Abstract
Asthma is a leading cause of morbidity in children. Risk factors include chronic exposure to allergens and air pollution. While chronically activated mast cells contribute to the pathophysiology of asthma in part through their proteases such as chymase and tryptase, previous studies of airway mast cell abundance and distribution in asthmatics have been inconsistent. To determine whether repeated episodic exposures to environmental pollutants during postnatal lung development alter airway mast cell abundance and distribution, we exposed infant rhesus monkeys to a known human allergen, house dust mite antigen (HDMA), and/or a known environmental pollutant, ozone (O3), and quantitatively compared the abundance of tryptase- or chymase-positive mast cells in three airway levels. Mast cells are resident in multiple compartments of the airway wall in infant rhesus monkeys raised from birth in filtered air. Tryptase- and chymase-positive cells were most abundant in trachea and least in terminal bronchioles. The majority of tryptase-positive and almost all chymase-positive cells were in extracellular matrix and smooth muscle bundles. Chronic exposure to HDMA elevated the abundance of both tryptase- and chymase-positive cells in the trachea and intrapulmonary bronchi. Neither exposure to O3 nor HDMA + O3 increased mast cell accumulations in the airway wall. We conclude that during postnatal airway development (1) mast cells are a resident airway cell population even in the absence of toxic air contaminants; (2) aeroallergen exposure alters large airway mast cell distribution and abundance, increasing chymase-positive mast cells; and (3) this response is attenuated by exposure to oxidant air pollutants.
Keywords: HDMA, lung development, mast cells, ozone, childhood asthma
Asthma is a major cause of childhood illness in the United States, affecting almost 1 in 10 children between the ages of 5 and 17. There has been a 65% increase in asthma since 1982 and a 6% increase since 2001 (ALA, 2009). Approximately half of all current asthma cases can be linked to specific allergies. Atopy, genetic factors (McCunney, 2005), exposure to allergens (Lemanske, 2002), and air pollution, including ozone (O3) (McConnell et al., 2002; Triche et al., 2006), are all risk factors for the development of childhood asthma. O3 has been shown both to exacerbate asthmatic symptoms (Thurston et al., 1997) and to increase the risk of developing asthma, particularly in children subjected to high levels during outdoor exercise (McConnell et al., 2002).
Within the asthmatic lung, numerous cell types have been linked to the disease, which is characterized by chronic inflammation and exuberant mucous production. Mast cells are implicated as key players involved in several hallmarks of asthma including mucous production, changes in smooth muscle organization, remodeling of extracellular matrix, and altered lung physiology (Bradding et al., 2006; Cho et al., 2002; Lazaar et al., 2002), primarily because they secrete biological mediators that play a central role in tissue homeostasis, wound healing, and host defense and, with T lymphocytes, are thought to regulate mucosal inflammation (Bradding and Holgate, 1999). However, whether elevations of chronically activated mast cells within airways contribute to the pathophysiology of asthma is still unclear. Studies have reported increases (Brightling et al., 2002; Pesci et al., 1993), no change (Beasley et al., 1989; Bradley et al., 1991; Djukanovic et al., 1990; Vignola et al., 1998), and even decreases (Connell, 1971) of mast cell quantities in human asthmatics. A lack of consensus may be because most studies do not define the airway generation–specific distribution of mast cells along the tracheobronchial airway tree or that spatial abundance is altered by environmental exposures during development. Normal variance in the etiology of asthma and asthma treatment in human populations likely further contributes to the diversity of mast cell abundance that has been observed. Furthermore, it is unknown whether the significantly altered microenvironment found in the airway wall in the presence of underlying asthma alters mast cell abundance and/or protease expression in airway mast cells.
For the current study, we employed our experimental model of childhood asthma using infant rhesus monkeys. The rhesus monkey is a species where the lungs of adults share the cellular and anatomical characteristics of adult human lungs (Plopper and Harkema, 2005). We have found that exposure to a known human allergen, house dust mite, along with the oxidant air pollutant O3, beginning soon after birth and continuing through the period of active lung development, reproduces the characteristics used by the National Heart, Lung and Blood Institute guidelines as criteria for defining asthma in children (Plopper et al., 2007; Schelegle et al., 2003). Alterations include basement membrane thickening, mucous cell hyperplasia, changes in smooth muscle organization, and altered lung physiology (Fanucchi et al., 2006; Joad et al., 2006; Schelegle et al., 2003). Our previous studies have shown that often the pathophysiology within the lung is site specific within the airway tree. This has been found in respect to vascular remodeling (Avdalovic et al., 2006), influx of immune cells (Miller et al., 2005), and airway responsiveness (Joad et al., 2006). Exposure to allergen and O3 also has a negative effect on airway growth and the organization of the cellular and acellular components of the airway wall, which participate in normal airway function as part of the epithelial mesenchymal trophic unit (Evans et al., 1999).
Using our experimental model of allergic airways disease in infant rhesus monkeys exposed to house dust mite with O3 (Schelegle et al., 2001, 2003), this study was designed to establish the role of toxic air contaminants in modulating mast cell populations in the developing airways during the early postnatal period by addressing the following questions: (1) Do mast cells become a resident cell population in the airways when early postnatal development occurs in an atmosphere free of toxic air contaminants? (2) Does exposure to a common household allergen (house dust mite) during the period of active early postnatal airway development, in a pattern that produces allergic airways disease, increase the abundance of mast cells in tracheobronchial airways? and (3) Does exposure to the most common oxidant air pollutant, O3, during the period of active early postnatal airway development increase the abundance of mast cells in tracheobronchial airways?
MATERIALS AND METHODS
Animals.
All protocols were approved by the Institutional Animal Care and Use Committee in compliance with the Animal Welfare Act and Public Health Service Policy on Human Care and Use of Laboratory Animals. Monkeys selected for these studies were California National Primate Research Center colony-born rhesus macaques (Macaca mulatta). Care and housing of animals before, during, and after treatment were performed by the California National Primate Research Center, which is accredited by the Association for Accreditation and Assessment of Laboratory Animal Care.
