nitrogen (40 μTorr). Data were collected using an Information Directed Acquisition protocol that triggered the collection of an enhanced product ion spectrum (MSMS) following the detection of a peptide of interest by the MRM algorithm. The enhanced product ion spectrum was taken using the trap function of the QTRAP mass spectrometer. Collision energy was 30 V, collision gas was pure nitrogen (40 μTorr), scan rate was 4000 Da/s, and 10 enhanced product ion scans were summed for each spectrum.
LCMSMS on the QTRAP 4000 mass spectrometer.
Samples that gave a positive result in the MRM method were also analyzed by LCMSMS. In addition, the LCMSMS method was used to test unfractionated pepsin-digested serum. The digested serum was diluted 2000-fold with 5% acetonitrile, 0.1% formic acid to reduce the protein concentration to 0.5 μg/μl (∼7 pmol albumin per μl). Protein concentration was estimated from absorbance at 280 nm in the NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE) relative to albumin standards. Five microliter of the diluted digest were separated on an HPLC nanocolumn and electrosprayed into the QTRAP 4000 mass spectrometer as described for the MRM experiment.
An information directed acquisition protocol triggered the collection of an enhanced resolution spectrum and an enhanced product ion spectrum on the four most intense ions entering the mass spectrometer having an m/z between 200 and 1500, a charge state of +2 to +4 and an intensity greater than 500,000 counts per second. After an ion was analyzed twice, it was excluded from analysis for 60 s. Collision energy was determined by the mass spectrometer based on mass and charge state of the ion. Collision gas was pure nitrogen (40 μTorr), and the scan rate was 4000 Da/s. Because of the time required to obtain this series of scans, only one enhanced product ion scan was taken for each ion. An ion spray voltage of 1900 V was maintained between the emitter and the mass spectrometer. The mass spectrometer was calibrated using MSMS fragments of Glu-Fibrinopeptide B.
The MSMS data files were searched for spectra that included a singly charged mass of 330 amu. The 330 amu mass is the y3 ion in both labeled and unlabeled albumin peptides VRYTKKVPQVSTPTL and LVRYTKKVPQVSTPTL. The extracted ion chromatogram feature of the Analyst software (version 1.4) was used for the search. MSMS spectra were accepted if the other masses in the spectra matched the fragment masses for the albumin peptides. Predicted fragment masses, for comparison with the observed fragment masses, were calculated with the aid of the fragment ion calculator in the Proteomics Toolkit (http://db.systemsbiology.net:8080/proteomicsToolkit/FragionServlet.html).
Infusion into the QTRAP 4000 mass spectrometer.
The MRM and LCMSMS protocols proved that the VRYTKKVPQVSTPTL and LVRYTKKVPQVSTPTL peptides had a mass 108 amu higher than the mass of the unlabeled peptides. However, the MSMS spectra did not show the b ion masses that proved the adduct was on tyrosine 411. To obtain proof that the reaction of dichlorvos with albumin resulted in covalent modification of tyrosine 411, we treated 0.1 ml of a 50 mg/ml solution of pure human albumin in 25mM NH4HCO3 pH 8.5 with 1.5mM dichlorvos overnight. The albumin was digested with pepsin and the peptides purified by offline HPLC. Purified peptides were dried and dissolved in 50% acetonitrile, 25% methanol, and 1% acetic acid in preparation for infusion. Samples were infused into the QTRAP mass spectrometer because infusion allows one to sum hundreds of MSMS spectra into one final spectrum, whereas the MRM method sums ten spectra and the LCMSMS method only one MSMS spectrum. The improved signal-to-noise ratio after summing 500 MSMS spectra in the infusion method reveals low-intensity ions.
Initially, a mass spectrum was taken to identify the ions in the sample. Masses consistent with those expected for dichlorvos-labeled peptic peptides were then fragmented in the mass spectrometer. Collision gas was nitrogen (40 μTorr), collision energy was optimized for maximum fragment information, and the scan rate was 4000 Da/s. The final spectrum was the sum of 500 MSMS spectra.
RESULTS
MRM for Detection of Dichlorvos-Albumin Adducts
Use of the MRM feature of the mass spectrometer requires one to know the masses of the parent and product ions that best indicate the presence of the desired peptide. This information was obtained from MSMS spectra of dichlorvos-labeled pure human albumin peptic peptides. The MSMS spectra acquired in the QTRAP 4000 mass spectrometer showed that the parent ions were most readily detectable in the triply charged state. The b12 product ion (also called transition and daughter ion) in charge state + 2 was intense for the OP-labeled VRYTKKVPQVSTPTL peptide. The b13 product ion in charge state + 2 was intense for the OP-labeled LVRYTKKVPQVSTPTL peptide. Therefore, the triply charged parent ion masses and the doubly charged product ion masses listed in Table 1 were selected for MRM.
