Abstract
Combustion-derived nanoparticles, such as diesel engine exhaust particles, have been implicated in the adverse health effects of particulate air pollution. Recent studies suggest that inhaled nanoparticles may also reach and/or affect the brain. The aim of our study was to comparatively evaluate the effects of short-term diesel engine exhaust (DEE) inhalation exposure on rat brain and lung. After 4 or 18 h recovery from a 2 h nose-only exposure to DEE (1.9 mg/m3), the mRNA expressions of heme oxygenase-1 (HO-1), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and cytochrome P450 1A1 (CYP1A1) were investigated in lung as well as in pituitary gland, hypothalamus, olfactory bulb, olfactory tubercles, cerebral cortex, and cerebellum. HO-1 protein expression in brain was investigated by immunohistochemistry and ELISA. In the lung, 4 h post-exposure, CYP1A1 and iNOS mRNA levels were increased, while 18 h post-exposure HO-1 was increased. In the pituitary at 4 h post-exposure, both CYP1A1 and HO-1 were increased; HO-1 was also elevated in the olfactory tuberculum at this time point. At 18 h post-exposure, increased expression of HO-1 and COX-2 was observed in cerebral cortex and cerebellum, respectively. Induction of HO-1 protein was not observed after DEE exposure. Bronchoalveolar lavage analysis of inflammatory cell influx, TNF-α, and IL-6 indicated that the mRNA expression changes occurred in the absence of lung inflammation. Our study shows that a single, short-term inhalation exposure to DEE triggers region-specific gene expression changes in rat brain to an extent comparable to those observed in the lung.
Keywords: Diesel engine exhaust, Nanoparticles, Brain, Oxidative stress, CYP1A1, Heme oxygenase-1
Introduction
Diesel engine exhaust (DEE) emissions represent a major source of ambient particulate matter (PM) and combustion-derived nanoparticles in most urban settings (Donaldson et al. 2005). Several lines of research have led to the concern that the brain represents a relevant target for the effects of such particles. Initial clues for potential neuropathological effects of ambient air particles have originated from comparative histopathology studies of brains of mongrel dogs, and more recently of postmortem tissues of lifelong residents from cities with strongly contrasting air pollution (Calderon-Garciduenas et al. 2004). These investigations revealed signs of oxidative stress and inflammation in the brain in association with high air pollution, characterized for instance by an increased expression of the transcription factor Nuclear Factor kappa B (NFκB) and the inflammatory genes cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (reviewed in Peters et al. 2006).
Controlled inhalation studies in mice have confirmed that ambient PM and nanoparticles may trigger oxidative stress, toxicity, and inflammation in brain tissue (Campbell et al. 2005; Elder et al. 2006; Kleinman et al. 2008; Veronesi et al. 2005). Moreover, it has been demonstrated that small fractions of inhaled nanoparticles may actually reach the brain (Elder et al. 2006; Oberdorster et al. 2004). For this, two principal routes have been proposed. In the one route, particles are considered to reach brain tissue following deposition on the nasal olfactory epithelium and translocation along the olfactory nerve; the other route involves alveolar deposition and subsequent crossing of the lung blood barrier and the blood–brain barrier (BBB) (Oberdorster et al. 2005; Peters et al. 2006). Under these assumptions, several in vitro studies demonstrated that nanoparticles, including diesel engine exhaust particles, may cause neurotoxic effects to specific brain cells and disturb BBB functions (Block et al. 2004; Hartz et al. 2008; Long et al. 2007).
The objective of the present study was to compare in the rat, the effects of a single, short-term DEE inhalation exposure on both lung and potentially sensitive regions of the brain. The pituitary and hypothalamus were selected because, on the basis of their incomplete BBB, they might be more prone to effects of blood-borne substances. As representative regions for the olfactory translocation route of carbonaceous nanoparticles (Oberdorster et al. 2004), we included olfactory bulb and tubercles. Cerebral cortex and cerebellum were analyzed to obtain a more complete picture of effects in the brain. In each of these brain regions, as well as the lung, the mRNA expression of iNOS, COX-2, heme oxygenase-1 (HO-1) and cytochrome p450 1a1 (CYP1A1) was determined. These genes were selected a priori on the fulfillment of two criteria: (1) their demonstrated relevance in diesel exhaust exposure and lung disease (Ahn et al. 2008; Risom et al. 2003; Takano et al. 2002; Zhao et al. 2006) and (2) their known inducibility in rat brain during oxidative stress, inflammation and/or exposure to polycyclic aromatic hydrocarbons (PAH) (Calderon-Garciduenas et al. 2004; Collino et al. 2006; Colombrita et al. 2003; Maeda et al. 2008; Shimada et al. 2003).
Materials and methods
Animals
Nine-week-old male Fischer F344 rats, obtained from Charles River (Germany), were used in the present study. Experiments were approved by the Animal Ethics Committee (IUCAC) of the Dutch National Vaccine Institute (NVI). In the week prior to exposure, animals were trained in nose-only tubes for 1 h daily during 5 days.
Exposure
The rats were exposed for 2 h, nose-only, to diluted DEE or purified air, followed by an exposure recovery of a further 4 or 18 h (N = 8 per time point). DEE from a stationary (1,500 rpm) diesel engine (35KVA genset, Deutz F3M2011 engine, Bredenoord, Apeldoorn the Netherlands) was mixed with conditioned purified air.