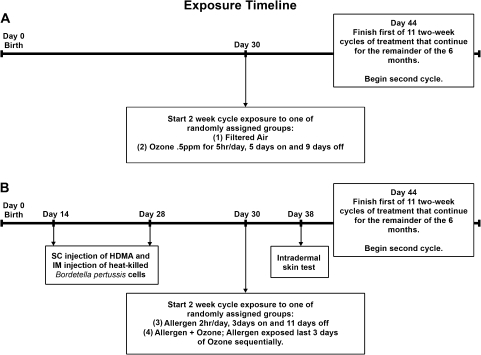
Male infant rhesus monkeys (30 days old) were exposed to 11 cyclic episodes of chemical-, biological-, and radiological-filtered air (FA), house dust mite (Dermatophagoides farinae) allergen (HDMA), O3, or a combination of HDMA allergen and O3. The exposures were designed to mimic the episodic exposures experienced by human infants and have been previously described (Schelegle et al., 2003). Briefly, 24 infant rhesus macaque monkeys born over two seasons at the California National Primate Research Center were studied. At 1 month of age, 12 nonsensitized monkeys were randomly assigned to receive 11 cycles of exposure to either (1) FA or (2) 0.5 ppm O3 for 6 h/day, 5 days on and 9 days off (see Figure 1A). Twelve separate infant monkeys were exposed to aerosolized house dust mite allergen (HDMA, 435 ± 96 μg/m3 protein [mean ± SD], D. farinae, Greer Laboratories, Inc., Lenoir, NC; n = 12) and were sensitized by subcutaneous injection of HDMA in alum and intramuscular injection of heat-killed Bordetella pertussis cells at 14 and 28 days of age. Dermatophygoides farinae was diluted in PBS and aerosolized with a nebulizer (Miniheart, Westmed, Inc., Tuscson, AZ) as previously described (Schelegle et al., 2003). Sensitization was confirmed by a positive intradermal skin test to HDMA on day 38 of the exposure protocol in 11 of 12 sensitized infants (Schelegle et al., 2003). The HDMA-sensitized macaques were randomly assigned to receive 11 cycles of (3) allergen, 2 h/day, 3 days on and 11 days off, or (4) allergen + O3 (allergen exposure on last 3 days of O3 exposure) (see Figure 1B). O3 was generated as previously described (Schelegle et al., 2003) and monitored using a Dasibi 1003-AH O3 analyzer (Dasibi Environmental Corporation, Glendale, CA). The studies were performed 3–5 days after the last exposure, at 6 months of age. Monkeys were euthanized with an overdose of pentobarbital after being sedated with Telazol (8 mg/kg im) and deeply anesthetized with Diprivan (0.1–0.2 mg/kg/min, iv) and were necropsied following exsanguination via the inferior vena cava.
FIG. 1.
Exposure time line of the experiment. Arrows indicate specific events within the 6-month exposure protocol. (A) Thirty-day-old rhesus monkeys were exposed to eleven 14-day cycles of either (1) FA or (2) 0.5 ppm O3. (B) Rhesus monkeys were sensitized through subcutaneous and intramuscular injections at 2 and 4 weeks of age. Thirty-day-old postnatal monkeys sensitized to HDMA were exposed to 11 rounds of 14-day aerosol exposure cycles of either (3) HDMA allergen or (4) a combination of HDMA allergen + 0.5 ppm O3. Exposure cycles are continuous within all groups, terminating at 6 months of age.
Tracheal samples were collected starting 2 cm distal of the larynx and were fixed in 4% paraformaldehyde for 4 h. The right cranial lobe was inflation fixed in 4% paraformaldehyde under 30-cm hydrostatic pressure. The axial path of the airway tree was exposed through microdissection, allowing airway generations at bifurcation sites to be defined, diagrammed, and numbered. Intrapulmonary airway samples were taken from the axial airway path between intrapulmonary airway generations 1 and 5, while terminal airways were classified as the last airway segment proximal to the respiratory bronchioles, usually distal to intrapulmonary airway generation 15. Following fixation, samples were embedded in paraffin and sectioned for staining.
Tryptase immunohistochemistry.
The presence of mast cell tryptase was visualized using a horse anti-mouse mast cell tryptase antibody AA1 (Vector Laboratories, Burlingame, CA). The optimal concentration and primary antibody at which there was minimal background staining was determined using a series of dilutions. The procedure was performed according to manufacturer's instructions (Vector Laboratories) with several alterations as outlined. Following tissue hydration, endogenous peroxidase activity was quenched with a 3% solution of hydrogen peroxide followed by antigen retrieval with hot 0.01M citrate buffer. To eliminate nonspecific primary antibody binding, tissue sections were blocked with horse serum. Primary tryptase antibody was allowed to incubate overnight at 4°C at a 1:500 dilution. Signal was visualized using the Vectastain ABC kit and NovaRED chromagen. Negative controls were substituted with PBS for the primary antibody to ensure specific positive staining.
Chymase histochemistry.
Mast cell chymase was detected using naphthol AS-D chloroacetate–specific esterase enzyme histochemistry as previously described (Yam et al., 1971). Briefly, paraffin tissue sections were deparaffinized and hydrated. Sections were incubated in a solution consisting of 4% new fuchsin acidified in 2N HCl, 4% sodium nitrite, 10 mg/ml naphthol AS-D chloroacetate in NN-dimethyl formamide, and combined in 0.1M, pH 7.6, phosphate buffer. Cellular staining and morphology were used to identify positive mast cells in tissue sections.
Morphometry.