TABLE 1.
MRM Transitions for the Albumin Peptides
| Albumin peptide VRYTKKVPQVSTPTL | Parent ion, charge + 3 m/z | Product ion b12, charge + 2 m/z |
| No label | 573.3 | 694.8 |
| Dichlorvos labeled; added mass + 108 | 609.4 | 748.8 |
| Albumin peptide LVRYTKKVPQVSTPTL | Parent ion, charge + 3 m/z | Product ion b13, charge + 2 m/z |
| No label | 611.1 | 751.4 |
| Dichlorvos labeled; added mass + 108 | 647.4 | 805.3 |
Note. Average masses are listed. The accession number for human albumin in the NCBI nonredundant database is gi:122920512.
Sera from Dichlorvos-Poisoned Patients Contain Dichlorvos Adducts on Tyrosine 411 of Albumin
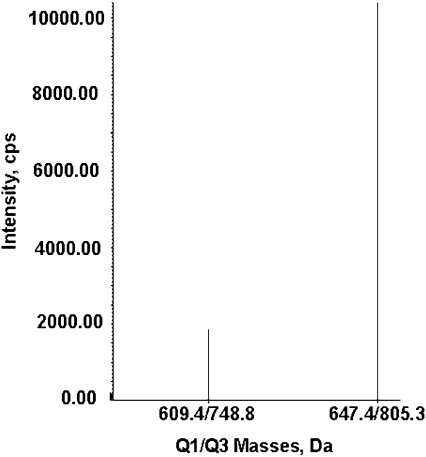
Albumin peptides partially purified from 0.010 ml patient serum were subjected to reverse-phase liquid chromatography followed by electrospray ionization and fragmentation in the triple quadrupole linear ion trap mass spectrometer. The mass spectrometer was programmed to search for the ion partners listed in Table 1. An example of an MRM hit is given in Figure 2, where two parent ions coeluted from the nanocolumn at the same time. Parent ion 609.4 m/z had product ion 748.8 m/z. Parent ion 647.4 had product ion 805.3. A positive MRM signal does not prove that the ion represents the peptide of interest. Proof comes from the MSMS spectrum, which is automatically acquired following the appearance of an MRM signal.
FIG. 2.
MRM transitions for dichlorvos-labeled albumin peptides. Two parent ions eluted at the same time from the nanocolumn. The triply charged parent ion at 609.4 m/z has a product ion at 748.8 m/z. The triply charged parent ion at 647.4 m/z has a product ion at 805.3 m/z. Q1 is the mass of the parent ion. Q3 is the mass of the product ion. MRM spectra, such as this, for dichlorvos-labeled albumin were obtained from the blood of two humans poisoned by dichlorvos.
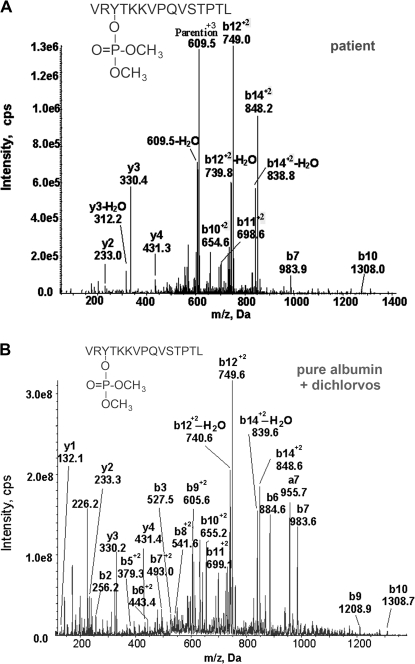
The MSMS spectrum for the triply charged parent ion at 609.4 m/z is given in Figure 3A. The mass of the parent ion is consistent with the peptide VRYTKKVPQVSTPTL plus an added mass from dichlorvos of 108 amu. The y and b ion series fit the predicted peptide sequence and appear with approximately the same relative intensities as the ions from a control sample of dichlorvos-labeled peptide (Figure 3B). These observations provide strong evidence that the 609.4 amu mass is dichlorvos-labeled albumin peptide VRYTKKVPQVSTPTL.
FIG. 3.
