Abstract
We review the literature on interoception as it relates to depression and anxiety, with a focus on belief, and alliesthesia. The connection between increased but noisy afferent interoceptive input, self-referential and belief-based states, and top-down modulation of poorly predictive signals is integrated into a neuroanatomical and processing model for depression and anxiety. The advantage of this conceptualization is the ability to specifically examine the interface between basic interoception, self-referential belief-based states, and enhanced top-down modulation to attenuate poor predictability. We conclude that depression and anxiety are not simply interoceptive disorders but are altered interoceptive states as a consequence of noisily amplified self-referential interoceptive predictive belief states.
Keywords: Anxiety, Depression, Interoception, Insula, Belief, Alliesthesia
Depression and anxiety
Depression is a common mental health condition that affects many aspects of daily life. Recent epidemiological data estimate the lifetime prevalence of major depressive disorder (MDD) at about 16% and the 12-month prevalence at 6% (Kessler et al. 2003). MDD is about twice as common in females relative to males (Kessler et al. 1993), manifests as an episodic but often recurrent illness with a mean duration of 16 weeks, and is comorbid with many other mental health conditions especially anxiety and alcohol use disorders. The current classification of depressive disorders (American Psychiatric Association 1994) is based on operational criteria that have been arrived at via consensus decisions by subject matter experts (Kendler and Gardner Jr. 1998). However, there is little evidence that these criteria delineate distinct biological entities or subsume similar biological conditions in one disorder (Sullivan et al. 1998). Nevertheless, there is substantial evidence from twin studies (Kendler et al. 1993), linkage studies (Hill et al. 1988), and association studies using single nucleotide polymorphisms (Liu et al. 2006) for strong genetic contribution to the susceptibility for depression. Other investigators have suggested that certain temperamental such as harm avoidance (Ongur et al. 2005) or personality traits such as neuroticism are associated with increased propensity to develop depressive disorders (Bienvenu et al. 2001). Neuropsychological investigations have revealed that an increased bias for remembering negative events (Watkins et al. 1996) and exaggerated response to negative feedback (Elliott et al. 1997) may constitute vulnerability factors for depression.
Anxiety disorders are the most common mental health problem (Kessler et al. 1994). These disorders comprise a heterogeneous group of conditions such as generalized anxiety disorder characterized by chronic worrying, panic disorder with a sudden onset of autonomic symptoms associated with a flooding of thoughts and anxious experiences, post-traumatic stress disorder characterized by intrusive recollections of exposure to extreme and potentially life-threatening events, obsessive compulsive disorder characterized by ruminative thinking and stereotyped actions aimed at relieving anxious thoughts, social phobia with fear of situations involving the potential for negative evaluation, and specific phobias with object-related anxiety symptoms. A common feature of all anxiety disorders is a profound avoidance of the event, object, or context, which has become associated with the experience or exacerbation of the anxiety symptoms.
Twin studies support the idea that anxiety disorders share a common genetic vulnerability that has been termed anxious-misery and fear (Kendler et al. 2003). For example, generalized anxiety disorder characterized by apprehensive expectation or chronic worry focused on multiple life situations (Barlow et al. 1986) is a chronic condition with a 12-month prevalence of approximately 3% that is commonly comorbid with MDD and associated with significant functional impairment (Stein 2004). Moreover, twin studies indicate that genetic and environmental risk factors for the anxiety disorders are similar between men and women and that the propensity for the development of anxiety falls into panic-generalized-agoraphobic anxiety versus specific phobias (Hettema et al. 2005). These studies, however, have also emphasized the individual environment experience as a contributing factor to the development of the disorder (Hettema et al. 2001). Neuropsychological studies have proposed that an increased emotional awareness (Novick-Kline et al. 2005), intolerance of uncertainty (Dugas et al. 1998), and a cognitive style termed looming vulnerability (Williams et al. 2005), i.e., mental representations of dynamically intensifying danger, appear to be among the cognitive predispositions to generalized anxiety disorder.
Interoception: the basis for “how I feel”
Interoception comprises sensing the physiological condition of the body (Craig 2002), as well as the representation of the internal state (Craig 2009) within the context of ongoing activities, and is closely associated with motivated action to homeostatically regulate the internal state (Craig 2007). Interoception includes a range of sensations such as pain (LaMotte et al. 1982), temperature (Craig and Bushnell 1994), itch (Schmelz et al. 1997), tickle (Lahuerta et al. 1990), sensual touch (Vallbo et al. 1995; Olausson et al. 2002), muscle tension (Light and Perl 2003), air hunger (Banzett et al. 2000), stomach discomfort related to low pH (Feinle 1998), and intestinal tension (Robinson et al. 2005), which together provide an integrated sense of the body’s physiological condition (Craig 2002). There are two pathways from the periphery to the insular cortex. First, these sensations travel via small diameter primary afferent fibers, which are thought to comprise a cohesive system for homeostatic afferent activity that parallels the efferent sympathetic nervous system (Craig 2007), and terminate on projection neurons in the most superficial layer of the spinal dorsal horn. The modality-selective lamina I spinothalamic neurons project to a specific thalamo-cortical relay nucleus, which in turn projects to a discrete portion of dorsal posterior insular cortex. Second, afferents traveling with the vagal and glossopharyngeal nerve synapse at the nucleus of the solitary tract (van der Kooy et al. 1984), which projects indirectly via ventro-posterior thalamic nucleus and bi-directionally directly to the posterior insula (Shipley 1982). Moreover, the insular cortex projects directly to the specific “gastric” part of the dorsal vagal complex (Bagaev and Aleksandrov 2006) as well as other brainstem autonomic nuclei (Ruggiero et al. 1987). Studies in the rat have shown that the insular cortex is an important part of a highly interconnected central autonomic system, which may be organized in a viscerotopic manner (Saper 1982).
