Abstract
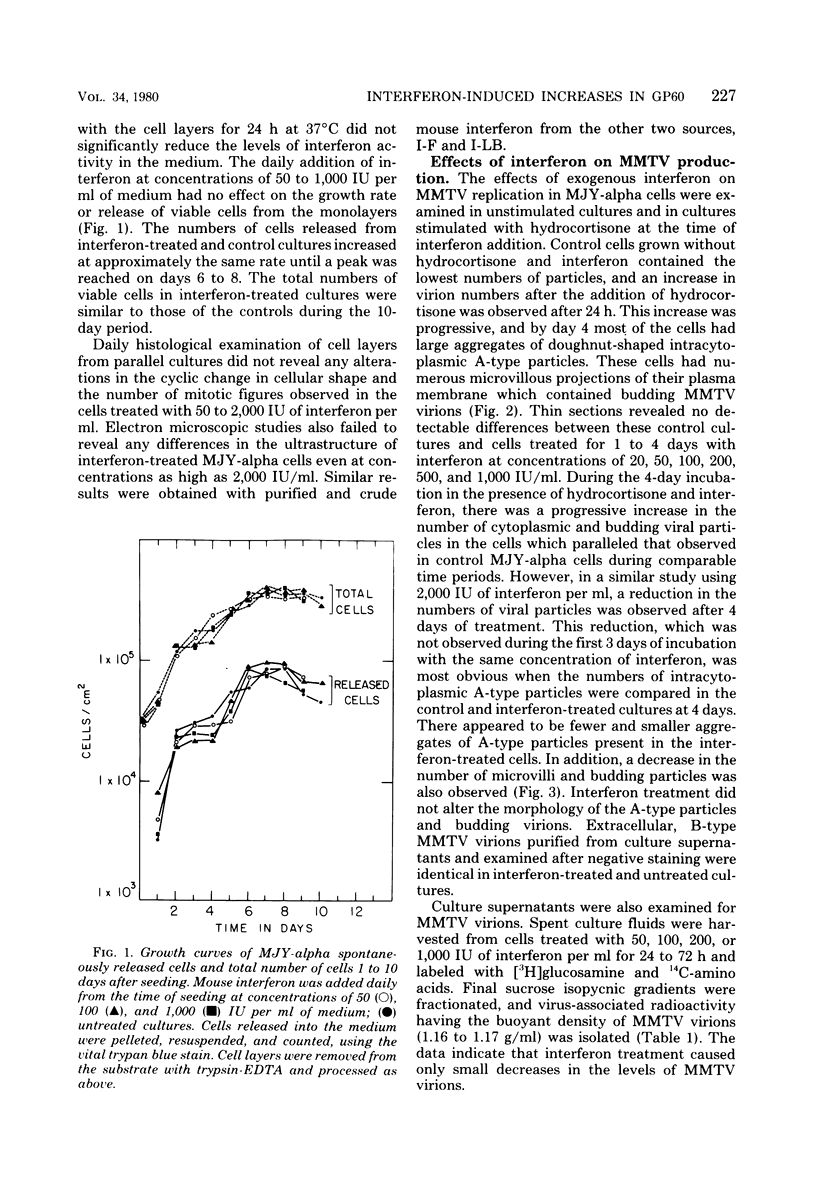
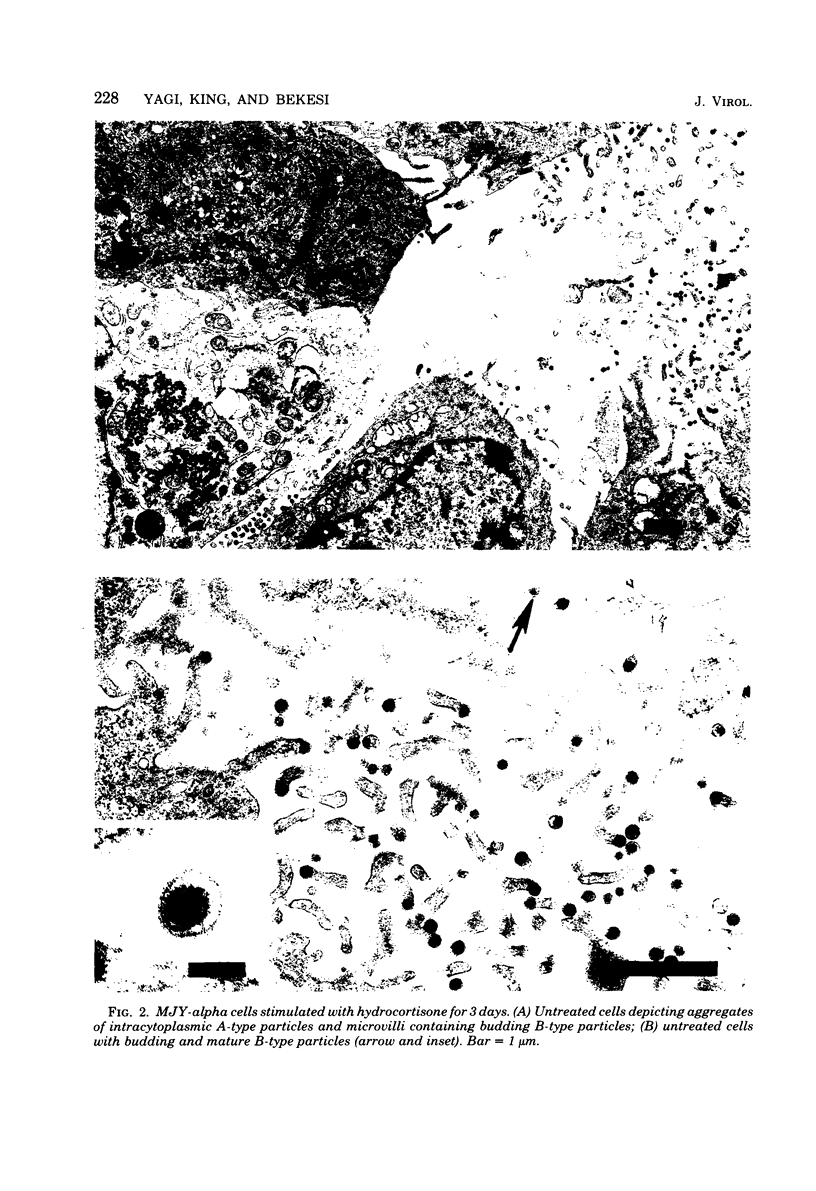
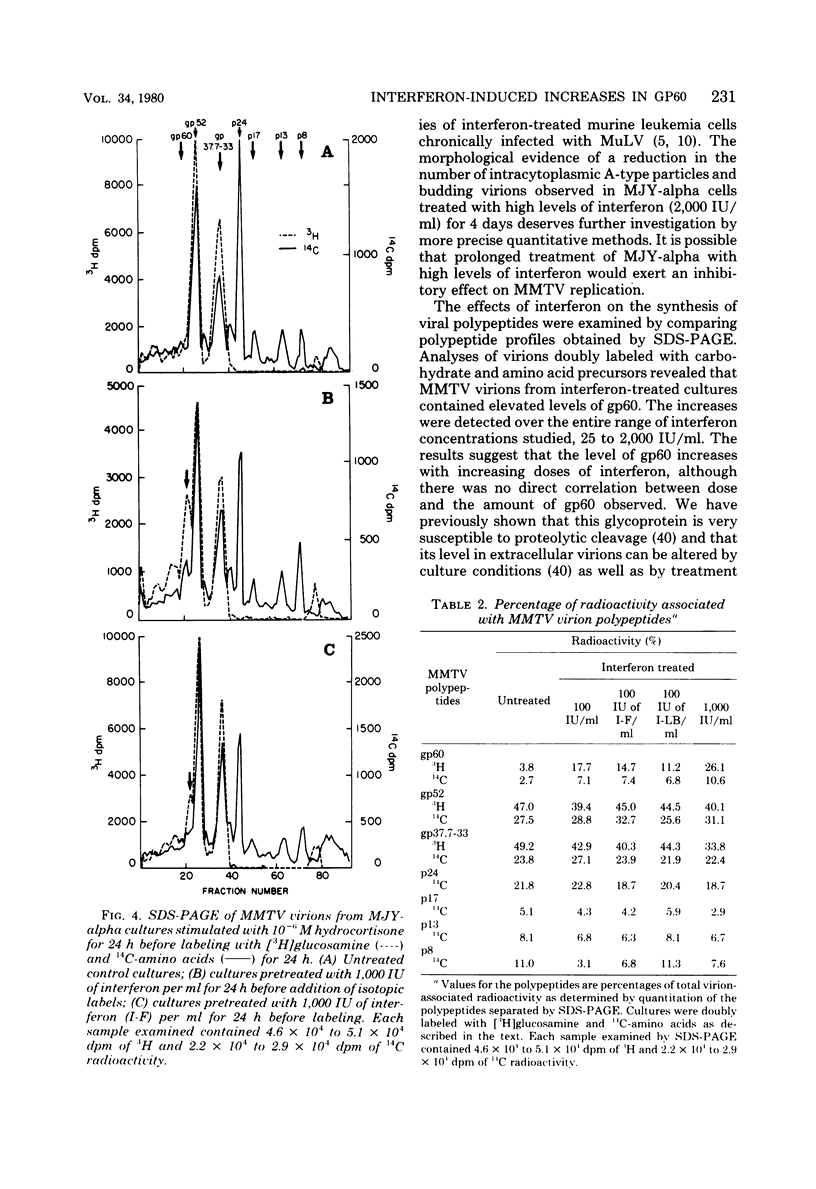
The effects of exogenous mouse interferon on the MJY-alpha mammary tumor cell line chronically infected with mouse mammary tumor virus (MMTV) were examined. Interferon at concentrations of 25 to 2,000 IU/ml in culture medium did not alter the growth rate or morphology of the cell layers. Electron microscopic examination of interferon-treated cells indicated a decrease in the numbers of A-type and budding B-type particles of MMTV. However, the levels of extracellular MMTV virions in the culture supernatants were not significantly reduced. Profiles of MMTV glycoproteins and nonglycosylated polypeptides obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of virions purified from interferon-treated cultures revealed increases in the relative levels of the 60,000-dalton glycoprotein, gp60.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Shoor R., Salzberg S. Effect of interferon on exogenous murine leukemia virus infection. Virology. 1978 Jan;84(1):134–141. doi: 10.1016/0042-6822(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Ankel H., Chany C., Galliot B., Chevalier M. J., Robert M. Antiviral effect of interferon covalently bound to sepharose. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2360–2363. doi: 10.1073/pnas.70.8.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekesi J. G., Roboz J. P., Zimmerman E., Holland J. F. Treatment of spontaneous leukemia in AKR mice with chemotherapy, immunotherapy, or interferon. Cancer Res. 1976 Feb;36(2 Pt 2):631–639. [PubMed] [Google Scholar]
- Billiau A., Edy V. G., Sobis H., de Somer P. Influence of interferon on virus-particle synthesis in oncornavirus-carrier lines. II. Evidence for a direct effect on particle release. Int J Cancer. 1974 Sep 15;14(3):335–340. doi: 10.1002/ijc.2910140306. [DOI] [PubMed] [Google Scholar]
- Billiau A., Heremans H., Allen P. T., De Maeyer-Guignard J., De Somer P. Trapping of oncornavirus particles at the surface of interferon-treated cells. Virology. 1976 Sep;73(2):537–542. doi: 10.1016/0042-6822(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Murphy P., Richardson L. C. Procedure for embedding in situ selected cells cultured in vitro. J Cell Biol. 1967 Oct;35(1):279–283. doi: 10.1083/jcb.35.