MSMS spectra of dichlorvos-labeled albumin peptide VRY411TKKVPQVSTPTL where dimethoxyphosphate is on tyrosine 411. (A) MSMS spectrum of the dichlorvos-labeled albumin peptide from the serum of a patient poisoned with dichlorvos. The spectrum was acquired in conjunction with the MRM method and is the sum of 10 MSMS spectra. The triply charged parent ion shows an m/z of 609.5 in this spectrum. (B) MSMS spectrum of the dichlorvos-labeled peptide prepared by in vitro treatment of pure human albumin with dichlorvos. The spectrum is the sum of 500 MSMS spectra acquired during infusion of purified peptides. The mass at 226.2 m/z is the dimethoxyphosphotyrosine immonium ion minus water. Masses are average.
There is no direct proof in this spectrum that the dimethoxyphosphate is attached to tyrosine because there are no signals for the b2 and b3 ions that define tyrosine 411. However, the masses of the remainder of the b ions in the spectrum (b7), (b10), (b11)+2, (b12)+2, and (b14)+2 are all consistent with the presence of the label. Therefore, the labeled residue must reside on the VRYTKKVP portion of the peptide. There are four nucleophilic residues in that portion of the peptide that could theoretically react with dichlorvos: tyrosine, threonine, and two lysines. The interpretation that the OP is covalently bound to tyrosine relies on comparison with the MSMS spectrum of pure albumin treated with dichlorvos where the b2 ion at 256.2 amu carries no label but the b3 ion at 527.5 amu has a mass consistent with dimethoxyphosphorylation of tyrosine (Figure 3B).
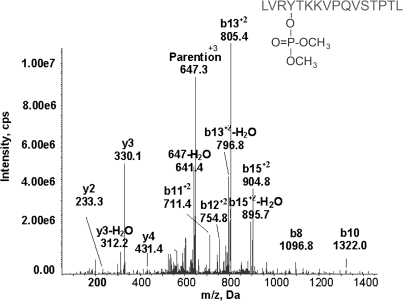
The MSMS spectrum for the second dichlorvos-labeled peptide isolated from a poisoned patient is given in Figure 4. The triply charged parent ion has a mass of 647.4 m/z, consistent with the peptide LVRYTKKVPQVSTPTL plus an added mass of 108 from dichlorvos. The y and b ion series fit the predicted peptide sequence. The masses of all observed b ions are consistent with an added mass of 108 amu on tyrosine.
FIG. 4.
MRM triggered MSMS spectrum of the dichlorvos-labeled albumin peptide, LVRY411TKKVPQVSTPTL. Serum from a patient poisoned with dichlorvos yielded the peptide modified on tyrosine 411 by dimethoxyphosphate. The triply charged parent ion has m/z 647.3.
The patient samples were reanalyzed by LCMSMS without using the MRM feature of the QTRAP 4000. The output mass spectrometry spectra were manually searched for the dichlorvos-labeled parent ions 609.4 and 647.4 m/z. Both parent ions were found. They eluted from the nanocolumn at about 40 min. The MSMS spectra acquired from LCMSMS analysis supported an added mass of 108 on peptides VRYTKKVPQVSTPTL and LVRYTKKVPQVSTPTL. However, peak intensities were lower than MSMS spectra acquired by MRM or by infusion. Peak intensities were 4 e4 for unfractionated digest analyzed by LCMSMS, 1.5 e5 for HPLC-purified digest analyzed by LCMSMS, 1.3 e6 and 1 e7 for HPLC-purified digest analyzed by MRM, and 3 e8 for infused sample. The difference in signal intensity is the result of being able to collect only one scan per MSMS spectrum in the LCMSMS protocol, as compared with 10 scans per MSMS spectrum in the MRM protocol and 500 scans per MSMS spectrum in the infusion protocol.
The Chlorpyrifos-Poisoned Patient
The pesticide sold for agricultural use is chlorpyrifos. Patients exposed to chlorpyrifos are analyzed for adducts from chlorpyrifos oxon because poison symptoms are caused by the oxon. Chlorpyrifos is a precursor of the active metabolite chlorpyrifos oxon. Chlorpyrifos undergoes oxidative desulfuration by liver cytochrome P450 enzymes to become the highly toxic chlorpyrifos oxon (Sams et al., 2004; Tang et al., 2001).
HPLC-purified peptides from a pepsin digest of serum from the patient poisoned with chlorpyrifos were analyzed on the QTRAP using LCMSMS and infusion methods. The diethoxyphosphate adduct on albumin was not observed.