The posterior insula provides topographic and modality-specific interoceptive signals to the anterior insular cortex for integration (Craig 2003). These topographically organized and modality-specific pathways that carry interoceptive signals are integrated in the anterior insula cortex (Craig 2003), which is integrally connected with subcortical (Chikama et al. 1997), limbic (Reynolds and Zahm 2005), and executive control brain systems (for review, see Augustine 1985, 1996). An organism’s interoceptive state and hedonic state are integrated via reciprocal connections of the anterior insular cortex to corticolimbic and striatal reward circuit components such as (a) anterior cingulate (Augustine 1996), which is important for error processing (Critchley et al. 2005; Carter et al. 1998) and evaluation of action selection (Rushworth and Behrens 2008; Goldstein et al. 2007), to (b) amygdala (Augustine 1985; Jasmin et al. 2004; Reynolds and Zahm 2005; Jasmin et al. 2003), which is critical for processing stimulus salience (Paton et al. 2006), (c) nucleus accumbens (Reynolds and Zahm 2005), which processes the incentive motivational aspects of rewarding stimuli (Robinson and Berridge 2008), and (d) orbitofrontal cortex (Ongur and Price 2000b), which has been implicated in context-dependent evaluation of environmental stimuli (O’Doherty et al. 2001; Rolls 2004a, b; Bechara et al. 2000; Schoenbaum et al. 2003, 2006; Kringelbach 2005; Rolls and Grabenhorst 2008).
Thus, the anterior insula has access to a multi-dimensional representation and integration of the current and possibly anticipated (Paulus and Stein 2006) feeling state and is important for the capacity to be aware (Craig 2002; Critchley et al. 2004) of oneself, others, and the environment (Craig 2009). The columnar organization of the insular cortex shows a highly organized anterior-inferior to posterior-superior gradient (for example, see Mesulam and Mufson 1982), similar to other parts of the brain where cortical representations are based on modulatory or selective feedback circuits (Shipp 2005). Therefore, it is not surprising that anterior insula is involved in a wide range of processes, including pain (Tracey et al. 2000), interoceptive (Critchley et al. 2004), emotion-related (Phan et al. 2002), cognitive (Huettel et al. 2004), and social functions (Eisenberger et al. 2003). In summary, the anterior insula is important for subjective feeling states (Craig 2002; Critchley et al. 2004), and takes part in modulatory control of decision-making by interacting with other limbic, and cortical areas (Garavan et al. 1999).
Self: depression and anxiety
An important aspect of both anxiety and depression is the altered experience of the individual with respect to self, others and the future. Altered self-related processing, e.g., reduced tendency to self-favoring (de Jong 2002) and/or exaggerated negative self-image (Hirsch et al. 2003), has been implicated in the development of anxiety in general (Bruch et al. 1995) and social anxiety in particular (Clark and McManus 2002). Moreover, the anticipation of future harm to the self has been proposed as a key process in anxiety (Reiss 1997). Similarly, low self-esteem has been found using rigorous statistical methodology as a critical pathway for the development of depression (Kendler et al. 2006). This negative evaluative bias for self-relevant information persists even when overt symptoms of depression are absent (Gemar et al. 2001). Beck (1967) proposed that depression is a manifestation of an unrealistic negative view about the relationship between the self and the world.
An important aspect of self is corporeal awareness, which has been defined as the perception, knowledge and evaluation of one’s own body as well as of other bodies (Berlucchi and Aglioti 2009). The meaning and implications of the concept of a “self” has been the focus of study for many philosophers and psychologists (James 1961). More recently, other disciplines such as psychiatry, neurology and neuroscience have begun to examine the psychological and biological basis for the existence of the “self” (Gusnard 2005). Reflecting upon oneself relates to an individual’s self-concept and plays a crucial role in the maintenance of emotional and physical equilibrium. Being aware of our internal state modulates approach and distancing behaviors which, in turn, help us maintain and regain homeostasis (i.e., regulation of internal body state).
The neuroanatomy of self-relevant processing has recently been linked to the anterior cingulate cortex and surrounding midline cortical structures. Evaluative judgment produced significant activation in the anterior frontomedian cortex (Zysset et al. 2002). Others have reported that self-reassurance, i.e., positive evaluation of one self, was associated with left temporal pole and insula activation (Longe et al. 2009). Results revealed that activity in medial prefrontal cortex (mPFC) predicted both subsequent memory performance and judgments of self-relevance (Macrae et al. 2004). The mPFC activates during self- and other-evaluation versus the baseline semantic positivity-evaluation condition (Schmitz et al. 2004). Processing of information relevant to self activated superior medial parietal areas in comparison to other, whereas other led to greater activation in inferior medial parietal and left lateral frontal areas than self (Seger et al. 2004). Moreover, the correct recognition of self-encoded personality traits engaged dorso-medial prefrontal cortex and lateral prefrontal regions, premotor cortex, parietal and occipital cortex, caudate and cerebellum (Fossati et al. 2004). Finally, the mPFC (Amodio and Frith 2006) is often found active during resting conditions when individuals presumably engage in internally directed attention, processing self-relevant thoughts (Kelley et al. 2002) and beliefs (Wicker et al. 2003). This area is also involved in cognitive control, i.e., coordinating thought and action in accordance with internal goals (Miller and Cohen 2001), and is part of the “default mode” network involved in self-regulation and monitoring of the internal milieu (Gusnard et al. 2001).