Particle and NO2 levels were closely monitored and regulated to ensure a constant exposure. Particle number and mass concentrations were determined using a condensation particle counter (CPC 3022A, TSI St. Paul, Minn., USA) and a nephelometer (DATARAM 2000, MIE, Billerica, Mass., USA). Time-integrated particle concentrations were determined by gravimetric analysis. A carbon sampler tube was placed downstream of one of the PTFE filters at the outlet to collect the volatile organic compounds (VOC), which were measured by means of GC–MS (RIVM, Bilthoven the Netherlands). The final particle mass concentration in diluted DEE was 1.9 mg/m3 with a geometric mean diameter of 65 nm. Total VOC content was 0.53 mg/m3. The concentrations of CO, NOx, NO, and NO2 measured in the mixing chamber were on average 14.23 ppb CO, 2.38 ppb NOx, 1.43 ppb NO, and 0.94 ppb NO2.
Necropsy
Animals were anesthetized 4 or 18 h post-exposure with a mixture of Ketamin (100 mg/ml) and Xylazine (20 mg/ml) in a 10:8 ratio. Animals were then killed through exsanguinations and after saline perfusion of the lungs via the right cardiac ventricle bronchoalveolar lavage was performed. Subsequently, brains were removed. Brains of three DEE-exposed and three control animals per time point were immediately incubated in Zamboni’s fixative while brains of five animals per treatment and time point were dissected into six regions, i.e., pituitary gland, olfactory bulb, olfactory tubercles, hypothalamus, cerebral cortex, and cerebellum. Additionally, the accessory lung lobe was removed from the same animals. Brain and lung tissue samples were snap-frozen in liquid nitrogen and stored at −80°C until further processing.
Bronchoalveolar lavage
The trachea was cannulated and the right lung was lavaged gently in three cycles of inserting and reclaiming the solution with 27 ml saline per kilogram body weight. Bronchoalveolar lavage fluid (BALF) was put on ice immediately and centrifuged at 4°C and 400g for 10 min. For differential cell counts, the cell pellet was resuspended in 1 ml saline. Cytospin slides were prepared in duplicate for each animal, and after staining with May-Grünwald-Giemsa, 200 cells were differentiated per slide. Total cell numbers in BALF were determined by Coulter Counter. To determine pulmonary toxicity and inflammation, BALF was analyzed for total protein using a reagent kit from Pierce (Etten-Leur, The Netherlands), LDH and alkaline phosphatase using assay kits from Roche Nederland, Almere, The Netherlands. Total Glutathione was measured as described previously (Cassee et al. 2005). Concentrations of the inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in BALF were determined with ELISA kits from Biosource (Etten-Leur, The Netherlands).
Quantitative real-time PCR
Brain sections and lung tissues were ground and homogenized in Trizol. RNA was extracted according to the manufacturer’s instructions and purified using the RNeasy® mini kit in combination with DNAse treatment (RNAse-free DNAse set, Qiagen). From 0.5 μg of RNA, cDNA was synthesized using the iScript cDNA Synthesis kit (BioRad, CA, USA) and diluted 15× in water. qRT-PCR primers were designed for rat HO-1, iNOS, COX-2, CYP1A1, and the housekeeping gene GAPDH, using Primer express software (Applied Biosystems). Sequences are shown in Table 1. The MyiQ single-color real-time PCR detection system (BioRad) coupled to SYBR© Green Supermix (Biorad), 5 μl diluted cDNA, and 0.3 μM primers in a total volume of 25 μl was used to perform real-time PCR. Denaturation at 95°C for 3 min was followed by 40 cycles at 95°C (15 s) and 60°C (45 s). Melt curves (60–95°C) were produced for product identification and purity. PCR efficiencies of all primer sets were in the range of 90–100%. Gene expression was analyzed using MyiQ Software (BioRad) and normalized to the expression of GAPDH. For the lung, data are expressed as fold-change in expression compared to the expression in air-exposed animals, using the 2-ΔΔCt method (Livak and Schmittgen 2001). For the brain, results are shown as expression relative to the mean expression in cerebellum of the air-exposed animals, to allow for comparative evaluation of brain region-specific expression as well as of the effect of DEE.
Table 1.
Primer sequences used in this study
| Gene | Forward | Reverse |
|---|---|---|
| HO-1 | 5′-GGGAAGGCCTGGCTTTTTT-3′ | 5′-CACGATAGAGCTGTTTGAACTTGGT-3′ |
| iNOS | 5′-AGGAGAGAGATCCGGTTCACAGT-3′ | 5′-ACCTTCCGCATTAGCACAGAA-3′ |
| COX-2 | 5′-GCACAAATATGATGTTCGCATTCT-3′ | 5′-GAACCCAGGTCCTCGCTTCT-3′ |
| CYP1A1 | 5′-TGGAGACCTTCCGACATTCATC-3′ | 5′-GCCATTCAGACTTGTATCTCTTATGG-3′ |
| GAPDH | 5′-TGATTCTACCCACGGCAAGTT-3′ | 5′-TGATGGGTTTCCCATTGATGA-3′ |
Brain fixation and immunohistochemistry
For immunohistochemistry, rat brains were immediately incubated in Zamboni’s fixative (24 h) and subsequently sucrose (48 h), and then frozen in isopentane at a temperature of −40°C. Brains were stored at −80°C until tissue sections were prepared for the determination of heme oxygenase-1 immunoreactivity as described elsewhere (Bidmon et al. 2001).