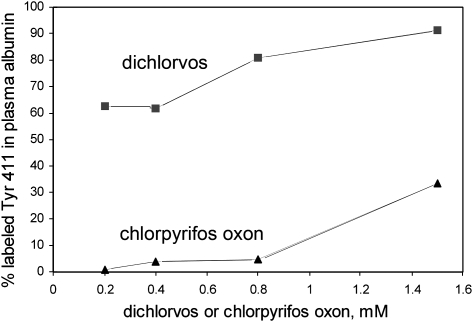
In an effort to understand why no diethoxyphosphate albumin adducts were detected, the relative reactivity of albumin with dichlorvos and chlorpyrifos oxon was examined. Plasma was incubated with various concentrations of chlorpyrifos oxon and dichlorvos for 16 h at 37°C. Cluster areas of labeled and unlabeled peptides observed in MALDI-TOF mass spectra were used to calculate percent labeling of tyrosine 411 (see the “Materials and Methods” section on “Reaction of human plasma with chlorpyrifos oxon and dichlorvos” for quantitation details). Figure 5 shows that 60% of the tyrosine 411 residues in albumin were labeled when human plasma was treated with 0.2mM dichlorvos. In contrast, less than 1% labeling occurred with 0.2mM chlorpyrifos oxon. It was not until 1.5mM chlorpyrifos oxon was used in the reaction that a significant amount of albumin adduct was observed (about 30%). Thus, it appears that chlorpyrifos oxon is substantially less reactive than dichlorvos toward albumin. The decreased reactivity of chlorpyrifos oxon indicates that a lower fraction of labeled albumin would be expected from sera of chlorpyrifos oxon–poisoned individuals. This, in turn, argues that a volume of plasma larger than the 0.010 ml used in the present study would need to be analyzed to detect the low level of albumin expected to have been modified by chlorpyrifos oxon.
FIG. 5.
Reactivity of tyrosine 411 in human albumin with dichlorvos and chlorpyrifos oxon. Percent labeling was calculated from cluster areas observed in the MALDI-TOF mass spectrometer of pepsin-digested plasma that had been treated with OP. The unlabeled peptides VRYTKKVPQVSTPTL and LVRYTKKVPQVSTPTL have masses of 1717 and 1830 amu. The dichlorvos-labeled peptides have masses of 1825 and 1938 amu. The chlorpyrifos oxon–labeled peptides have masses of 1853 and 1966 amu.
DISCUSSION
Comparison of Peptide Analyses for BChE and Albumin from Dichlorvos- and Chlorpyrifos-Poisoned Patients
Sera from the group of patients described in this report were previously analyzed for BChE adducts (Li et al., 2010). In those experiments, partially purified BChE was digested with trypsin and analyzed by mass spectrometry. Adducts on serine 198 were found. Table 2 summarizes the results for adducts on both BChE and albumin from the sera of four poisoned patients. The three patients who attempted suicide by drinking pesticides have low serum BChE activity, with inhibition levels from 62 to 84%. BChE in the accidental exposure case was only slightly inhibited (8%). OP adducts on BChE were found for all three samples where BChE inhibition was high but not for the sample where BChE inhibition was low.
TABLE 2.
Detection of OP Adducts on BChE and Albumin in Poisoned Patients
| Pesticide | Time between poisoning and blood draw | BChE activity U/ml | Inhibition of BChE % | Labeled BChE peptide found | Labeled albumin peptide found |
| Dichlorvos | 10 h | 0.41 | 84 | Yes | Yes |
| Dichlorvos | 9 h | 0.50 | 80 | Yes | Yes |
| Dichlorvos | 11 h | 2.30 | 8 | No | No |
| Unknown (chlorpyrifos)a | Unknown | 0.95 | 62 | Yes | No |
| Control—none | None | 2.50 | 0 | No | No |
Chlorpyrifos oxon was identified by mass spectrometry of the BChE adduct in a sample for which the poison was originally unknown. Results for BChE have been previously reported (Li et al., 2010). Mass spectrometry analysis used 2 ml serum for detection of BChE adducts and 0.010 ml serum for detection of albumin adducts. The concentration of BChE in human plasma is 4 mg/l, whereas that of albumin is 40,000 mg/l. This difference in protein abundance explains the necessity of using a larger plasma sample for detection of BChE adducts.