Considering the impact that our own self-concept has on the way we behave and interact with our surroundings, it is not surprising to find the mPFC involved not only in the judgment of one’s emotional state (Ochsner et al. 2004) but also in self-reflection (Johnson et al. 2002). According to Damasio, the “core self” is the summation of extero- and interoceptive stimuli that form the experience of the self as one integrated entity (Damasio 1999, 2003). Interoception or self-perception of bodily signals is an important component for the preservation of homeostasis via adaptive attention allocation, contextual evaluation, and action planning. The insula, considered the interoceptive center of the brain, is critical for evaluating the potential impact of stimuli on the body (Paulus and Stein 2006). Along with the mPFC, the insula plays a crucial role in the detection of emotionally salient stimuli (Morris et al. 1998; Phillips et al. 1998), as well as in the generation and regulation of affective responses (Phillips et al. 2003). Anatomically, the insular cortex shares afferent and efferent connections with the mPFC cortex (Ongur and Price 2000a) as well as the anterior cingulate gyrus (Reynolds and Zahm 2005).
Thus, in understanding the dysfunctions of interoception in anxiety and depression, one has to consider how interoceptive afferents and processes within the insular cortex are integrated with the neural representation of the self. In particular, because the self is a manifestation of past and present cognitive, affective and body state experiences, there must be a critical link between interoceptive processes and so-called dysfunctional schemas, i.e., organized elements of past reactions and experience that form a relatively cohesive and persistent representation, which guides perception and appraisals (Segal 1988). Belief-related processes provide this important link between the self and interoception.
Belief: a key dysfunctional process in depression and anxiety
Belief can be defined as a propositional mental construct that affirms or denies the truth of a state of affairs and is closely linked to basic judgment processes (Harris et al. 2007). The maintenance of a large and stable set of beliefs is essential for intelligent behavior, since this forms the basis for any actions which one may take to achieve one’s goals (Elliott et al. 1995). More importantly, beliefs are frequently used to build mental models of the state of the world and are therefore important constructs to guide decision-making. The psychological approach to characterizing beliefs is complex and has focused recently on developing testable process theories, which can be disambiguated using modern neuroscience. To understand recent neuroscience findings of belief-based decision-making, it is important to briefly review a few relevant constructs.
The theory-of-mind and mentalizing has been an important construct for the conceptualization of beliefs. Theory-of-mind refers to the everyday ability to attribute independent mental states of self and others to predict and explain behavior (Happe 2003). The mechanisms underlying theory-of-mind have been extensively investigated via reasoning tasks that require participants to predict the action of agents based on information about beliefs and desires. Others have investigated the ability to explain the action of others and found systematic reasoning errors (Wertz and German 2007). Typically, we infer other people’s beliefs from what they tell us. However, behavioral experiments have the advantage that the experimenter does not have to rely on the degree to which an individual is reporting truthfully. Behavioral experiments show that false beliefs engage greater cognitive resources resulting in longer response times, which may be due to the fact that subsequent judgments and decision-making need to take into account false beliefs as a conflict with the individual’s perception of reality (Apperly et al. 2008).
Several neural substrates have been found to underlie belief-related processes. First, the temporo-parietal junction (TPJ) has been implicated not only in processing the attribution of beliefs to other people but also in the redirection of attention to task-relevant stimuli. Recent studies support the notion that the overlap between theory-of-mind and attentional reorienting suggests the need for new accounts of right TPJ function that integrate across these disparate task comparisons (Mitchell 2008). Others have found that the right TPJ differentially activated as a function of belief and outcome and was highest for cases of possible negative outcome based on the belief that action of a protagonist would cause harm to others, even though the harm did not occur (Young et al. 2007). In comparison to neuroimaging studies, a report of brain-damaged individuals revealed that, in addition to frontal cortex, left TPJ is necessary for reasoning about the beliefs of others (Samson et al. 2004). A study examining the contribution of mPFC and TPJ in false versus true belief reasoning showed that the dorsal part of the anterior cingulate cortex, the right lateral rostral prefrontal cortex and the right TPJ associated with false belief. The authors suggest that cognitive control areas are involved in action monitoring and stimulus-independent cognitive processing whereas the activation of the TPJ might be related to the computation of mental representations that create perspective differences, such as a person’s false belief that contrasts with reality and therefore might be centrally involved in the decoupling mechanism (Sommer et al. 2007). Taken together, the TPJ appears to be crucial for the representation of a mental state associated with belief formation, possibly focused on disambiguating true from false beliefs.
The mPFC as pointed out above is often found to be active during “resting” conditions when individuals presumably engage in internally directed attention processing self-relevant (Kelley et al. 2002) thoughts and beliefs (Wicker et al. 2003). Some investigators have found that whereas mPFC is recruited for processing belief valence, TPJ and precuneus are recruited for processing beliefs in moral judgment mediating both encoding beliefs and integrating beliefs with outcome for moral judgment (Young and Saxe 2008). These findings are consistent with those of others showing greater activation of anteromedial prefrontal cortex and rostral anterior cingulate while participants implicitly made associations consistent with gender and racial biases. In contrast, associations incongruent with stereotypes recruited dorsolateral prefrontal cortex (Knutson et al. 2007).