Heme oxygenase-1 ELISA
For all brain sections from the 18 h recovery time point, except for the pituitary gland due to insufficiency of available material, protein was extracted according to assay kit recommendations (Assay Designs, Ann Arbor, USA). Protein concentrations of all samples were determined using the bicinchoninic protein assay, and all concentrations were adjusted to 2.38 μg/μl. Samples were then diluted 2× for the final assay, and 100 μl of sample, standard, or buffer control was added per well. The ELISA method was performed according to the manufacturer’s guidelines; absorbance was measured immediately at 450 nm. Data are extrapolated from the standard curve and expressed as pg/mg total protein.
Statistical evaluation
Data are expressed as mean ± SEM unless stated otherwise. For the evaluation of treatment-related differences (DEE versus air exposure) in lung as well as within specific regions of the brain, data were evaluated by Student t test using SPSS version 15.0 for Windows. A difference was considered to be statistically significant when p < 0.05.
Results
Markers of lung toxicity, inflammation, and oxidative stress in BALF
The effects of DEE on BALF markers of cytotoxicity, inflammation, and oxidative stress are shown in Table 2. LDH, total protein, and total glutathione, indicative of toxicity, increased permeability of the alveolar capillary barrier and oxidative stress, respectively, were not significantly increased at 4 or 18 h post-exposure. Inflammation, measured as increases in total cell number, percentages of neutrophils or macrophages, and levels of TNF-α and IL-6, was also absent at either time point. Alkaline phosphatase, considered as a marker of damage to lung type II epithelial cells, was significantly increased after 18 h of exposure recovery. Together, these observations demonstrate limited toxicity and the absence of a marked inflammatory response in rat lungs.
Table 2.
Bronchoalveolar lavage parameters from rats after 4 or 18 h recovery from a 2 h exposure to diesel engine exhaust (DEE) or filtered air
| BALF | Air, 4 h | DEE, 4 h | Air, 18 h | DEE, 18 h |
|---|---|---|---|---|
| Total cells (×106) | 1.17 ± 0.14 | 1.34 ± 0.13 | 1.14 ± 0.07 | 1.05 ± 0.13 |
| % Macrophages | 97.60 ± 0.17 | 98.60 ± 0.32 | 96.25 ± 1.15 | 97.55 ± 0.45 |
| % Neutrophils | 1.30 ± 0.37 | 0.90 ± 0.27 | 1.80 ± 0.40 | 1.50 ± 0.45 |
| Total protein (mg/l) | 215 ± 12 | 189 ± 17 | 189 ± 6 | 205 ± 14 |
| LDH (U/l) | 72.6 ± 6.6 | 78.9 ± 5.9 | 70.2 ± 1.7 | 75.7 ± 7.0 |
| AP (U/l) | 53.9 ± 4.3 | 45.5 ± 1.8 | 40.3 ± 6.6 | 67.3 ± 5.5* |
| Total glutathione (μM) | 1.06 ± 0.07 | 0.92 ± 0.10 | 1.28 ± 0.12 | 0.94 ± 0.09 |
| TNF-α (pg/ml) | 81.9 ± 22.0 | 85.7 ± 19.4 | 73.6 ± 18.4 | 50.8 ± 9.4 |
| IL-6 (pg/ml) | 15.6 ± 4.1 | 22.7 ± 2.7 | 16.1 ± 6.6 | 22.3 ± 3.2 |
Data represent mean ± SEM from n = 5 rats per treatment group and time point, * p < 0.05
Gene expression in rat lungs
The effects of DEE exposure on HO-1, COX-2, iNOS, and CYP1A1 mRNA expression in lung are shown in Fig. 1. The mRNA expression of HO-1 was found to be significantly increased in the lungs from DEE-exposed animals after 18 h of recovery (1.7-fold increase). For CYP1A1, a statistically significant increase in mRNA was measured after 4 h recovery (4.1-fold). An induction was also observed at 18 h post-exposure (1.8-fold), but this did not reach statistical significance. A small but significant increase in iNOS mRNA was detected in the lungs of the rats after 4 h recovery. No changes in COX-2 mRNA expression were observed.
Fig. 1.
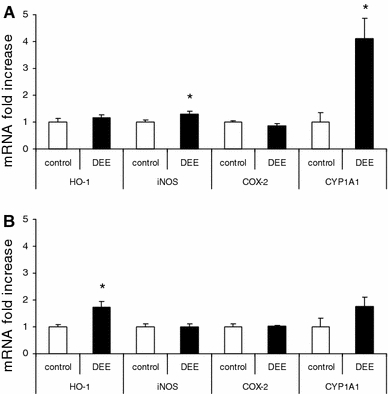
mRNA expression of HO-1, iNOS, COX-2 and CYP1A1 in rat lungs after 4 h (a) or 18 h (b) recovery from diesel engine exhaust (DEE) inhalation exposure. Data represent mean values and standard errors (n = 5) and are expressed as GADPH-adjusted fold-increase mRNA expression compared to controls. * p < 0.05 versus air-exposed group
Gene expression in specific regions of the rat brain
The mRNA expression of HO-1, COX-2, iNOS, and CYP1A1 in various sections of the rat brain is shown in Figs. 2 and 3. Data were corrected for GAPDH and expressed as fold expression relative to the expression in the cerebellum of the air-exposed rats. For each gene, considerable brain region-specific expression contrasts were found. For HO-1, mRNA expression was found to be highest in pituitary and lowest in both cerebral cortex and cerebellum. In relation to the DEE exposure, HO-1 expression tended to be enhanced in most of the brain regions, both at 4 h and at 18 h post-treatment. Statistically significant increases after DEE exposure were found in the pituitary gland and olfactory tubercles after 4 h and in the cerebral cortex after 18 h. For iNOS, similar to HO-1, the strongest mRNA expression was found in the pituitary glands. In the DEE-exposed animals an approximate twofold increase in the pituitary gland was observed after 4 h. However, this effect did not reach statistical significance because of considerable variation. COX-2 expression was found to be highest in the cerebral cortex and lowest in hypothalamus. At 4 h post-exposure, COX-2 expression tended to be reduced in cerebral cortex, olfactory bulb and tubercles compared to controls. In contrast, at 18 h post-exposure, a general tendency of enhanced COX-2 mRNA was observed, reaching statistical significance in the cerebellum only. For CYP1A1 the expression variability in brain was found to be most pronounced. Its expression was about 18–20-fold higher in pituitary gland and fourfold higher in olfactory bulb relative to cerebellum. DEE-exposed animals showed a significantly higher CYP1A1 expression in the pituitary gland after 4 h. Notable, but non-significant increases were also observed in cerebellum and olfactory tubercles after 4 and 18 h.