Dichlorvos adducts on albumin were found for the two patients who ingested a relatively large dose of dichlorvos, based on BChE inhibition of 84 and 80%. No dichlorvos adduct was found on albumin for the patient whose BChE was inhibited 8%. No chlorpyrifos oxon adduct was found on albumin for the patient whose BChE was inhibited 62%. The absence of a detectable chlorpyrifos oxon adduct on albumin can be explained as follows. (1) Chlorpyrifos oxon added to plasma is less reactive with tyrosine 411 of albumin than dichlorvos. (2) Chlorpyrifos oxon is hydrolyzed by paraoxonase in plasma and is sequestered by albumin in noncovalent binding sites from which it can be extracted with pentane (Eyer et al., 2009; Heilmair et al., 2008). This diminishes the concentration of chlorpyrifos oxon available for covalent reaction with tyrosine 411 of albumin. (3) The chlorpyrifos poison ingested by patients is converted to the toxic oxon by hepatic cytochrome P450 enzymes. There is a 10-fold variability in the efficiency of this step, as shown in studies that measured plasma levels of both the chlorpyrifos and the oxon (Eyer et al., 2009). Patients with high CYP2B6 and CYP3A4 levels would produce more of the oxon and therefore might have detectable levels of OP-albumin adducts in 0.01 ml plasma. However, detection of albumin adducts in other patients is expected to require plasma volumes larger than 0.01 ml.
OP-Albumin Adduct in Poisoned Humans
This is the first report to identify OP-albumin adducts in humans poisoned by OP. The amino acid modified by covalent attachment of dichlorvos is tyrosine 411. In previous work, we have found that many proteins can be modified by OP on tyrosine and that the OP-tyrosine adduct is stable and does not undergo aging (Li et al., 2008; Schopfer et al., 2010). Identification of the dichlorvos-albumin adduct in human serum required only 0.010 ml of serum because the exposure levels were high, as indicated by plasma BChE inhibition levels of 80%. It is anticipated that detection of OP-albumin adducts in people exposed to low doses will also be possible but that larger volumes of plasma will need to be processed in preparation for analysis by mass spectrometry. OP-albumin adducts have been found in the plasma of guinea pigs treated in vivo with the nerve agents tabun, soman, sarin, and cyclosarin (Read et al., 2010; Williams et al., 2007).
OP-Albumin as a Biomarker of Exposure
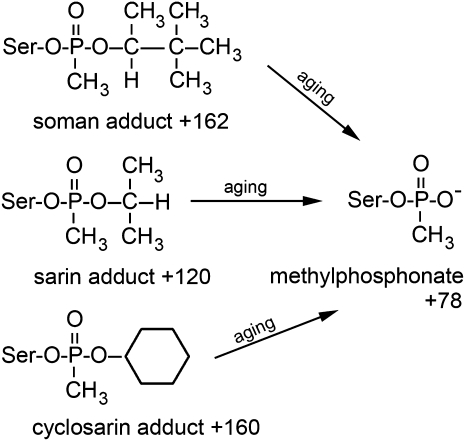
Butyrylcholinesterase in human plasma reacts rapidly with a wide range of OP. Upon reaction with OP, the enzymatic activity of BChE is inhibited. Loss of activity has long served as an indicator of OP exposure (Eddleston et al., 2008; Namba et al., 1971). However, use of BChE activity as a biomarker for OP exposure has major drawbacks. (1) The normal activity of BChE can vary widely so that only severe exposure can be confidently diagnosed (Eddleston et al., 2008; Kalow and Staron, 1957). (2) Hepatocarcinoma and malnutrition can cause depression of BChE activity (Whittaker, 1980). (3) Other compounds, such as carbamates, can inhibit BChE activity (Li et al., 2009) so that the true identity of the inhibitor is always in question. Consequently, efforts have shifted to the application of mass spectrometry for identification of the actual adducts formed upon reaction of BChE with inhibitors (Fidder et al., 2002; Li et al., 2009). Mass spectrometry provides a direct measure of the nature of the inhibitor attached to BChE. However, analysis of OP adducts on BChE as biomarkers for OP exposure also has drawbacks. The principal problem is that OP adducts on the active site serine of BChE are unstable. Instability arises from two sources. (1) Treatment with oximes releases the OP from serine, and oxime treatment is part of the normal medical response to OP poisoning. (2) Spontaneous dealkylation of the OP on BChE occurs via the aging process (Masson and Lockridge, 2010). Dealkylation reduces the information content of the resulting adduct. For example, aging converts adducts with soman, sarin, and cyclosarin to identical methylphosphonate structures, as indicated in Figure 6. The products of the aging of dichlorvos and chlorpyrifos oxon adducts on BChE are illustrated in Figure 1. To circumvent these drawbacks, new OP targets have been sought. Serum albumin is a promising candidate (Li et al., 2007, 2008; Means and Wu, 1979; Noort et al., 2009; Ortigoza-Ferado et al., 1984; Read et al., 2010; Sogorb et al., 2008; Tarhoni et al., 2008; Williams et al., 2007; Yeung et al., 2008).
FIG. 6.
Aging yields the identical methylphosphonate adduct on BChE. The reactions of soman, sarin, and cyclosarin with serine 198 of BChE yield distinct initial covalent adducts with added masses of + 162 for soman, + 120 for sarin, and + 160 for cyclosarin. These adducts age to the identical methylphosphonate structure with an added mass of + 78.