Several studies have shown that direct appraisals of the self as compared to others more strongly recruited mPFC and right rostrolateral prefrontal cortex. Moreover, direct appraisals as compared to reflected appraisals recruited regions associated with a first-person perspective, e.g., posterior cingulate, whereas reflected as compared to direct appraisals recruited regions associated with emotion and memory, e.g., insula, orbitofrontal, and temporal cortex (Ochsner et al. 2005). Using event-related potential studies investigating the neural substrates of false belief reasoning revealed a specific late negative component which could be located in the middle cingulate cortex, which may be related to conflict or error detection (Wang et al. 2008). Another approach is to examine individuals suffering from dysfunctional belief systems. For example, individuals with delusions or fixed false beliefs provide an important clinical sample to examine the rigidity to which false beliefs are held. In comparison, subjects with delusions show significantly less rostral-ventral anterior cingulate activation but increased posterior cingulate activation during the determining of self-relevance, which may contribute to persecutory belief formation and maintenance (Blackwood et al. 2004). Taken together, the mPFC appears to be important for processing the degree to which beliefs are self-relevant, possibly with emphasis on the correct or incorrect, or acceptable versus unacceptable valence of the belief.
The strength to which one holds to a belief is an important regulator of human behavior and emotion. A recent neuroimaging study shows that states of belief, disbelief, and uncertainty differentially activated distinct regions of the prefrontal and parietal cortices, as well as the basal ganglia. These investigators propose that the final acceptance of a statement as “true” or its rejection as “false” relies on more primitive, hedonic processing in the mPFC and the anterior insula (Harris et al. 2007). Contrasting belief-based versus belief-neutral reasoning, some researchers have reported left temporal lobe versus parietal lobe activation, respectively, which is thought to be modulated by lateral prefrontal cortex in case of overcoming belief bias and ventral medial prefrontal cortex when succumbing to belief bias (Goel and Dolan 2003). Examining belief-based decision-making in a navigation task under uncertain conditions showed distinct regions of prefrontal cortex activation which was consistent with an underlying Bayesian model of decision-making that permits efficient goal-oriented navigation (Yoshida and Ishii 2006). Thus, dorsolateral and ventromedial prefrontal cortex may have important regulatory functions in moderating belief intensity and the degree to which beliefs influence decision-making. In comparison, when the observers judged the actions to reflect a false belief, there was activation in the superior temporal sulcus, orbitofrontal, paracingulate cortex and cerebellum (Grezes et al. 2004). Others have related belief-based decision-making to altruistic behavior and found that medial orbitofrontal-subgenual and lateral orbitofrontal areas mediate decisions to donate or to oppose societal causes whereas more anterior sectors of the prefrontal cortex are distinctively recruited when altruistic choices prevail over selfish material interests (Moll et al. 2006). Therefore, additional brain regions may modulate belief-based processing by contributing resources to (1) over-ride beliefs or (2) enforce beliefs, (3) engage in reward-related processing associated with beliefs (i.e., how good it feels to be right), or (4) determine how one’s own belief-based decision affects others.
Thus, altered interoceptive processing and anxiety or depression is closely linked to the view of the self, which—in turn—critically depends on belief-based processes. Before integrating these findings into a interoceptive mode of these disorders, however, one needs to consider that evaluative processes are not static and absolute but are dynamic and relative to the current internal state of the individual. In other words, body state-related information that affects the self and is being evaluated relative to one’s belief about oneself is subject to the momentary internal state of the individual. Alliesthesia is an important construct that links these processes together.
Alliesthesia: a process connecting the inside world to the outside world in depression and anxiety
Alliesthesia is a physiological construct introduced in 1973 to connect the stimulations that come from the “milieu exterieur” and affect the “milieu interieur” (Cabanac 1971). Alliesthesia critically links the subjective experience of external stimuli to the internal state of the individual. Cabanac (1971) proposed that a given external stimulus can be perceived as either pleasant or unpleasant, depending upon interoceptive signals. In particular, negative alliesthesia refers to the notion that repeated administration of a stimulus will affect the internal milieu in such a way that the valence of the stimulus is attenuated, e.g., the second or third stimulation by a painful stimulus is not experienced as aversely. In contrast, positive alliesthesia is observed if the repeated administration of a stimulus changes the internal milieu in such a way as to increase its valence. The constructs of negative and positive alliesthesia add the relative body state of the individual to the notion of habituation or sensitization. The relative body state has a profound effect on the experienced intensity and valence of the stimulus. Evidence for positive alliesthesia in anxiety comes from studies with socially anxious individuals. For example, repeated exposure to a social stressor resulted in a significantly greater increase of the skin conductance in a high anxious group relative to a comparison group (Eckman and Shean 1997). Moreover, individuals with higher trait anxiety showed less habituation during a repeated affective go/no-go paradigm (Hare et al. 2008). And some have argued that differential habituation may contribute to differences in self-reported anxiety (Houtveen et al. 2001).
Based on the alliesthesia construct, investigators have proposed a dynamic, homeostatic hypothesis of mood regulation (Cabanac et al. 2002; Ramirez and Cabanac 2003). This hypothesis proposes that (1) we experience the intensity of a positively valenced stimulus based on the evaluation of the stimulus relative to our internal state (Cabanac 1992), and (2) we engage regulatory mechanisms to maximize the hedonic aspect of the internal state. The alliesthesia construct can be applied to both rewarding and aversive stimuli, but it also provides an explicit connection between the internal state (i.e., interoception) and rewarding or aversive stimuli in the environment. As pointed out above, the construct of positive and negative alliesthesia is similar to the notion of sensitization and habituation. However, the latter constructs do not necessarily take into account the internal state of the individual and focus instead on an increase or decrease of frequency or intensity of an observable behavior. Thus, alliesthesia provides a direct link between the outer world and the inner world. Moreover, the reference to an internal state identifies additional circuitry, e.g., insular cortex and its connectivity to frontal cortical and extended amygdala circuits, which can be empirically tested for its role in processing appetitive or aversive stimuli.