Fig. 2.
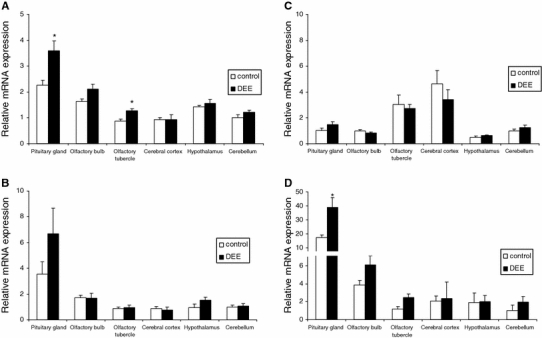
mRNA expression of HO-1 (a), iNOS (b), COX-2 (c) and CYP1A1 (d) in specific rat brain regions after 4 h recovery from diesel engine exhaust (DEE) inhalation exposure. Data are shown as mean and SEM (n = 5 per treatment) of the GAPDH adjusted mRNA expression, relative to the mean mRNA expression as measured in the cerebellum of the air-exposed animals for each gene. * p < 0.05 versus air exposure in the same brain region
Fig. 3.
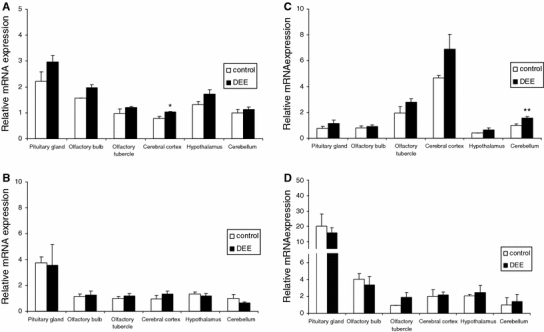
mRNA expression of HO-1 (a), iNOS (b), COX-2 (c) and CYP1A1 (d) in specific rat brain regions after 18 h recovery from diesel engine exhaust (DEE) inhalation exposure. Data are mean and SEM (n = 5 per treatment) of GAPDH adjusted mRNA expression, relative to the mean mRNA expression as measured in the cerebellum. * p < 0.05 versus air exposure in the same brain region
HO-1 protein expression by immunohistochemistry and ELISA
To further evaluate the effects of DEE on rat brain, we also measured HO-1 protein expression by immunohistochemistry and ELISA. Although specific expression profiles were observed for different brain regions, no treatment-related differences were evident upon full evaluation of brain tissue slices. Exemplary, Fig. 4a and b show HO-1 staining in cerebellum of, respectively, a control and a DEE-exposed animal. A clear pattern of HO-1 immunoreactivity was observed irrespective of the exposure, particularly in Purkinje cells. ELISA measurement results are shown in Fig. 4c. Because of insufficiently available material, pituitary gland could not be evaluated. Marked differences were observed in HO-1 concentrations between the different brain regions, with the highest levels in cerebellum and hypothalamus. In relation to the DEE exposure, no significant differences were found, although tendencies toward upregulation of HO-1 protein were noted in the cerebral cortex and the hypothalamus.
Fig. 4.
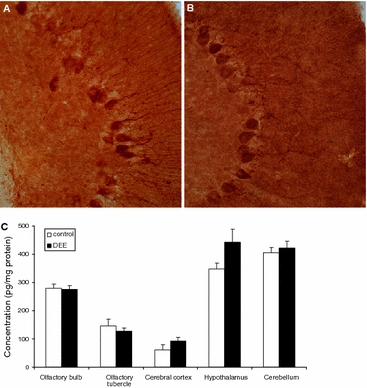
HO-1 protein expression by immunohistochemistry (a, b) and ELISA (c) in rat brain tissue after diesel engine exhaust (DEE) inhalation exposure. Representative HO-1 staining in rat cerebellum after 24 h recovery from a 2 h exposure to filtered air (a) or DEE (b). Original magnification ×25. Panel C shows HO-1 expression presented as pg/mg total protein, in brain section lysates
Discussion
The present study was undertaken to compare the effects of short-term inhalation of diesel engine exhaust on lung and different brain regions of the rat. Among the genes investigated, CYP1A1 tended to show the strongest mRNA expression changes. Our findings suggest a rapid, transient induction upon DEE exposure. The fold increase in CYP1A1 expression in the lung in this study was similar to investigations by Sato et al. (2000), who found a 5.5-fold induction in rat lungs following 4 weeks of inhalation exposure at 6 mg/m3. Since CYP1A1 induction is considered as a sensitive marker of exposure to diesel engine exhaust particles in the lung (Stoeger et al. 2009; Takano et al. 2002), our current findings suggest that the brain represents a target for DEE.