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Came P. E., Moore D. H. Inhibition of spontaneous mammary carcinoma of mice by treatment with interferon and poly I:C. Proc Soc Exp Biol Med. 1971 May;137(1):304–305. doi: 10.3181/00379727-137-35565. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Friedman R. M. A large glycoprotein of Moloney leukemia virus derived from interferon-treated cells. Biochem Biophys Res Commun. 1977 Jul 11;77(1):392–398. doi: 10.1016/s0006-291x(77)80210-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Mims S. J., Triche T. J., Friedman R. M. Interferon inhibits mouse leukaemia virus release: an electron microscope study. J Gen Virol. 1977 Feb;34(2):363–367. doi: 10.1099/0022-1317-34-2-363. [DOI] [PubMed] [Google Scholar]
- Chirigos M. A., Pearson J. W. Brief communication: Cure of murine leukemia with drug and interferon treatment. J Natl Cancer Inst. 1973 Oct;51(4):1367–1368. doi: 10.1093/jnci/51.4.1367. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Influenza virus proteins. II. Association with components of the cytoplasm. Virology. 1973 Jan;51(1):56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- Drohan W., Kettmann R., Colcher D., Schlom J. Isolation of the mouse mammary tumor virus sequences not transmitted as germinal provirus in the C3H and RIII mouse strains. J Virol. 1977 Mar;21(3):986–995. doi: 10.1128/jvi.21.3.986-995.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Chang E. H., Ramseur J. M., Myers M. W. Interferon-directed inhibition of chronic murine leukemia virus production in cell cultures: lack of effect on intracellular viral markers. J Virol. 1975 Sep;16(3):569–574. doi: 10.1128/jvi.16.3.569-574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Interferons and cancer. J Natl Cancer Inst. 1978 Jun;60(6):1191–1194. doi: 10.1093/jnci/60.6.1191. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Ramseur J. M. Inhibition of murine leukemia virus production in chronically infected AKR cells: a novel effect of interferon. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3542–3544. doi: 10.1073/pnas.71.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Gresser I. Antitumor effects of interferon. Adv Cancer Res. 1972;16:97–140. doi: 10.1016/s0065-230x(08)60339-5. [DOI] [PubMed] [Google Scholar]