Evidence for altered insula-related processing in depression and anxiety
Both depression and anxiety are multifactorial disorders that involve different brain processes and neural substrates. Here, we selectively review neuroimaging studies that provide evidence for the contribution of the insular cortex to the regulation and dysfunction of mood and anxiety. The results from these studies will be used to conceptualize the basic dysfunction in depression and anxiety as that of alliesthesia of internal body signals due to altered belief-based self-relevance. In other words, a change in the internal state of the individual, which may be due to an increased bias toward negative self-view (depression) or increased attentional biases toward threat (anxiety), sets up beliefs that are used to interpret afferent internal body signals. Moreover, external cues or internal thought processes generate an anticipation of aversive body states that sets up a body prediction error, i.e., the difference between the current and anticipated body state. This body prediction error acts as a motivating signal for individuals to withdraw (depression) or avoid (anxiety). There are several neuroimaging studies that provide evidence for an altered activation pattern of neural substrates that are important for interoceptive processing. For example, whereas increased self-criticism was associated with activity in lateral prefrontal cortex regions as well as anterior cingulate, self-reassurance was associated with—among other areas—increased insula activation. Moreover, lateral prefrontal cortex activity was positively correlated with high levels of self-criticism (Longe et al. 2009). Worrying, a set of self-relevant stereotypic cognitions, relative to neutral statements has been associated with increased brain activation in prefrontal cortex, striatum, and insula (Hoehn-Saric et al. 2004). Self-relevant aversive affective processing such as guilt was also found to involve left anterior insular cortex (Shin et al. 2000). Finally, sad self-relevant autobiographical memories were found to activate the ventral insula (Liotti et al. 2000). These examples provide clear evidence for the involvement of insular cortex during aversively valenced self-relevant processing.
The periacquaductal grey, amygdala, anterior cingulate cortex, and anterior insula are key neural substrates in pain processing (Ochsner et al. 2008; Wiech and Tracey 2009; Chua et al. 1999). However, aside from the direct experiencing of an aversive stimulus, the anticipation of aversive events and the interpretation of these events with respect to the construct of one self are important processes to consider in depression and anxiety. A number of studies have shown that the anticipation of aversive events involves the insular cortex. Several studies found that brain areas activated by anticipation of aversive pictures include dorsal amygdala, anterior insula, dorsal anterior cingulate cortex, right dorsolateral prefrontal cortex, and right posterior orbitofrontal cortex (Nitschke et al. 2006; Simmons et al. 2004). This network of activation in relation to the anticipation of aversive stimuli extends to other modalities such as monetary losses (Samanez-Larkin et al. 2008), conditioned stimulus predicting an aversive stimulus (Marschner et al. 2008), and the expectation of pain (Ploghaus et al. 1999). Moreover, insula activation is an important processing area for the affective elements to an individual’s pain experience in subjects with depression (Giesecke et al. 2005). Some have argued that the insula and anterior cingulate are relatively more sensitive to the expectation of unpleasant stimuli relative to expecting pleasant or neutral stimuli (Herwig et al. 2007). Finally, uncertainty or ambiguity is an important aspect of anticipatory processing. Thus, it is not surprising that the degree to which an individual is sensitive to uncertainty correlates positively with activation in bilateral insula during affective ambiguity (Simmons et al. 2008). Others have reported that unpredictable stimulus presentations were correlated to brain activity in the anterior insula (Carlsson et al. 2006). Taken together, these data suggest that the insula plays an important role in processing the anticipation and subjective experience of aversive stimuli across a number of different modalities.
Another approach to identify brain processing differences is to examine the neural substrate activation differences in individuals that differ on their level of depression or anxiety. There is converging evidence from these kinds of studies that the insula plays a central role in anxiety and depression (Phan et al. 2002). Specifically, responding to sad mood distractors was found to be related to activation in anterior cingulate, ventromedial and orbital prefrontal cortex and insula (Wang et al. 2006). Increased sadness induced greater blood flow in subgenual cingulate and anterior insula (Mayberg et al. 1999). Transient sadness was associated with significant loci of activation in the anterior temporal pole and the midbrain, bilaterally, as well as in the left amygdala, left insula, and right ventrolateral prefrontal cortex, which some have related to the degree of sadness (Levesque et al. 2003), the degree of insomnia (Perico et al. 2005), or to the degree of stress-related ACTH regulation (Ottowitz et al. 2004). Dysthymic individuals relative to comparison subjects show greater brain activation during negatively valenced images in amygdala, anterior cingulate and insula (Ravindran et al. 2009). Patients with MDD show an enhanced responsivity in insula, anterior cingulate, and amygdala to non-painful stimuli and an increased activation in the right amygdala during anticipation of pain which is associated with more self-perceived helplessness (Strigo et al. 2008). The insula was also more sensitive in MDD patients compared to controls when presented with emotional images (Mitterschiffthaler et al. 2003). Studies examining the role of the insula in anxiety find a similarly increased sensitivity. For example, symptom provocation in anxiety activates, among other structures, bilateral insular cortex (Rauch et al. 1997). Anxiety-prone relative to comparison subjects show greater bilateral amygdala and insula activation to emotional faces (Stein et al. 2007). These individuals also exhibit greater bilateral insula activation during the anticipation of emotionally valenced pictures (Simmons et al. 2006). The enhanced insular response is also found in patients with specific phobia (Wright et al. 2003). Moreover, panic disorder patients show relatively less inhibitory (GABAergic) receptors in the insula, which is correlated with severity of illness (Cameron et al. 2007). Finally, several studies have highlighted the role of increased insula activation during aversive, anxiety-related processes such as combat-relevant stimuli (Vermetten et al. 2007) or heat temperature (Geuze et al. 2007), in patients with post-traumatic stress disorder. Taken together, these studies show clear evidence for altered insular cortex activation during a variety of different processes that share in common three features. First, they involve a stimulus that is evaluated with respect to one’s self; second, the stimulus is typically viewed as aversive; and third, these processes occur even if the actual stimulus is not yet being perceived but is being anticipated.