Organic constituents adsorbed on diesel particles, PAH in particular, are thought to be crucially responsible for pulmonary CYP1A1 induction (Bonvallot et al. 2001; Zhao et al. 2006). Whether these are also responsible for CYP1A1 induction in the brain remains to be demonstrated. PAH are highly potent inducers of CYP1A1 in the brain (Shimada et al. 2003). However, deposition and translocation calculations (Oberdorster et al. 2004) indicate that the amount of particles, and hence the ultimate concentration of particle-bound PAH and other organic constituents, that may have reached this organ within 6 h after DEE exposure initiation, will be rather low. Effects of PAH will also depend on their bioavailability, which is known to depend on adsorption strength as well as the amount loaded onto the carbonaceous carrier particles (Gerde et al. 2001). Finally, one should consider a role for (semi)volatile compounds in the present study. For instance, VOC within PM have recently been shown to induce CYP1A1 in vitro (Abbas et al. 2009). Neurotoxic effects of specific VOC are well known. To the best of our knowledge, the CYP1A1-inducing potential in the brain has not been investigated for VOC.
DEE exposure was also associated with expression changes of COX-2 and iNOS. Diesel engine exhaust particles have been shown to increase the mRNA expression of both these genes after instillation in rat or mouse lung (Ahn et al. 2008; Lim et al. 1998; Zhao et al. 2006). In the current 2 h inhalation study, COX-2 mRNA expression in lung was unaffected, whereas iNOS was rapidly increased. In contrast, this short exposure led in the brain to a significantly increased expression of COX-2, but not of iNOS. Enhanced COX-2 mRNA expression has been observed in the frontal cortex and hippocampus of lifelong inhabitants of cities with high levels of air pollution (Calderon-Garciduenas et al. 2004; Peters et al. 2006). Obviously, these autopsy investigations cannot be directly linked to our study, which addressed potential effects after a single, short-term exposure to DEE. In the human brain autopsy investigations many pollutants other than DEE, as well as independent lifestyle factors might have been responsible for, or contributing to the observed COX-2 expression profiles.
The most consistent effects in our study were found for HO-1, also known as Heat shock protein-32. In concordance with our findings, increased HO-1 mRNA expression has been described in mouse lungs after single and repeated DEE inhalations (Li et al. 2007; Risom et al. 2003). In vitro studies have demonstrated that diesel engine exhaust particles as well as their organic extracts can induce HO-1 in various cell lines (Li et al. 2000, 2002). The main mechanism of HO-1-mediated cellular protection is rooted in the powerful antioxidant and anti-inflammatory potential of its metabolic products, like carbon monoxide and bilirubin (Stocker et al. 1987). HO-1 is also well known for its capability to protect brain from oxidative injury and recognized as a sensitive marker of cellular stress responses in this organ (Abraham et al. 1996). Astrocytes highly express HO-1 in response to injury and the HO pathway has been shown to be a critical defense mechanism for neurons against oxidant aggressors (Chen et al. 2000; Le et al. 1999). In the ischemic rat brain, a common model for oxidative brain damage, a strong rapid induction of HO-1 has been shown (Collino et al. 2006). As such, our current observations indicate that the DEE inhalation triggered a rapid, albeit mild oxidative stress in specific rat brain regions.
Expression changes in HO-1 after DEE exposure could not be confirmed on the protein level by immunohistochemistry. Subsequent analysis by ELISA demonstrated strong region-specific contrasts in expression which were in line with the patterns as observed by immunohistochemistry and described in the literature (e.g. Colombrita et al. 2003). In association with DEE exposure, the ELISA measurements indicated a slight upregulation of HO-1 protein in the hypothalamus and the cerebral cortex, although this did not reach statistical significance. Unfortunately, insufficient material was available for protein expression measurement in the pituitary gland, for which the HO-1 mRNA expression changes after DEE exposure appeared to be the most pronounced.
The overall picture derived from the present study is that short-term exposure of rats to DEE causes mRNA expression changes of genes related to oxidative stress and inflammation in specific brain regions. Moreover, DEE exposure was found to increase the expression of the xenobiotic metabolism enzyme CYP1A1 in the brain. The pituitary gland, which is in direct contact with the hypothalamus, appeared to be most sensitive to the DEE exposure, as indicated by an increased expression of all genes investigated. This suggests that the observed effects of DEE involve the BBB exposure route. At this point it should be noted that the pituitary represents a neuroendocrine organ involved in the orchestration of multiple organs and their functions in a circadian manner via regulatory hormones (Diamanti-Kandarakis et al. 2009; Melmed 2002). Thus, our data indicate an important route by which effects of DEE may be transmitted to various organs.
However, at 4 h post-exposure, the expression of HO-1 and CYP1A1 also tended to be higher in olfactory bulb and tubercles. This indicates that the DEE effects may, at least in part, also be mediated via the olfactory route. Interestingly, the pituitary gland and olfactory bulb, both representing “windows of the brain to the outside world” (Feron et al. 2001), also tended to show the highest constitutive mRNA expression for all investigated genes, with the exception of COX-2, for which constitutive expression was highest in the cerebral cortex. Likewise, differences in constitutive expression of these genes throughout the brain have been reported by others (e.g. Huang et al. 2000; Maeda et al. 2008; Morse et al. 1998; Quan et al. 1998).