The approximate abundance of tryptase- and chymase-positive mast cells were quantified using the Olympus computer-assisted stereology grid system (Computer Assisted Stereological Toolbox grid, Olympus, Denmark) on defined airway generations as previously described (Miller et al., 2005). Briefly, axial airways were outlined at ×4 magnification for determination of surface area of epithelial basement membrane per reference volume (Sv) calculated using as follows:
where Ibl is the number of intersections with the epithelial basal lamina, Pt is the number of points hitting the epithelium, and l/p is the length per test point on four lines oriented either horizontally or vertically in the counting frame. Subsequently, airways were systematically sampled with a random start point at ×100 magnification for volume fraction (Vv) determination. Chymase- and tryptase-positive volume fractions were determined using point (P) and intercept (I) counts with a lattice grid and Stereology Toolbox (Morphometrix, Davis, CA) at a minimum of 60 fields per airway level in each animal. Vv was calculated using the formula:
where Pi is the number of test points hitting the structure of interest divided by Pt, the total number of points hitting the reference space. Finally, the volume (Vs) of tryptase (MCT) and chymase (MCTC) within the surface area of epithelial basal lamina (cubic microns/square microns) was calculated using the formula:
Statistics.
All data are reported as the mean ± SEM. Statistical outliers were eliminated using the extreme studentized deviate method. Comparisons between groups were done using one-way ANOVA. Post hoc analysis with Fisher's Protected Least Significant Difference method was used to determine significance between control and exposed groups. All statistical functions were performed using Statview (SAS, Cary, NC). A value of p < 0.05 was considered statistically significant.
RESULTS
Animals Reared in FA
Tryptase (MCT)-positive mast cells and chymase (MCTC)-positive mast cells were detected in airway paraffin cross sections from defined airway generations using immunohistochemical detection of tryptase (Figure 2A) and histochemical detection of chymase (Figure 2B) in three specific airway regions: tracheal, proximal intrapulmonary airways, and terminal bronchioles (Figure 3A). In animals reared in FA, MCT and MCTC cells were most abundant in the trachea, half as abundant in proximal intrapulmonary bronchi, and least in terminal bronchioles (Figure 3B). Tracheal MCTC were a fifth as abundant compared to tracheal MCT and very low in both proximal intrapulmonary airways and terminal bronchioles (Figure 3C). In the tracheas of FA animals, MCT cells were localized mainly within the extracellular matrix (47%) and distributed evenly between the glands (21%) and smooth muscle (22%) with a minor amount in the blood vessels (9%) (Figure 4A). MCT cells were rarely detected within the epithelium (< 1%). Tracheal MCTC cells were distributed primarily within the extracellular matrix (57%), and smooth muscle (42%) MCTC cells were rarely observed in blood vessels (1%) and undetectable in both glands and epithelium (Figure 4B). Within the intrapulmonary region, MCT cells were predominantly observed in the smooth muscle (63%) and extracellular matrix (31%). Insignificant amounts were present in the epithelium (< 1%), blood vessels (< 1%), and glands (2%) (Figure 4C). A majority of the intrapulmonary MCTC cells were distributed within the smooth muscle (58%) and extracellular matrix (42%). As with tracheal MCTC cells, intrapulmonary MCTC cells were undetected in the epithelium, blood vessels, or glands (Figure 4D).
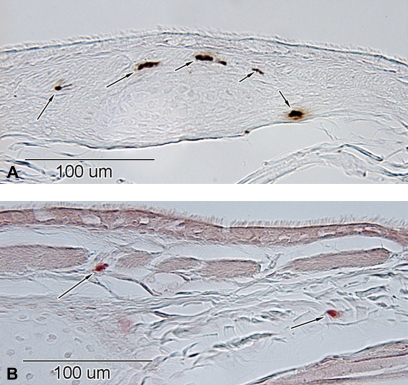
FIG. 2.
Representative micrographs of mast cell stains in the intrapulmonary airway level from the rhesus monkey. (A) Arrows indicate immunohistochemically positive tryptase-positive mast cells (MCT) and (B) histochemically positive chymase mast cells (MCTC). Scale bar is 100 μm.
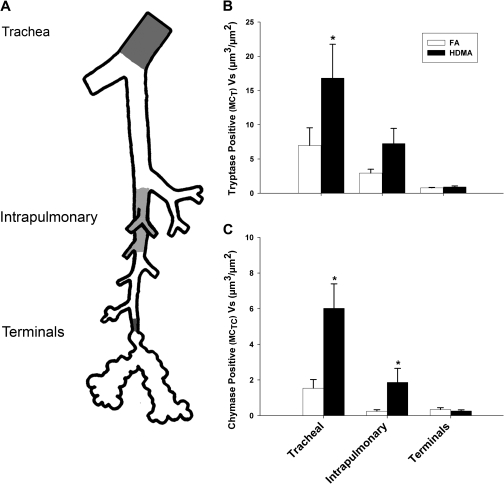
FIG. 3.
Abundance and distribution of MCT- and MCTC-positive cell (Vs) mass in specific regions in the airway wall. (A) The lung was microdissected open along the axial pathway, and airway branch points were marked. Trachea, intrapulmonary (generations 1–5), and terminal bronchioles (defined as proximal to the first alveolar outpocketings) were measured and compared. (B) MCT- and MCTC-positive cells were most abundant in the trachea and declined in the intrapulmonary and terminal airways. The trend is similar but exaggerated following HDMA exposure; both MCT- and MCTC-positive cell mass within the trachea was significantly greater than either intrapulmonary or terminal airway groups. Compared to FA controls, MCT cell mass was significantly increased in the trachea following the HDMA exposure regimen. (C) MCTC cell mass was significantly increased in both the trachea and the intrapulmonary airways. Terminal bronchiolar mast cells were not increased in response to HDMA exposure. Data are means ± SEM (n = 5–6 monkeys per group). *p < 0.05, as compared with FA controls in the same airway region.
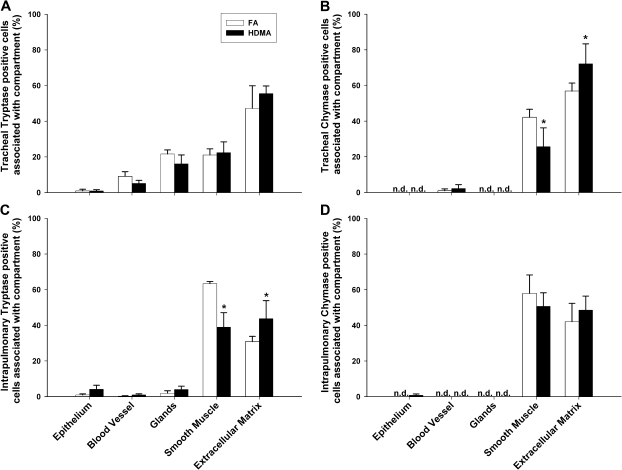
FIG. 4.