The OP-albumin adduct has several advantages as a biomarker of OP exposure. (1) OP binds to tyrosine 411 on albumin, and tyrosine adducts are not reversed by oximes (Read et al., 2010). (2) OP-tyrosine adducts do not age (Li et al., 2007; Williams et al., 2007). This means that exposure to soman can be distinguished from exposure to sarin and to cyclosarin. (3) The OP-albumin adduct persists longer in the blood than the OP-BChE adduct. Studies in guinea pigs treated with nerve agents found OP-tyrosine adducts 24 days after exposure to OP, at a time when OP-BChE adducts were undetectable (Read et al., 2010)
The major drawback for use of albumin-OP adducts as a biomarker for exposure to OP is the slow reaction of OP with albumin (Li et al., 2008). However, despite the low reactivity, OP-albumin adducts form in vivo and can be detected using mass spectral techniques, as has been demonstrated in this report.
The principal advantage of working with albumin is the stability of its OP-tyrosine adduct. Another significant factor is that the OP adduct is located on the surface of albumin, as opposed to the adduct on BChE that is located at the bottom of a deep pocket in the protein (Nachon et al., 2005). A novel outgrowth of the stability and accessibility of the albumin adduct is the potential to develop antibodies to OP-tyrosine. Such antibodies could be used for detection of OP exposure.
Hypothesis to Explain Neurotoxicity because of Chronic Low-Dose Exposure to OP
It is generally agreed that acute toxicity because of exposure to OP comes from inhibition of acetylcholinesterase in the synapses and nerve muscle junctions (Mileson et al., 1998). However, inhibition of acetylcholinesterase does not explain all of the clinical sequelae that arise from exposure to OP, especially low-dose exposure. It has been proposed that excess acetylcholine or inhibition of serine hydrolases with greater OP reactivity than acetylcholinesterase may explain low-dose neurotoxicity (Pernot et al., 2009; Richards et al., 2000). Symptoms of low-dose toxicity are neurological in nature (e.g., headache, memory loss, anxiety, fatigue) (Ray and Richards, 2001; Salvi et al., 2003). Investigation into the causes of this neurotoxicity is ongoing.
OP binding to albumin would not be expected to explain the neurotoxicity of OP but can serve as a model for what could be happening to other proteins. The special reactivity of tyrosine 411 in human albumin suggests that other proteins may have similarly reactive tyrosine residues. In fact, we have demonstrated that OP react with tyrosine on tubulin and that this reaction can disrupt the structure of microtubules in vitro and in vivo (Grigoryan and Lockridge, 2009; Jiang et al., 2010). If the function of key proteins important for axonal transport (such as tubulin) is disrupted by OP, the neuron could lose synaptic connectivity and nerve function (Gearhart et al., 2007; Terry et al., 2007).
Epidemiologists have linked chronic low-dose OP exposure to Parkinson's disease, neurologic dysfunction, Gulf War illness, and depression (Beseler et al., 2008; Hancock et al., 2008; Kamel et al., 2007; Toomey et al., 2009). Disruption of axonal transport has been suggested as the mechanism to explain neurodegenerative diseases, including Parkinson's, Alzheimer disease, and amyotrophic lateral sclerosis (Morfini et al., 2009). Our finding that tyrosine in albumin is covalently labeled by OP in clinically relevant human cases suggests that OP labeling of tyrosine in other proteins may also be occurring under these conditions. This concept provides a new direction in the search for a mechanism of OP-induced chronic neurotoxicity.
FUNDING
The U.S. Army Medical Research and Materiel Command (W81XWH-07-2-0034 to O.L.); the National Institutes of Health (U01 NS058056 to O.L.) and CA36727; Délégation Générale pour l'Armement (DGA/PEA 03CO10-05/01 08 7 to P.M.) (DGA/PEA 08CO501 to F.N.); Agence Nationale de la Recherche (ANR-06-BLAN-0163 and ANR-09-BLAN-0192 to F.N.).
Acknowledgments
Mass spectra were obtained with the support of the Mass Spectrometry and Proteomics core facility at the University of Nebraska Medical Center.