It remains to be investigated whether the observed effects result from direct actions of translocated particles or specific components (e.g. PAH, metals, VOC) carried on the surface of these particles (Donaldson et al. 2005), or whether they are caused indirectly by mediators released from respiratory tract and/or the blood (Peters et al. 2006). In an in vitro experimental model of isolated brain capillaries, diesel engine exhaust particles were recently found to disturb BBB functions through oxidative stress and pro-inflammatory cytokine production (Hartz et al. 2008). Exposure to diesel exhaust particles can induce systemic inflammation and oxidative stress (Inoue et al. 2006; Yokota et al. 2005), and circulating cytokines are known to evoke inflammatory effects in the brain, including COX-2 induction (Rivest 2001). Although we did not measure specific markers of systemic inflammation or toxicity, such effects are unlikely to provide an explanation for the observed mRNA responses in brain tissue. The DEE exposure did not lead to a notable pulmonary inflammation, measured in BALF as total leukocyte amount, percentage of neutrophils, or the content of the pro-inflammatory cytokines TNF-α and IL-6. None of these well-recognized markers for inflammation showed any differences between DEE and control groups. A significant increase was found only for alkaline phosphatase at 18 h post-exposure, indicative of a delayed selective toxicity to type II lung epithelial cells. However, this does not completely explain the rapid (4 h post-exposure) expression changes in the brain tissue.
At present we do not know whether a single, short exposure to DEE can trigger the effects in human brains that currently were observed in rats. Notably, however, a DEE exposure in human volunteers as brief as 1 h (0.3 mg/m3 particles) has been shown to induce the pulmonary influx of inflammatory cells as well as to elicit the production and systemic release of inflammatory mediators (Salvi et al. 1999, 2000). As for the genes found to be affected in the brain in our current study, COX-2 has been considered to play a role in the progression of neurodegenerative disorders (Heneka and O’Banion 2007; Teismann et al. 2003) and a deregulation of the HO enzyme system has been linked to Alzheimer’s disease and Multiple Sclerosis (Calabrese et al. 2006; Schipper et al. 2000). Cytochrome P450 enzymes like CYP1A1 are, apart from their specific involvement in xenobiotic-induced neurotoxicity, nowadays also considered to play a role in normal brain physiology (e.g. neurotransmitter regulation) as well as in the pathogenesis of Alzheimer’s and Parkinson’s diseases (Dutheil et al. 2008).
Associations between high (particulate) air pollution and Alzheimer-like pathology features in brain autopsies were shown by Calderón-Garcidueñas et al. (2004). Epidemiological evidence has revealed that increased PM levels may also be involved in Multiple Sclerosis relapses (Oikonen et al. 2003). Recently, in a cohort of elderly women mild cognitive function impairments were observed in association with traffic-related exposure, suggesting a role for combustion-derived nanoparticles (Ranft et al. 2009).
Obviously, it is too premature to conclude from our present study that inhalation of diesel engine exhaust particles may contribute to neuropathology. The data from our current study should be viewed alongside the fact that the well-known pulmonary and cardiovascular health impairments associated with exposure to PM are considered to result from chronic exposures characterized by multiple peak episodes. Therefore, it will be highly relevant to determine whether repeated exposures to combustion-derived nanoparticles can trigger a more persistent oxidative stress and inflammation in brain, and whether such effects can actually cause or accelerate neurodegenerative and neurobehavioral effects.
Our study indicates that short-term exposure to DEE may trigger rapid stress responses and CYP1A1 induction in specific brain regions, most notably in the pituitary. We hypothesize that even short-term exposures to DEE may exert effects in peripheral organs via the pituitary-endocrine axis, which could lead to a more widespread (though not necessarily region-specific) pattern of adverse peripheral reactions. Interestingly, mRNA expression changes in the brains of DEE-exposed rats were similar in extent to those observed in their lungs, a well-known target of DEE. While far lower concentrations of potentially harmful DEE components are expected to reach the brain upon inhalation, this organ is known to be highly sensitive to oxidative stress because of its high energy demands, and its relatively low content of antioxidants (Floyd 1999). Hence, this may explain that even small amounts of toxic substances might induce relevant effects in the brain.