- Gresser I., Maury C., Brouty-Boyé D. Mechanism of the antitumour effect of interferon in mice. Nature. 1972 Sep 15;239(5368):167–168. doi: 10.1038/239167a0. [DOI] [PubMed] [Google Scholar]
- King N. W., Jr, Barahona H., Daniel M. D., Bekesi G., Jones T. C. The effect of phosphonoacetic acid on the ultrastructure of cell cultures infected with simian oncogenic herpesviruses. Lab Invest. 1978 Feb;38(2):181–188. [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the specific cytotoxicity of sensitized lymphocytes. Proc Natl Acad Sci U S A. 1972 Mar;69(3):721–725. doi: 10.1073/pnas.69.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Vlahakis G., Schlom J. A biochemical approach to the study of the transmission of mouse mammary tumor viruses in mouse strains RIII and C3H. Int J Cancer. 1976 Jul 15;18(1):105–115. doi: 10.1002/ijc.2910180114. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Rowe W. P., Oxman M. N. Effect of interferon on exogenous, endogenous, and chroniv murine leukemia virus infection. Virology. 1976 Apr;70(2):324–338. doi: 10.1016/0042-6822(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Rossi G. B., Matarese G. P., Grappelli C., Belardelli F., Benedetto A. Interferon inhibits dimethyl sulphoxide-induced erythroid differentiation of Friend leukaemia cells. Nature. 1977 May 5;267(5606):50–52. doi: 10.1038/267050a0. [DOI] [PubMed] [Google Scholar]
- Sato T. A modified method for lead staining of thin sections. J Electron Microsc (Tokyo) 1968;17(2):158–159. [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schellekens H., Weimar W., Cantell K., Stitz L. Antiviral effect of interferon in vivo may be mediated by the host. Nature. 1979 Apr 19;278(5706):742–742. doi: 10.1038/278742a0. [DOI] [PubMed] [Google Scholar]
- Schlom J., Colcher D., Drohan W., Kettmann R., Michalides R., Vlahakis G., Young J. Differences in mouse mammary tumor viruses. Relationship to early and late occurring mammary tumors. Cancer. 1977 Jun;39(6 Suppl):2727–2733. doi: 10.1002/1097-0142(197706)39:6<2727::aid-cncr2820390660>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Sheffield J. B., Zacharchuk C. M., Taraschi N., Daly T. M. Effect of trypsin on mouse mammary tumor virus. J Virol. 