References
- Beseler CL, Stallones L, Hoppin JA, Alavanja MC, Blair A, Keefe T, Kamel F. Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study. Environ. Health Perspect. 2008;116:1713–1719. doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SJ, Carr J, Carlson JE, Tong L, Xue W, Li Y, Schopfer LM, Li B, Nachon F, Asojo O, et al. Five tyrosines and two serines in human albumin are labeled by the organophosphorus agent FP-biotin. Chem. Res. Toxicol. 2008;21:1787–1794. doi: 10.1021/tx800144z. PMCID:2646670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Eyer P, Worek F, Sheriff MH, Buckley NA. Predicting outcome using butyrylcholinesterase activity in organophosphorus pesticide self-poisoning. QJM. 2008;101:467–474. doi: 10.1093/qjmed/hcn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eyer F, Roberts DM, Buckley NA, Eddleston M, Thiermann H, Worek F, Eyer P. Extreme variability in the formation of chlorpyrifos oxon (CPO) in patients poisoned by chlorpyrifos (CPF) Biochem. Pharmacol. 2009;78:531–537. doi: 10.1016/j.bcp.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem. Res. Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol. Appl. Pharmacol. 2007;218:20–29. doi: 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Grigoryan H, Lockridge O. Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: implications for neurotoxicity. Toxicol. Appl. Pharmacol. 2009;240:143–148. doi: 10.1016/j.taap.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmair R, Eyer F, Eyer P. Enzyme-based assay for quantification of chlorpyrifos oxon in human plasma. Toxicol. Lett. 2008;181:19–24. doi: 10.1016/j.toxlet.2008.06.868. [DOI] [PubMed] [Google Scholar]
- Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol. Sci. 2010;115:183–193. doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalow W, Staron N. On distribution and inheritance of atypical forms of human serum cholinesterase, as indicated by dibucaine numbers. Can. J. Med. Sci. 1957;35:1305–1320. [PubMed] [Google Scholar]
- Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum. Exp. Toxicol. 2007;26:243–250. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- Li B, Nachon F, Froment MT, Verdier L, Debouzy JC, Brasme B, Gillon E, Schopfer LM, Lockridge O, Masson P. Binding and hydrolysis of soman by human serum albumin. Chem. Res. Toxicol. 2008;21:421–431. doi: 10.1021/tx700339m. [DOI] [PubMed] [Google Scholar]
- Li B, Ricordel I, Schopfer LM, Baud F, Megarbane B, Masson P, Lockridge O. Dichlorvos, chlorpyrifos oxon, and aldicarb adducts of butyrylcholinesterase detected by mass spectrometry, in human plasma following deliberate overdose. J. Appl. Toxicol. 2010 doi: 10.1002/jat.1526. Advance Access published on April 13, 2010; doi: 10.1002/jat.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Schopfer LM, Hinrichs SH, Masson P, Lockridge O. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry assay for organophosphorus toxicants bound to human albumin at Tyr411. Anal. Biochem. 2007;361:263–272. doi: 10.1016/j.ab.2006.11.018. PMCID:1828685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ricordel I, Tong L, Schopfer LM, Baud F, Megarbane B, Maury E, Masson P, Lockridge O. Carbofuran poisoning detected by mass spectrometry of butyrylcholinesterase adduct in human serum. J. Appl. Toxicol. 2009;29:149–155. doi: 10.1002/jat.1392. [DOI] [PubMed] [Google Scholar]
- Masson P, Froment MT, Bartels CF, Lockridge O. Importance of aspartate-70 in organophosphate inhibition, oxime re-activation and aging of human butyrylcholinesterase. Biochem. J. 1997;325(Pt 1):53–61. doi: 10.1042/bj3250053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P, Lockridge O. Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior. Arch. Biochem. Biophys. 2010;494:107–120. doi: 10.1016/j.abb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means GE, Wu HL. The reactive tyrosine residue of human serum albumin: characterization of its reaction with diisopropylfluorophosphate. Arch. Biochem. Biophys. 1979;194:526–530. doi: 10.1016/0003-9861(79)90647-7. [DOI] [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, et al. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol. Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, et al. Axonal transport defects in neurodegenerative diseases. J. Neurosci. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachon F, Asojo OA, Borgstahl GE, Masson P, Lockridge O. Role of water in aging of human butyrylcholinesterase inhibited by echothiophate: the crystal structure suggests two alternative mechanisms of aging. Biochemistry. 2005;44:1154–1162. doi: 10.1021/bi048238d. [DOI] [PubMed] [Google Scholar]