Acknowledgments
We thank the members of the Dutch National Vaccine Institute and RIVM, especially John Boere, Daan Leseman and Paul Fokkens, for their experimental assistance. This study is supported by grants from the German Federal Ministry of Environment (BMU), and RIVM, Netherlands (S630111). Sponsors did not participate in study design; collection, analysis, and interpretation of data; and writing of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- BALF
Bronchoalveolar lavage fluid
- BBB
Blood–brain barrier
- COX-2
Cyclooxygenase-2
- CYP1A1
Cytochrome P450 1A1
- DEE
Diesel engine exhaust
- HO-1
Heme oxygenase-1 (HO-1)
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- PM
Particulate matter
- TNF-α
Tumor necrosis factor-alpha
- VOC
Volatile organic compound
References
- Abbas I, Saint-Georges F, Billet S, Verdin A, Mulliez P, Shirali P, Garcon G. Air pollution particulate matter (PM2.5)-induced gene expression of volatile organic compound and/or polycyclic aromatic hydrocarbon-metabolizing enzymes in an in vitro coculture lung model. Toxicol In Vitro. 2009;23:37–46. doi: 10.1016/j.tiv.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–168. doi: 10.1159/000154819. [DOI] [Google Scholar]
- Ahn EK, Yoon HK, Jee BK, Ko HJ, Lee KH, Kim HJ, Lim Y. COX-2 expression and inflammatory effects by diesel exhaust particles in vitro and in vivo. Toxicol Lett. 2008;176:178–187. doi: 10.1016/j.toxlet.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Bidmon HJ, Emde B, Oermann E, Kubitz R, Witte OW, Zilles K. Heme oxygenase-1 (HSP-32) and heme oxygenase-2 induction in neurons and glial cells of cerebral regions and its relation to iron accumulation after focal cortical photothrombosis. Exp Neurol. 2001;168:1–22. doi: 10.1006/exnr.2000.7456. [DOI] [PubMed] [Google Scholar]
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. Faseb J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- Bonvallot V, Baeza-Squiban A, Baulig A, Brulant S, Boland S, Muzeau F, Barouki R, Marano F. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am J Respir Cell Mol Biol. 2001;25:515–521. doi: 10.1165/ajrcmb.25.4.4515. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, Butterfield DA. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxid Redox Signal. 2006;8:1975–1986. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt AC, Altenburg M, Torres-Jardon R, Swenberg JA. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, Misra C, Mendez LB, Kleinman M. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Cassee FR, Boere AJ, Fokkens PH, Leseman DL, Sioutas C, Kooter IM, Dormans JA. Inhalation of concentrated particulate matter produces pulmonary inflammation and systemic biological effects in compromised rats. J Toxicol Environ Health A. 2005;68:773–796. doi: 10.1080/15287390590930171. [DOI] [PubMed] [Google Scholar]
- Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–313. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med. 2006;41:579–589. doi: 10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Colombrita C, Calabrese V, Stella AM, Mattei F, Alkon DL, Scapagnini G. Regional rat brain distribution of heme oxygenase-1 and manganese superoxide dismutase mRNA: relevance of redox homeostasis in the aging processes. Exp Biol Med (Maywood) 2003;228:517–524. doi: 10.1177/15353702-0322805-16. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, MacNee W, Stone V. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutheil F, Beaune P, Loriot MA. Xenobiotic metabolizing enzymes in the central nervous system: contribution of cytochrome P450 enzymes in normal and pathological human brain. Biochimie. 2008;90:426–436. doi: 10.1016/j.biochi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdorster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron VJ, Arts JH, Kuper CF, Slootweg PJ, Woutersen RA. Health risks associated with inhaled nasal toxicants. Crit Rev Toxicol. 2001;31:313–347. doi: 10.1080/20014091111712. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Gerde P, Muggenburg BA, Lundborg M, Dahl AR. The rapid alveolar absorption of diesel soot-adsorbed benzo[a]pyrene: bioavailability, metabolism and dosimetry of an inhaled particle-borne carcinogen. Carcinogenesis. 2001;22:741–749. doi: 10.1093/carcin/22.5.741. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. Faseb J. 2008;22:2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Huang P, Rannug A, Ahlbom E, Hakansson H, Ceccatelli S. Effect of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin on the expression of cytochrome P450 1A1, the aryl hydrocarbon receptor, and the aryl hydrocarbon receptor nuclear translocator in rat brain and pituitary. Toxicol Appl Pharmacol. 2000;169:159–167. doi: 10.1006/taap.2000.9064. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Sakurai M, Oda T, Tamura H, Yanagisawa R, Shimada A, Yoshikawa T. Pulmonary exposure to diesel exhaust particles enhances coagulatory disturbance with endothelial damage and systemic inflammation related to lung inflammation. Exp Biol Med (Maywood) 2006;231:1626–1632. doi: 10.1177/153537020623101007. [DOI] [PubMed] [Google Scholar]
- Kleinman MT, Araujo JA, Nel A, Sioutas C, Campbell A, Cong PQ, Li H, Bondy SC. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol Lett. 2008;178:127–130. doi: 10.1016/j.toxlet.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le WD, Xie WJ, Appel SH. Protective role of heme oxygenase-1 in oxidative stress-induced neuronal injury. J Neurosci Res. 1999;56:652–658. doi: 10.1002/(SICI)1097-4547(19990615)56:6<652::AID-JNR11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Li N, Venkatesan MI, Miguel A, Kaplan R, Gujuluva C, Alam J, Nel A. Induction of heme oxygenase-1 expression in macrophages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element. J Immunol. 2000;165:3393–3401. doi: 10.4049/jimmunol.165.6.3393. [DOI] [PubMed] [Google Scholar]