Distribution of MCT- and MCTC-positive cells within compartments in the trachea and intrapulmonary regions. Mast cells were quantified in FA and house dust mite antigen (HDMA)-exposed animals within the epithelium, blood vessel, glands, smooth muscle, and extracellular matrix compartments. (A) Within tracheas of FA animals, MCT cells were predominantly observed within the extracellular matrix followed by an approximately equal distribution within the glands and smooth muscle. MCT cells were rarely detected within the epithelium. MCT cell distribution was not changed significantly following HDMA sensitization and aerosol exposure. (B) The majority of MCTC cells in the trachea were distributed in the extracellular matrix and smooth muscle. Cells were rarely detected within blood vessels and undetected in the epithelium and glands. Upon HDMA exposure, distribution of MCTC cells changed significantly in the smooth muscle and the extracellular matrix. (C) Intrapulmonary MCT cells were predominantly observed in the smooth muscle and extracellular matrix. Following HDMA exposure, MCT cells were significantly decreased in the smooth muscle and increased in the extracellular matrix. (D) Similar to the trachea, a majority of intrapulmonary MCTC cells were distributed within the smooth muscle and extracellular matrix. Mast cells were undetected within the epithelial, blood vessel, and glandular compartments. Distribution between compartments did not change significantly following HDMA treatment. Data are plotted as means ± SEM (n = 5–6 monkeys per group). *p < 0.05, as compared with FA controls in the same compartment.
Animals Reared in High Allergen (HDMA) Air
Compared to FA controls, there was a significant increase in tracheal MCT cells (Figure 3B) (p = 0.010) in HDMA-exposed animals. Marked increases of MCTC cells were found in both tracheal (p = 0.001) and intrapulmonary (p = 0.018) airways (Figure 3C). Exposure to HDMA did not affect MCT abundance in intrapulmonary airways. There were no significant differences in the terminal airways for either MCT or MCTC cells. In addition to significantly increased MCTC cell mass, the distribution of tracheal MCTC cells also significantly changed within the smooth muscle and extracellular matrix compartments compared to FA controls. MCTC cells within the smooth muscle decreased from 42 to 26% (p = 0.034), while MCTC cells in the extracellular matrix rose from 57 to 72% (p = 0.048) (Figure 4B). In proximal intrapulmonary airways, the proportion of MCT cells in smooth muscle significantly decreased from 63 to 41% (p < 0.001) and increased from 31 to 46% (p = 0.009) in extracellular matrix (Figure 4C).
Animals Reared in O3 with or without Allergen (HDMA)
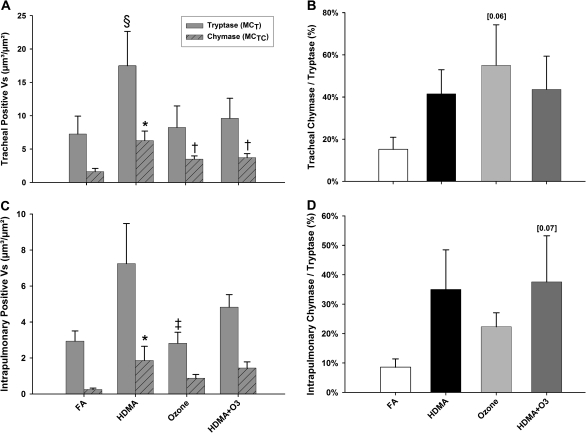
Due to a marked increase in MCT and MCTC cells following HDMA exposure, we compared the effect of a common oxidant pollutant, O3, in addition to or separately from HDMA on MCT and MCTC cell abundance in the trachea and proximal intrapulmonary airways. In contrast to animals exposed to HDMA alone (which resulted in an increased MCT and MCTC cells relative to FA controls), there were no significant increases in either MCT or MCTC in the trachea (Figure 5A) or intrapulmonary airways (Figure 5C) of animals exposed to O3 or a combination of O3 and HDMA compared to FA controls. However, there were exposure-related differences between animals exposed to O3 or a combination of HDMA plus O3 when compared to animals exposed to HDMA alone. Tracheal MCTC abundance, while insignificantly different from FA controls, are significantly decreased in O3 and HDMA + O3 groups when compared to HDMA alone. A similar trend is seen in intrapulmonary airways; O3 treatment significantly decreased MCT abundance compared to HDMA alone. Within the trachea, MCTC cells accounted for 55% of total mast cells in O3-exposed animals and 44% in animals exposed to both HDMA and O3. However, due to a large variance in the data, these differences were not statistically significant (Figure 5B). In intrapulmonary airways, MCTC cells account for 22% in O3-exposed animals and 38% in HDMA + O3 treatment. Similar to the percentages seen in the trachea, these differences were not statistically significant due to large animal variances (Figure 5D).
FIG. 5.
MCT and MCTC cell mass (Vs) in the trachea and intrapulmonary conducting airways. (A) HDMA allergen–exposed animals had both significantly greater MCT and MCTC cell mass compared to FA controls. Interestingly, HDMA MCTC cells are also significantly greater compared to all other aerosol exposures in the trachea. (B) Individual MCTC Vs plotted as a percentage of total mast cells in the trachea. A trend showing increased percentage of MCTC/MCT can be seen following all aerosol treatments compared to FA controls. A large increase in the MCTC/MCT ratio is seen between O3-treated (55%) compared to FA animals (15%). But due to a large variance within the data, the comparison was insignificant (p = 0.06). (C) Within the intrapulmonary component, MCTC Vs increased significantly following HDMA exposure compared with both FA controls and O3 aerosol treatments. Surprisingly, MCT Vs was not significantly increased following HDMA exposure, as compared to FA controls. (D) Comparable to the trachea, the percentage of intrapulmonary MCTC/MCT also trended in an increasing fashion following aerosol exposures compared to FA controls. The combined HDMA + O3–exposed group had the greatest MCTC/MCT ratio compared to FA controls (38 vs. 9%) but failed to reach significance (p = 0.07). Data are shown as means ± SEM (n = 5–6 monkeys per group). *p < 0.05, MCTC Vs as compared with FA controls in the same airway region. †p < 0.05, as compared with HDMA treatment in the trachea. §p < 0.05, MCT Vs as compare with FA treatment in the trachea. ‡p < 0.05, as compared with HDMA treatment in the intrapulmonary region.