- Namba T, Nolte CT, Jackrel J, Grob D. Poisoning due to organophosphate insecticides. Acute and chronic manifestations. Am. J. Med. 1971;50:475–492. doi: 10.1016/0002-9343(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Noort D, Hulst AG, van Zuylen A, van Rijssel E, van der Schans MJ. Covalent binding of organophosphorothioates to albumin: a new perspective for OP-pesticide biomonitoring? Arch. Toxicol. 2009;83:1031–1036. doi: 10.1007/s00204-009-0456-5. [DOI] [PubMed] [Google Scholar]
- Ortigoza-Ferado J, Richter RJ, Hornung SK, Motulsky AG, Furlong CE. Paraoxon hydrolysis in human serum mediated by a genetically variable arylesterase and albumin. Am. J. Hum. Genet. 1984;36:295–305. [PMC free article] [PubMed] [Google Scholar]
- Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol. Sci. 2005;83:303–312. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- Pernot F, Carpentier P, Baille V, Testylier G, Beaup C, Foquin A, Filliat P, Liscia P, Coutan M, Pierard C, et al. Intrahippocampal cholinesterase inhibition induces epileptogenesis in mice without evidence of neurodegenerative events. Neuroscience. 2009;162:1351–1365. doi: 10.1016/j.neuroscience.2009.05.068. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Casida JE. Blood acylpeptide hydrolase activity is a sensitive marker for exposure to some organophosphate toxicants. Toxicol. Sci. 2005;86:291–299. doi: 10.1093/toxsci/kfi195. [DOI] [PubMed] [Google Scholar]
- Ray DE, Richards PG. The potential for toxic effects of chronic, low-dose exposure to organophosphates. Toxicol. Lett. 2001;120:343–351. doi: 10.1016/s0378-4274(01)00266-1. [DOI] [PubMed] [Google Scholar]
- Read RW, Riches JR, Stevens JA, Stubbs SJ, Black RM. Biomarkers of organophosphorus nerve agent exposure: comparison of phosphylated butyrylcholinesterase and phosphylated albumin after oxime therapy. Arch. Toxicol. 2010;84:25–36. doi: 10.1007/s00204-009-0473-4. [DOI] [PubMed] [Google Scholar]
- Richards PG, Johnson MK, Ray DE. Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol. Pharmacol. 2000;58:577–583. doi: 10.1124/mol.58.3.577. [DOI] [PubMed] [Google Scholar]
- Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol. Sci. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- Sams C, Cocker J, Lennard MS. Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP) Xenobiotica. 2004;34:861–873. doi: 10.1080/00498250400017273. [DOI] [PubMed] [Google Scholar]
- Schopfer LM, Grigoryan H, Li B, Nachon F, Masson P, Lockridge O. Mass spectral characterization of organophosphate-labeled, tyrosine-containing peptides: characteristic mass fragments and a new binding motif for organophosphates. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010;878:1297–1311. doi: 10.1016/j.jchromb.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogorb MA, Garcia-Arguelles S, Carrera V, Vilanova E. Serum albumin is as efficient as paraxonase in the detoxication of paraoxon at toxicologically relevant concentrations. Chem. Res. Toxicol. 2008;21:1524–1529. doi: 10.1021/tx800075x. [DOI] [PubMed] [Google Scholar]
- Tang J, Cao Y, Rose RL, Brimfield AA, Dai D, Goldstein JA, Hodgson E. Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab. Dispos. 2001;29:1201–1204. [PubMed] [Google Scholar]
- Tarhoni MH, Lister T, Ray DE, Carter WG. Albumin binding as a potential biomarker of exposure to moderately low levels of organophosphorus pesticides. Biomarkers. 2008;13:343–363. doi: 10.1080/13547500801973563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Beck WD, Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J. Pharmacol. Exp. Ther. 2007;322:1117–1128. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- Toomey R, Alpern R, Vasterling JJ, Baker DG, Reda DJ, Lyons MJ, Henderson WG, Kang HK, Eisen SA, Murphy FM. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. J. Int. Neuropsychol. Soc. 2009;15:717–729. doi: 10.1017/S1355617709990294. [DOI] [PubMed] [Google Scholar]
- Whittaker M. Plasma cholinesterase variants and the anaesthetist. Anaesthesia. 1980;35:174–197. doi: 10.1111/j.1365-2044.1980.tb03800.x. [DOI] [PubMed] [Google Scholar]
- Williams NH, Harrison JM, Read RW, Black RM. Phosphylated tyrosine in albumin as a biomarker of exposure to organophosphorus nerve agents. Arch. Toxicol. 2007;81:627–639. doi: 10.1007/s00204-007-0191-8. [DOI] [PubMed] [Google Scholar]
- Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Arch. Toxicol. 1999;73:7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- Yeung DT, Smith JR, Sweeney RE, Lenz DE, Cerasoli DM. A gas chromatographic-mass spectrometric approach to examining stereoselective interaction of human plasma proteins with soman. J. Anal. Toxicol. 2008;32:86–91. doi: 10.1093/jat/32.1.86. [DOI] [PubMed] [Google Scholar]