- Li N, Wang M, Oberley TD, Sempf JM, Nel AE. Comparison of the pro-oxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. J Immunol. 2002;169:4531–4541. doi: 10.4049/jimmunol.169.8.4531. [DOI] [PubMed] [Google Scholar]
- Li YJ, Kawada T, Matsumoto A, Azuma A, Kudoh S, Takizawa H, Sugawara I. Airway inflammatory responses to oxidative stress induced by low-dose diesel exhaust particle exposure differ between mouse strains. Exp Lung Res. 2007;33:227–244. doi: 10.1080/01902140701481062. [DOI] [PubMed] [Google Scholar]
- Lim HB, Ichinose T, Miyabara Y, Takano H, Kumagai Y, Shimojyo N, Devalia JL, Sagai M. Involvement of superoxide and nitric oxide on airway inflammation and hyperresponsiveness induced by diesel exhaust particles in mice. Free Radic Biol Med. 1998;25:635–644. doi: 10.1016/S0891-5849(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, Hester S, Lowry GV, Veronesi B. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007;115:1631–1637. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Nakatsuka I, Hayashi Y, Higuchi H, Shimada M, Miyawaki T. Heme oxygenase-1 induction in the brain during lipopolysaccharide-induced acute inflammation. Neuropsychiatr Dis Treat. 2008;4:663–667. doi: 10.2147/ndt.s3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S. The pituitary. Cambridge: Blackwell; 2002. [Google Scholar]
- Morse DC, Stein AP, Thomas PE, Lowndes HE. Distribution and induction of cytochrome P450 1A1 and 1A2 in rat brain. Toxicol Appl Pharmacol. 1998;152:232–239. doi: 10.1006/taap.1998.8477. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonen M, Laaksonen M, Laippala P, Oksaranta O, Lilius EM, Lindgren S, Rantio-Lehtimaki A, Anttinen A, Koski K, Eralinna JP. Ambient air quality and occurrence of multiple sclerosis relapse. Neuroepidemiology. 2003;22:95–99. doi: 10.1159/000067108. [DOI] [PubMed] [Google Scholar]
- Peters A, Veronesi B, Calderon-Garciduenas L, Gehr P, Chen LC, Geiser M, Reed W, Rothen-Rutishauser B, Schurch S, Schulz H. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Herkenham M. Cyclooxygenase 2 mRNA expression in rat brain after peripheral injection of lipopolysaccharide. Brain Res. 1998;802:189–197. doi: 10.1016/S0006-8993(98)00402-8. [DOI] [PubMed] [Google Scholar]
- Ranft U, Schikowski T, Sugiri D, Krutmann J, Kramer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Risom L, Dybdahl M, Bornholdt J, Vogel U, Wallin H, Moller P, Loft S. Oxidative DNA damage and defence gene expression in the mouse lung after short-term exposure to diesel exhaust particles by inhalation. Carcinogenesis. 2003;24:1847–1852. doi: 10.1093/carcin/bgg144. [DOI] [PubMed] [Google Scholar]
- Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2001;26:761–788. doi: 10.1016/S0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Salvi SS, Nordenhall C, Blomberg A, Rudell B, Pourazar J, Kelly FJ, Wilson S, Sandstrom T, Holgate ST, Frew AJ. Acute exposure to diesel exhaust increases IL-8 and GRO-alpha production in healthy human airways. Am J Respir Crit Care Med. 2000;161:550–557. doi: 10.1164/ajrccm.161.2.9905052. [DOI] [PubMed] [Google Scholar]
- Sato H, Sone H, Sagai M, Suzuki KT, Aoki Y. Increase in mutation frequency in lung of Big Blue rat by exposure to diesel exhaust. Carcinogenesis. 2000;21:653–661. doi: 10.1093/carcin/21.4.653. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Chertkow H, Mehindate K, Frankel D, Melmed C, Bergman H. Evaluation of heme oxygenase-1 as a systemic biological marker of sporadic AD. Neurology. 2000;54:1297–1304. doi: 10.1212/wnl.54.6.1297. [DOI] [PubMed] [Google Scholar]
- Shimada T, Sugie A, Shindo M, Nakajima T, Azuma E, Hashimoto M, Inoue K. Tissue-specific induction of cytochromes P450 1A1 and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in engineered C57BL/6J mice of arylhydrocarbon receptor gene. Toxicol Appl Pharmacol. 2003;187:1–10. doi: 10.1016/S0041-008X(02)00035-2. [DOI] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Stoeger T, Takenaka S, Frankenberger B, Ritter B, Karg E, Maier K, Schulz H, Schmid O. Deducing in vivo toxicity of combustion-derived nanoparticles from a cell-free oxidative potency assay and metabolic activation of organic compounds. Environ Health Perspect. 2009;117:54–60. doi: 10.1289/ehp.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano H, Yanagisawa R, Ichinose T, Sadakane K, Inoue K, Yoshida S, Takeda K, Yoshino S, Yoshikawa T, Morita M. Lung expression of cytochrome P450 1A1 as a possible biomarker of exposure to diesel exhaust particles. Arch Toxicol. 2002;76:146–151. doi: 10.1007/s00204-002-0323-0. [DOI] [PubMed] [Google Scholar]
- Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, Przedborski S. COX-2 and neurodegeneration in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Veronesi B, Makwana O, Pooler M, Chen LC. Effects of subchronic exposures to concentrated ambient particles. VII. Degeneration of dopaminergic neurons in Apo E−/− mice. Inhal Toxicol. 2005;17:235–241. doi: 10.1080/08958370590912888. [DOI] [PubMed] [Google Scholar]
- Yokota S, Seki T, Furuya M, Ohara N. Acute functional enhancement of circulatory neutrophils after intratracheal instillation with diesel exhaust particles in rats. Inhal Toxicol. 2005;17:671–679. doi: 10.1080/08958370500189628. [DOI] [PubMed] [Google Scholar]
- Zhao H, Barger MW, Ma JK, Castranova V, Ma JY. Cooperation of the inducible nitric oxide synthase and cytochrome P450 1A1 in mediating lung inflammation and mutagenicity induced by diesel exhaust particles. Environ Health Perspect. 2006;114:1253–1258. doi: 10.1289/ehp.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]