Depression and anxiety: alliesthesia of internal body signals and belief-based self-relevance
The basic model that is proposed here (Fig. 1) is an extension and refinement of the insular model of anxiety that we proposed earlier (Paulus and Stein 2006). We considered that anxiety is a result of an increased anticipatory response to the potential of aversive consequences, which manifests itself in enhanced anterior insular cortex processing. The main aim of this model was to incorporate interoceptive processing aspects into the conceptualization of anxiety. In the current formulation, we extend this model in three ways. First, we propose that beliefs, i.e., propositional statements that refer to the state of the individual, which are processed in the mPFC and TPJ, contribute significantly to the evaluation of the anticipatory interoceptive signals. Second, we assume that positive alliesthesia, i.e., an exaggeration of the valence (positive or negative) relative to the individual’s internal state, plays a role in enhancing the aversive aspects of predictive body signals, i.e., initially sub-threshold afferent interoceptive signals are amplified and associated with the prediction of potential aversive or negative outcomes. Third, we propose that individuals at risk for anxiety and depression exhibit a reduced signal to noise ratio of interoceptive afferents. Specifically, when these individuals receive body signals, they cannot easily differentiate between those, which are associated with potential aversive (or pleasant) consequences versus those, which are part of constantly ongoing and fluctuating interceptive afferents. As a consequence of positive alliesthesia, these individuals imbue afferent interoceptive stimuli with motivational significance, i.e., an increased tendency to plan and act upon the reception of this input. Specifically, an internal body signal, e.g., a heart beat or inspiratory breathing sensation, is associated with negative valence and linked to belief-based processes, e.g., “there is something wrong with my heart” or “I am not getting enough air”, which results in an increased “fight/flight” response and potential withdrawal or avoidance behaviors. As a consequence of this noisy amplification, top-down modulatory brain areas such as the anterior cingulate, dorsolateral prefrontal cortex, and orbitofrontal cortex are engaged constantly to differentially amplify or attenuate signals that are predictive or not predictive of future states, respectively. This relative “overactivity” of cognitive control-related brain areas is subjectively experienced as increased production of thoughts and associated beliefs, which provide prediction enhancing propositions. Practically, these cognitive processes result in “worrying”, which is aimed at providing increased prediction accuracy.
Fig. 1.
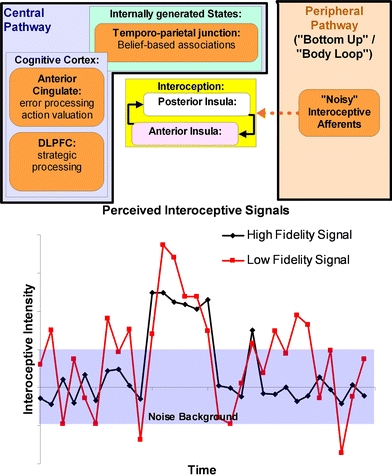
Proposed alterations in brain circuitry (top) and the resulting process abnormalities in anxiety and depression. Briefly, “noisy” afferent interoceptive information results combined belief-based associations lead to an attempt of the cognitive control apparatus to differentiate predictive from non-predictive signals. The resulting experience (bottom) consists of amplified interoceptive afferents that are associated with carry poor differentiation of stimuli that carry predictive outcomes and create constant uncertainty for the future. More details are described in the text
The proposed model integrates a network of processes and neural processing substrates; however, it is less definitive about the causal chain of events. As proposed recently (Ochsner et al. 2009), distinct but overlapping neural substrates are involved in both top-down and bottom-up affective processes. Feeling states can be altered by both exaggerated valence of interoceptive afferents as well as exaggerated belief systems and it is not clear based on the current literature, which alteration is primary. It may even be possible to separate individuals into those who experience a predominant bottom-up dysregulation versus those who are subject to a primary top-down dysfunction. However, this will require the construction of careful experimental approaches that integrate interoceptive stimulation, belief states, and modulation of anticipatory states. The application of interoceptive modulation provides a unique experimental approach that takes advantage of the firm neuroanatomical basis of these processes to better probe self-related evaluative processes that are so critical for depression and anxiety.
There are two components of interoceptive dysregulation in anxiety and depression. First, we implicate specific neural substrates in the pathological processes observed in anxiety and depression. Specifically, consistent with our prior view of the role of insula in anxiety (Paulus and Stein 2006), this neural structure provides the contextualized body prediction error signal to other brain structures. The mPFC evaluates this body prediction signal based on self-relevant and belief-based processes. The dorsolateral prefrontal cortex and anterior cingulate respond to these contextualized self-relevant and belief-based signals to differentiate those that are predictive from those that are not. The relative increase in anterior cingulate activity has been reported in a number of studies with anxiety disorder individuals (McClure et al. 2007; Nitschke et al. 2009; Paulus et al. 2004). Therefore, altered brain processing in both depression and anxiety is thought to comprise the concerted and increased synchronicity of these brain structures with the ultimate goal of adapting the individual internal milieu to the demands of the outside world.