DISCUSSION
The overall goal of this study was to establish whether exposure to aeroallergens and oxidant air pollutants during the early postnatal development of tracheobronchial airways would impact the organization and abundance of an airway cell population, mast cells, identified as potential key modulators of reactive airways disease. We used an exposure model of childhood allergic airways disease where normal postnatal airway growth and development and the pathobiology of reactive airways disease closely resemble humans and the infant rhesus monkeys exposed to a common human allergen, house dust mite, and/or the ubiquitous air pollutant, O3. Recruitment of mast cells is a normal feature of development. Our data show that mast cells become a resident cell population in the three airway generations we compared (trachea, proximal intrapulmonary bronchi, and terminal bronchioles) during the normal postnatal development of rhesus monkeys reared in an FA environment. The abundance and distribution of the mast cell population differs between different airway microenvironments. Postnatal airway development in the presence of an aeroallergen alters the character of airway mast cell populations. Chronic aeroallergen exposure greatly increases the abundance and alters the distribution of both tryptase- and chymase-positive airway mast cells in tracheal and proximal airways but not in terminal bronchioles. The response is not the same in tracheal and intrapulmonary proximal airways. The increases were associated primarily with extracellular matrix and not necessarily in areas adjacent to smooth muscle bundles or mucous glands. Postnatal airway development in the presence of an oxidant air pollutant, with or without the presence of an aeroallergen, has only a minor impact on the mast cell population of proximal airways.
The increase in chymase-positive mast cells (MCTC) in the extracellular matrix of aeroallergen (HDMA)-exposed rhesus monkeys may have implications for airway remodeling. Mast cell chymase may play a role in both tissue homeostasis as well as disease. Chymase has been shown to have a number of effects that are linked to asthma pathology such as degradation of extracellular matrix (Kofford et al., 1997; Lazaar et al., 2002) and activation of Protease-activate receptor 1 (Reed and Kita, 2004; Schechter et al., 1998). Other studies have indicated that chymase and chymase-positive mast cells have functions that may be protective such as inhibition of smooth muscle proliferation or reduction of airway hyperreactivity (Balzar et al., 2005; Lazaar et al., 2002). Chymase-positive mast cells have been reported as being beneficial for pulmonary function when located in the alveolar attachment region of small airways in adult humans with severe asthma (Balzar et al., 2005). In contrast to the study by Balzar et al. (2005) in adult severe asthmatic humans, we did not find abundant MCTC in peripheral regions. However, this might be due to the difference between a disease that is long established and one that is nascent. Mice that lack the MC protease 4 have more airway hyperresponsiveness and inflammation than wild-type control mice, suggesting that cells that contain this chymase (which were preferentially located in upper airways) are present to protect from hyperresponsiveness (Waern et al., 2009). It is possible that the increase in MCTC is an adaptive response to repeated exposure to an allergen and that the group that may be of concern is the group that does not mount this response, the HDMA + O3–exposed group. This is the exposure group (HDMA + O3) in our model that has the greatest increase in baseline airway resistance (Raw) (Schelegle et al., 2003), suggesting that perhaps a lack of MCTC in an allergic animal changes airway tone. However, attempts to correlate individual rhesus MCTC with Raw values in these same animals found no correlation (data not shown). These studies in aggregate indicate a complex role for mast cell chymase that may vary by position within the airway tree.
Mast cells have different effects on helper T cell response 1 (Tg1) and helper T cell response 2 (Tg2) responses depending on their type. In the human conjunctiva, the Tg1 cytokines interleukin (IL)5 and IL6 have been reported to preferentially localize to MCT, while the Tg2 cytokines IL4 and IL13 have been reported to preferentially localize to MCTC (Anderson et al., 2001). Mast cells that contain chymase can be functionally distinguished by their expression of CD88, the C5aR, in contrast to MCT which do not express CD88 (Oskeritzian et al., 2005). This suggests a different functional role for these mast cells in asthma. Again accenting the role of occasionally opposing functions of mast cells in the pathophysiology of asthma, recent work using C5aR-deficient mice and antagonists to the C5aR have shown that C5a plays a dual role. C5a provides protection from development of Tg2 responses while at the same time exacerbating airway inflammation and responsiveness in environments where airway inflammation is already established (Kohl et al., 2006). This suggests a further role for the mast cells, particularly MCTC, in these processes. Whether this is true in airways disease in primates is unknown, but our study suggests that it is likely and emphasizes the need to define the role of mast cells, inflammation, and airway responsiveness on an airway-by-airway–specific basis.
Chymase-positive mast cells, MCTC, may have a role in the vascular component of airway remodeling in asthma. MCTC correlate with the number of vascular endothelial growth factor (VEGF)-positive cells as well as the increased vascular area and vascular remodeling in asthmatic patients (Zanini et al., 2007). Increased bronchial vascular density has been found in rhesus monkeys exposed to HDMA (Avdalovic et al., 2006), associated with an increase in the VEGF121 splice variant, likely produced by epithelium. As an intermediary located between vasculature and epithelium, the mast cell is well positioned to regulate responses of the immune system and lung remodeling, and, in our model, the changes in mast cell populations occur in the same airways also associated with vascular remodeling (Avdalovic et al., 2006).