1976 Jul;19(1):255–266. doi: 10.1128/jvi.19.1.255-266.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strannegård O., Larsson I., Lundgren E., Miörner H., Persson H. Modulation of immune responses in newborn and adult mice by interferon. Infect Immun. 1978 May;20(2):334–339. doi: 10.1128/iai.20.2.334-339.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauchen J. A., Young N. A., Friedman R. M. Interferon-mediated inhibition of mouse mammary tumor virus expression in cultured cells. Virology. 1977 Oct 1;82(1):232–236. doi: 10.1016/0042-6822(77)90046-0. [DOI] [PubMed] [Google Scholar]
- Tilles J. G., Finland M. Microassay for human and chick cell interferons. Appl Microbiol. 1968 Nov;16(11):1706–1707. doi: 10.1128/am.16.11.1706-1707.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., MacLeod R., Chang E. H., Myers M. W., Friedman R. M. The effect of interferon on de novo infection of Moloney murine leukemia virus. Cell. 1977 Feb;10(2):245–252. doi: 10.1016/0092-8674(77)90218-5. [DOI] [PubMed] [Google Scholar]
- Yagi M. J., Blair P. B., Lane M. A. Modulation of mouse mammary tumor virus production in the MJY-alpha cell line. J Virol. 1978 Nov;28(2):611–623. doi: 10.1128/jvi.28.2.611-623.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M. J. Characteristics of mammary tumor cultures from four mouse strains infected with mammary tumor virus. Cancer Res. 1975 Feb;35(2):370–373. [PubMed] [Google Scholar]
- Yagi M. J., Compans R. W. Structural components of mouse mammary tumor virus. I. Polypeptides of the virion. Virology. 1977 Feb;76(2):751–766. doi: 10.1016/0042-6822(77)90256-2. [DOI] [PubMed] [Google Scholar]
- Yagi M. J. Cultivation and characterization of BALB-cfC3H mammary tumor cell lines. J Natl Cancer Inst. 1973 Dec;51(6):1849–1860. doi: 10.1093/jnci/51.6.1849. [DOI] [PubMed] [Google Scholar]
- Yagi M. J. Further observations on the production of oncornaviruses by MJY-alpha cell line. J Natl Cancer Inst. 1974 Nov;53(5):1383–1385. doi: 10.1093/jnci/53.5.1383. [DOI] [PubMed] [Google Scholar]
- Yagi M. J., Stutzman R. E., Robertson B. H., Compans R. W. Structural components of mouse mammary tumor virus. II. Isolation and purification of virion polypeptides. J Virol. 1978 May;26(2):448–456. doi: 10.1128/jvi.26.2.448-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]