Second, the processes that are implicated in this model extend beyond interoception to include belief-based and self-referential aspects. In this context, one could consider the combination of contextualized interoceptive afferents, belief-based and self-referential constructs as “interoceptive prediction schemas” of the individuals. For example, a rapid heart rate may fit into a “positive excitement schema” or into an “anxious potentially harmful schema”, i.e., the same afferent interoception can result in fundamentally different interpretation and therefore have completely different self-relevance. One important direction is to determine the degree to which these interoceptive prediction schemas evolve. In particular, one needs to consider the interplay between the disposition to experience a poorer signal to noise ratio of interoceptive afferents and specific experiences, which may result in greater uncertainty due to the catastrophic consequences of not predicting a significant aversive event. For example, an individual who is particularly sensitive to interoceptive afferents and is exposed to childhood mistreatment may need to engage all available top-down modulatory processes to differentiate interoceptive fluctuation from predicting an impending episode of mistreatment.
The proposed extension of the initial model is consistent with other conceptualizations of depression and anxiety. For example, altered self-schemas particularly as they relate to personal worth (Teasdale et al. 1995) have been proposed as a basic vulnerability for depression (Williams et al. 1990). Moreover, a mismatch between self-relevant schemas such as negative affectivity and physiological arousal has been observed in individuals with generalized anxiety disorder (Dibartolo et al. 1997). Practically, individuals with anxiety disorders show a marked tendency to underestimate their level of physical exertion (Vaitl 1996). Subjects with high emotional reactivity showed a higher degree of interoceptive awareness based on the heart beat detection task and higher trait anxiety, which has been taken to suggest that a chronically increased sympathetic outflow might be one variable contributing to the establishment of high interoceptive awareness (Pollatos et al. 2007a). Further analyses, however, revealed that the relationship between emotional arousal and trait anxiety was mediated by differences in interoceptive awareness (Pollatos et al. 2007b). Thus, there is some evidence that anxious individuals show increased interoceptive sensitivity, which may be associated with reduced interoceptive accuracy. Finally, enhanced fear-relevant schemas in the context of external stimuli have been proposed to contribute to the formation of excessive anxiety (Teachman et al. 2001).
The value of the current model is to dissect both processes and brain structures to be able to formulate and test novel hypotheses about the fundamental dysfunction in depression and anxiety. Specifically, we predict that individuals with anxiety and depression show (1) reduced ability to adequately report interoceptive afferents; (2) exaggerated response to aversive interoceptive afferents, even those that are not predictive of aversive outcomes. As a consequence, these individuals should perform more poorly on tasks that are guided by somatic signals, e.g., the Iowa gambling task. In summary, we propose that both depression and anxiety represent altered interoceptive states as a consequence of noisily amplified self-referential interoceptive predictive belief states. Due to the uncertain and noisy ascending interoceptive input, unstable interoceptive schemas are developed that foster predictive uncertainty and as a consequence an increased attempt to top-down control and predict these inputs.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders (4th edition): DSM-IV. Washington: The American Psychiatric Association; 1994. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Apperly IA, Back E, Samson D, France L. The cost of thinking about false beliefs: evidence from adults’ performance on a non-inferential theory of mind task. Cognition. 2008;106:1093–1108. doi: 10.1016/j.cognition.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Augustine JR. The insular lobe in primates including humans. Neurol Res. 1985;7:2–10. doi: 10.1080/01616412.1985.11739692. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bagaev V, Aleksandrov V. Visceral-related area in the rat insular cortex. Auton Neurosci. 2006;125:16–21. doi: 10.1016/j.autneu.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11:2117–2120. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Blanchard EB, Vermilyea JA, Vermilyea BB, DiNardo PA. Generalized anxiety and generalized anxiety disorder: description and reconceptualization. Am J Psychiatry. 1986;143:40–44. doi: 10.1176/ajp.143.1.40. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: clinical, experimental, and theoretical aspects. New York: Hoeber Medical Division, Harper & Row; 1967. [Google Scholar]
- Berlucchi G, Aglioti SM. The body in the brain revisited. Exp Brain Res. 2009;200:25–35. doi: 10.1007/s00221-009-1970-7. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Nestadt G, Samuels JF, Costa PT, Howard WT, Eaton WW. Phobic, panic, and major depressive disorders and the five-factor model of personality. J Nerv Ment Dis. 2001;189:154–161. doi: 10.1097/00005053-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ, Bentall RP, Ffytche DH, Simmons A, Murray RM, Howard RJ. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychol Med. 2004;34:591–596. doi: 10.1017/S0033291703008997. [DOI] [PubMed] [Google Scholar]
- Bruch MA, Hamer RJ, Heimberg RG. Shyness and public self-consciousness: additive or interactive relation with social interaction? J Pers. 1995;63:47–63. doi: 10.1111/j.1467-6494.1995.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Pleasure: the common currency. J Theor Biol. 1992;155:173–200. doi: 10.1016/s0022-5193(05)80594-6. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Guillaume J, Balasko M, Fleury A. Pleasure in decision-making situations. BMC Psychiatry. 2002;2:7. doi: 10.1186/1471-244X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG, Huang GC, Nichols T, Koeppe RA, Minoshima S, Rose D, et al. Reduced gamma-aminobutyric acid(A)-benzodiazepine binding sites in insular cortex of individuals with panic disorder. Arch Gen Psychiatry. 2007;64:793–800. doi: 10.1001/archpsyc.64.7.793. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32:1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Clark DM, McManus F. Information processing in social phobia. Biol Psychiatry. 2002;51:92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, editors. Handbook of emotions. 3. New York: Guilford Press; 2007. pp. 272–290. [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. New York, NY: Harcourt; 1999. [Google Scholar]