Smooth muscle in rhesus monkeys exposed to HDMA is hypertrophic in the cartilaginous airways (Tran et al., 2004). It is interesting that even though smooth muscle is increased in mass and bundle size in young animals exposed to HDMA that this does not correspond to increases in mast cell abundance within the smooth muscle layer. In fact, both intrapulmonary MCT and tracheal MCTC were significantly decreased in abundance in the smooth muscle tissue compartment in HDMA-treated animals. Our previous work has established that the rhesus monkey HDMA exposure model is a model of eosinophilic, allergic asthma (Chou et al., 2005; Schelegle et al., 2003). Therefore, mast cell infiltration of smooth muscle was expected as this has been shown in a previous study of bronchial biopsies of 17 human asthmatics (Brightling et al., 2002). Elevated smooth muscle mast cells distinguish allergic asthma from eosinophilic bronchitis, and these mast cells contain both chymase and tryptase (Brightling et al., 2002). Instead, we found increased mast cells in the peribronchial interstitium. It could be that smooth muscle infiltration by mast cells is a late event in the pathogenesis of asthma or that it is developmentally regulated. Since the monkeys were not screened for atopy or predisposition to allergic airway disease prior to enrollment in the study, it could also be that these animals do not have the correct genetic make up to completely mimic human asthma. Human asthma is a syndrome caused by a myriad of environmental and intrinsic, genetic, factors. Schema for reclassification of asthma based on subphenotypes has been proposed (Green et al., 2007). There is substantial heterogeneity in asthma observed in both children and adults, supporting a hypothesis of different subclassifications rather than a divergence in later life based solely on history of prior exposure.
The lack of consensus in the literature regarding the abundance of mast cells in the airways of human asthmatics is likely confounded by the observed spatial differences in the abundance of mast cells within the tracheobronchial airway tree and by differences in histories of exposure and allergy. Recent work in samples from human severe asthmatics (steroid dependent) has suggested that total mast cells increase in distal lung regions compared with large airways (Balzar et al., 2005). The induction of MCTC, particularly in small airways, may protect lung function in severe asthma (Balzar et al., 2005) and also in patients with chronic obstructive pulmonary disease (Gosman et al., 2008). We did not find increased MCTC in the small airways in our animal model of childhood airway disease. However, the differences in findings between our studies and those in humans may be due to the age of our animals at necropsy, 6 months. This would correspond to a human “toddler” age of approximately 1.5 years, an age not frequently examined in studies of human asthma. It is possible that the distal airway mast cell recruitment is a later event that occurs in chronic adult asthmatics who have had the disease for a longer term and in whom the airways are no longer developing. Our results would suggest that mast cells are recruited, initially, to the larger airways. Future studies are needed to further define the persistence of these mast cell changes and whether distal airways have more mast cells in older animals.
We determined whether changes in mast cell abundance favor specific compartments such as epithelium, blood vessels, glands, extracellular matrix, or smooth muscle in our model of pediatric allergic airways disease. The abundance of MCT and MCTC mast cells varied by location in the tracheobronchial airway tree, with significant increases in MCT cells between the tracheal airways and MCTC-positive mast cells in the tracheal and intrapulmonary airways of HDMA-exposed infant monkeys associated with the extracellular matrix. Microlocalization within specific lung regions is a key issue for recruitment, activation, and retention of immune cells. The mechanisms that account for the heterogeneous mast cell increases found in the allergic animals are unknown. It is likely that the significantly altered microenvironment of the airway wall in allergen-exposed infant monkeys is signaling the migration of circulating mast cell precursors, or possibly that the microenvironment surrounding the mast cell, has changed such that it increases the mast cell life span or a combination of both. Known mast cell chemotactic factors include stem cell factor (SCF) (Nilsson et al., 1994), nerve growth factor (NGF) (Sawada et al., 2000), eotaxin (de Paulis et al., 2001), and transforming growth factor beta (Berger et al., 2003). SCF is an important cytokine responsible for the recruitment and survival of mast cell progenitors from blood and is also produced, stored, and secreted by human lung mast cells (de Paulis et al., 1999). Mast cells also produce, store, and release NGF (Leon et al., 1994). NGF is reported to significantly decrease cell death in cultured mast cells (Horigome et al., 1994). Therefore, repeated activation and degranulation of mast cells may increase the abundance of mast cells in tissues through the release of mast cell chemotactic factors.
A consequence of mast cell accumulation can be an increased pool of available mediators, sitting in airway mast cell secretory granules, awaiting the signal for release into the airway wall. Mast cells interact with both sensory and parasympathetic neurons in multiple organs. Mast cell neural interactions in pulmonary tissues are supported by studies showing that substance P–positive nerves interact with mast cells to change lung solution and ion movement in the trachea and by colocalization of mast cells with calcitonin gene-related peptide-positive nerve fibers in rats (Bienenstock et al., 1988; Dimitriadou et al., 1994). Recent studies in transgenic animals indicate that mast cell–mediated bronchoconstriction is only observed when nerves are intact. Further, mast cell release of serotonin causes this response, and this was linked to cholinergic pathways using blocking agents in a mouse model (Cyphert et al., 2009). These studies and others suggest that mast cells act as sentinels and through their ability to secrete a variety of mediators contribute to immune and neural responses in the lung. Previous studies of innervation in rhesus monkeys exposed to the same HDMA exposure regimen found that the airways were hyperinnervated at 1 year of age (Kajekar et al., 2007). The interaction of airway epithelial hyperinnervation with elevated peribronchial mast cells containing abundant enzymes and serotonin is a fertile area for further study of bronchoconstrictive mechanisms in this model.
In summary, the present study establishes that the mast cells become resident in the tracheobronchial airways even when postnatal development occurs in an FA environment in the absence of aeroallergens and oxidant air pollutants. We further establish that aeroallergen exposure during this period of postnatal development elevates airway mast cell abundance and alters its distribution to favor more reactive airways. These increases do not occur uniformly in the tracheobronchial airway tree and do not favor the smooth muscle components of the airway wall. Whether these changes are permanent and persist into adulthood is unknown, but our study suggests that exposure during early postnatal life can profoundly alter an airway cell population thought to play a critical role in chronic allergic airways disease.
FUNDING
National Institutes of Health (P01 ES 00628, R01 ES 06700, NCRR RR00169); National Institute of Environmental Health Sciences Center (ES05707).
Acknowledgments
The authors thank Sarah Davis, Brain Tarkington, as well as the animal care staff and the members of the Respiratory Diseases Unit of the California National Primate Research Center for their assistance with these studies. We would also like to acknowledge Trenton Combs for his assistance and expertise in graphic design and digital image manipulation. University of California Davis was a National Institute of Environmental Health Sciences Center, and past support for core facilities used in this work is gratefully acknowledged.
References
- American Lung Association (ALA) 2009 In Trends in asthma morbidity and mortality January 2009 (R. a. P.S.D. Epidemiology and Statistics Unit, Eds.), pp. 4–9, 37–43. American Lung Association, Washington, DC. Available at: http://www.lungusa.org/finding-cures/for-professionals/asthma-trend-report.pdf. [Google Scholar]
- Anderson DF, Zhang S, Bradding P, McGill JI, Holgate ST, Roche WR. The relative contribution of mast cell subsets to conjunctival TH2-like cytokines. Invest. Ophthalmol. Vis. Sci. 2001;42:995–1001. [PubMed] [Google Scholar]
- Avdalovic MV, Putney LF, Schelegle ES, Miller L, Usachenko JL, Tyler NK, Plopper CG, Gershwin LJ, Hyde DM. Vascular remodeling is airway generation-specific in a primate model of chronic asthma. Am. J. Respir. Crit. Care Med. 2006;174:1069–1076. doi: 10.1164/rccm.200506-848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzar S, Chu HW, Strand M, Wenzel S. Relationship of small airway chymase-positive mast cells and lung function in severe asthma. Am. J. Respir. Crit. Care Med. 2005;171:431–439. doi: 10.1164/rccm.200407-949OC. [DOI] [PubMed] [Google Scholar]
- Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am. Rev. Respir. Dis. 1989;139:806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Berger P, Girodet PO, Begueret H, Ousova O, Perng DW, Marthan R, Walls AF, Tunon de Lara JM. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J. 2003;17:2139–2141. doi: 10.1096/fj.03-0041fje. [DOI] [PubMed] [Google Scholar]
- Bienenstock J, Perdue M, Blennerhassett M, Stead R, Kakuta N, Sestini P, Vancheri C, Marshall J. Inflammatory cells and the epithelium. Mast cell/nerve interactions in the lung in vitro and in vivo. Am. Rev. Respir. Dis. 1988;138:S31–S34. doi: 10.1164/ajrccm/138.6_Pt_2.S31. [DOI] [PubMed] [Google Scholar]
- Bradding P, Holgate ST. Immunopathology and human mast cell cytokines. Crit. Rev. Oncol. Hematol. 1999;31:119–133. doi: 10.1016/s1040-8428(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani AM, Schwartz LB, Durham SR, Jeffery PK, Kay AB. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J. Allergy Clin. Immunol. 1991;88:661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- Cho SH, Anderson AJ, Oh CK. Importance of mast cells in the pathophysiology of asthma. Clin. Rev. Allergy Immunol. 2002;22:161–174. doi: 10.1385/CRIAI:22:2:161. [DOI] [PubMed] [Google Scholar]
- Chou DL, Daugherty BL, McKenna EK, Hsu WM, Tyler NK, Plopper CG, Hyde DM, Schelegle ES, Gershwin LJ, Miller LA. Chronic aeroallergen during infancy enhances eotaxin-3 expression in airway epithelium and nerves. Am. J. Respir. Cell Mol. Biol. 2005;33:1–8. doi: 10.1165/rcmb.2004-0236RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell JT. Asthmatic deaths. Role of the mast cell. JAMA. 1971;215:769–776. doi: 10.1001/jama.215.5.769. [DOI] [PubMed] [Google Scholar]
- Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, Wess J, Koller BH. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J. Immunol. 2009;182:7430–7439. doi: 10.4049/jimmunol.0900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paulis A, Annunziato F, Di Gioia L, Romagnani S, Carfora M, Beltrame C, Marone G, Romagnani P. Expression of the chemokine receptor CCR3 on human mast cells. Int. Arch. Allergy Immunol. 2001;124:146–150. doi: 10.1159/000053694. [DOI] [PubMed] [Google Scholar]
- de Paulis A, Minopoli G, Arbustini E, de Crescenzo G, Dal Piaz F, Pucci P, Russo T, Marone G. Stem cell factor is localized in, released from, and cleaved by human mast cells. J. Immunol. 1999;163:2799–2808. [PubMed] [Google Scholar]
- Dimitriadou V, Rouleau A, Dam Trung Tuong M, Newlands GJ, Miller HR, Luffau G, Schwartz JC, Garbarg M. Functional relationship between mast cells and C-sensitive nerve fibres evidenced by histamine H3-receptor modulation in rat lung and spleen. Clin. Sci. 1994;87:151–163. doi: 10.1042/cs0870151. [DOI] [PubMed] [Google Scholar]
- Djukanovic R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, Howarth PH, Holgate ST. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am. Rev. Respir. Dis. 1990;142:863–871. doi: 10.1164/ajrccm/142.4.863. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am. J. Respir. Cell Mol. Biol. 1999;21:655–657. doi: 10.1165/ajrcmb.21.6.3807. [DOI] [PubMed] [Google Scholar]
- Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am. J. Physiol. 2006;291:L644–L650. doi: 10.1152/ajplung.00027.2006. [DOI] [PubMed] [Google Scholar]
- Gosman MM, Postma DS, Vonk JM, Rutgers B, Lodewijk M, Smith M, Luinge MA, Ten Hacken NH, Timens W. Association of mast cells with lung function in chronic obstructive pulmonary disease. Respir. Res. 2008;9:64. doi: 10.1186/1465-9921-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RH, Brightling CE, Bradding P. The reclassification of asthma based on subphenotypes. Curr. Opin. Allergy Clin. Immunol. 2007;7:43–50. doi: 10.1097/ACI.0b013e3280118a32. [DOI] [PubMed] [Google Scholar]
- Horigome K, Bullock ED, Johnson EM., Jr Effects of nerve growth factor on rat peritoneal mast cells. Survival promotion and immediate-early gene induction. J. Biol. Chem. 1994;269:2695–2702. [PubMed] [Google Scholar]
- Joad JP, Kott KS, Bric JM, Peake JL, Plopper CG, Schelegle ES, Gershwin LJ, Pinkerton KE. Structural and functional localization of airway effects from episodic exposure of infant monkeys to allergen and/or ozone. Toxicol. Appl. Pharmacol. 2006;214:237–243. doi: 10.1016/j.taap.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Kajekar R, Pieczarka EM, Smiley-Jewell SM, Schelegle ES, Fanucchi MV, Plopper CG. Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir. Physiol. Neurobiol. 2007;155:55–63. doi: 10.1016/j.resp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J. Biol. Chem. 1997;272:7127–7131. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J. Clin. Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaar AL, Plotnick MI, Kucich U, Crichton I, Lotfi S, Das SK, Kane S, Rosenbloom J, Panettieri RA, Jr, Schechter NM, et al. Mast cell chymase modifies cell-matrix interactions and inhibits mitogen-induced proliferation of human airway smooth muscle cells. J. Immunol. 2002;169:1014–1020. doi: 10.4049/jimmunol.169.2.1014. [DOI] [PubMed] [Google Scholar]
- Lemanske RF., Jr Issues in understanding pediatric asthma: epidemiology and genetics. J. Allergy Clin. Immunol. 2002;109:S521–S524. doi: 10.1067/mai.2002.124564. [DOI] [PubMed] [Google Scholar]
- Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- McCunney RJ. Asthma, genes, and air pollution. J. Occup. Environ. Med. 2005;47:1285–1291. doi: 10.1097/01.jom.0000188561.75578.bf. [DOI] [PubMed] [Google Scholar]
- Miller LA, Hurst SD, Coffman RL, Tyler NK, Stovall MY, Chou DL, Putney LF, Gershwin LJ, Schelegle ES, Plopper CG, et al. Airway generation-specific differences in the spatial distribution of immune cells and cytokines in allergen-challenged rhesus monkeys. Clin. Exp. Allergy. 2005;35:894–906. doi: 10.1111/j.1365-2222.2005.02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G, Butterfield JH, Nilsson K, Siegbahn A. Stem cell factor is a chemotactic factor for human mast cells. J. Immunol. 1994;153:3717–3723. [PubMed] [Google Scholar]
- Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci A, Foresi A, Bertorelli G, Chetta A, Olivieri D. Histochemical characteristics and degranulation of mast cells in epithelium and lamina propria of bronchial biopsies from asthmatic and normal subjects. Am. Rev. Respir. Dis. 1993;147:684–689. doi: 10.1164/ajrccm/147.3.684. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Harkema JR. The respiratory system and its use in research. In: Wolfe-Coote S, editor. The Laboratory Primate. London: Elsevier Academic Press; 2005. pp. 503–527. [Google Scholar]
- Plopper CG, Smiley-Jewell SM, Miller LA, Fanucchi MV, Evans MJ, Buckpitt AR, Avdalovic M, Gershwin LJ, Joad JP, Kajekar R, et al. Asthma/allergic airways disease: does postnatal exposure to environmental toxicants promote airway pathobiology? Toxicol. Pathol. 2007;35:97–110. doi: 10.1080/01926230601132030. [DOI] [PubMed] [Google Scholar]
- Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J. Allergy Clin. Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. quiz 1009. [DOI] [PubMed] [Google Scholar]
- Sawada J, Itakura A, Tanaka A, Furusaka T, Matsuda H. Nerve growth factor functions as a chemoattractant for mast cells through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Blood. 2000;95:2052–2058. [PubMed] [Google Scholar]
- Schechter NM, Brass LF, Lavker RM, Jensen PJ. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J. Cell. Physiol. 1998;176:365–373. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, et al. Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae) Am. J. Pathol. 2001;158:333–341. doi: 10.1016/S0002-9440(10)63973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, et al. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol. Appl. Pharmacol. 2003;191:74–85. doi: 10.1016/s0041-008x(03)00218-7. [DOI] [PubMed] [Google Scholar]
- Thurston GD, Lippmann M, Scott MB, Fine JM. Summertime haze air pollution and children with asthma [see comments] Am. J. Respir. Crit. Care Med. 1997;155:654–660. doi: 10.1164/ajrccm.155.2.9032209. [DOI] [PubMed] [Google Scholar]
- Tran MU, Weir AJ, Fanucchi MV, Rodriguez AE, Pantle LM, Smiley-Jewell SM, Van Winkle LS, Evans MJ, Miller LA, Schelegle ES, et al. Smooth muscle hypertrophy in distal airways of sensitized infant rhesus monkeys exposed to house dust mite allergen. Clin. Exp. Allergy. 2004;34:1627–1633. doi: 10.1111/j.1365-2222.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- Triche EW, Gent JF, Holford TR, Belanger K, Bracken MB, Beckett WS, Naeher L, McSharry JE, Leaderer BP. Low-level ozone exposure and respiratory symptoms in infants. Environ. Health Perspect. 2006;114:911–916. doi: 10.1289/ehp.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, Bousquet J. Airway inflammation in mild intermittent and in persistent asthma. Am. J. Respir. Crit. Care Med. 1998;157:403–409. doi: 10.1164/ajrccm.157.2.96-08040. [DOI] [PubMed] [Google Scholar]
- Waern I, Jonasson S, Hjoberg J, Bucht A, Abrink M, Pejler G, Wernersson S. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J. Immunol. 2009;183:6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- Yam LT, Li CY, Crosby WH. Cytochemical identification of monocytes and granulocytes. Am. J. Clin. Pathol. 1971;55:283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Zanini A, Chetta A, Saetta M, Baraldo S, D'Ippolito R, Castagnaro A, Neri M, Olivieri D. Chymase-positive mast cells play a role in the vascular component of airway remodeling in asthma. J. Allergy Clin. Immunol. 2007;120:329–333. doi: 10.1016/j.jaci.2007.04.021. [DOI] [PubMed] [Google Scholar]