- Damasio AR. Looking for Spinoza: joy, sorrow, and the feeling brain. New York, NY: Harcourt; 2003. [Google Scholar]
- de Jong PJ. Implicit self-esteem and social anxiety: differential self-favouring effects in high and low anxious individuals. Behav Res Ther. 2002;40:501–508. doi: 10.1016/s0005-7967(01)00022-5. [DOI] [PubMed] [Google Scholar]
- Dibartolo PM, Brown TA, Barlow DH. Effects of anxiety on attentional allocation and task performance: an information processing analysis. Behav Res Ther. 1997;35:1101–1111. [PubMed] [Google Scholar]
- Dugas MJ, Freeston MH, Ladouceur R, Rheaume J, Provencher M, Boisvert JM. Worry themes in primary GAD, secondary GAD, and other anxiety disorders. J Anxiety Disord. 1998;12:253–261. doi: 10.1016/s0887-6185(98)00013-9. [DOI] [PubMed] [Google Scholar]
- Eckman PS, Shean GD. Habituation of cognitive and physiological arousal and social anxiety. Behav Res Ther. 1997;35:1113–1121. [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Elliott R, Jobber D, Sharp J. Using the theory of reasoned action to understand organizational behaviour: the role of belief salience. Br J Soc Psychol. 1995;34:161–172. [Google Scholar]
- Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel ES. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J Neurol Neurosurg Psychiatry. 1997;63:74–82. doi: 10.1136/jnnp.63.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinle C. Role of intestinal chemoreception in the induction of gastrointestinal sensations. Dtsch Tierarztl Wochenschr. 1998;105:441–444. [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, et al. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemar MC, Segal ZV, Sagrati S, Kennedy SJ. Mood-induced changes on the Implicit Association Test in recovered depressed patients. J Abnorm Psychol. 2001;110:282–289. doi: 10.1037//0021-843x.110.2.282. [DOI] [PubMed] [Google Scholar]
- Geuze E, Westenberg HG, Jochims A, de Kloet CS, Bohus M, Vermetten E, et al. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Explaining modulation of reasoning by belief. Cognition. 2003;87:B11–B22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Frith CD, Passingham RE. Inferring false beliefs from the actions of oneself and others: an fMRI study. Neuroimage. 2004;21:744–750. doi: 10.1016/S1053-8119(03)00665-7. [DOI] [PubMed] [Google Scholar]
- Gusnard DA. Being a self: considerations from functional imaging. Conscious Cogn. 2005;14:679–697. doi: 10.1016/j.concog.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Happe F. Theory of mind and the self. Ann N Y Acad Sci. 2003;1001:134–144. doi: 10.1196/annals.1279.008. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go–nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Sheth SA, Cohen MS (2007) Functional neuroimaging of belief, disbelief, and uncertainty. Ann Neurol [DOI] [PubMed]
- Herwig U, Abler B, Walter H, Erk S. Expecting unpleasant stimuli—an fMRI study. Psychiatry Res. 2007;154:1–12. doi: 10.1016/j.pscychresns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Hill EM, Wilson AF, Elston RC, Winokur G. Evidence for possible linkage between genetic markers and affective disorders. Biol Psychiatry. 1988;24:903–917. doi: 10.1016/0006-3223(88)90225-9. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Clark DM, Mathews A, Williams R. Self-images play a causal role in social phobia. Behav Res Ther. 2003;41:909–921. doi: 10.1016/s0005-7967(02)00103-1. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131:11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Rietveld S, Schoutrop M, Spiering M, Brosschot JF. A repressive coping style and affective, facial and physiological responses to looking at emotional pictures. Int J Psychophysiol. 2001;42:265–277. doi: 10.1016/s0167-8760(01)00150-7. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Misiurek J, Jurkowski AJ, McCarthy G. Dynamic and strategic aspects of executive processing. Brain Res. 2004;1000:78–84. doi: 10.1016/j.brainres.2003.11.041. [DOI] [PubMed] [Google Scholar]
- James W. Psychology: the briefer course. New York: Harper and Row; 1961. [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol. 2004;468:425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO., Jr Boundaries of major depression: an evaluation of DSM-IV criteria. Am J Psychiatry. 1998;155:172–177. doi: 10.1176/ajp.155.2.172. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of 1-year prevalence of major depression in women. Arch Gen Psychiatry. 1993;50:843–852. doi: 10.1001/archpsyc.1993.01820230009001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. Am J Psychiatry. 2006;163:115–124. doi: 10.1176/appi.ajp.163.1.115. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I. Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Hum Brain Mapp. 2007;28:915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lahuerta J, Bowsher D, Campbell J, Lipton S. Clinical and instrumental evaluation of sensory function before and after percutaneous anterolateral cordotomy at cervical level in man. Pain. 1990;42:23–30. doi: 10.1016/0304-3959(90)91087-Y. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Torebjork HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci. 1982;2:765–781. doi: 10.1523/JNEUROSCI.02-06-00765.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Unmyelinated afferent fibers are not only for pain anymore. J Comp Neurol. 2003;461:137–139. doi: 10.1002/cne.10691. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, et al. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006;404:358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Longe O, Maratos FA, Gilbert P, Evans G, Volker F, Rockliff H, et al. Having a word with yourself: neural correlates of self-criticism and self-reassurance. Neuroimage. 2009;49:1849–1856. doi: 10.1016/j.neuroimage.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic–cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McClure EB, Parrish JM, Nelson EE, Easter J, Thorne JF, Rilling JK, et al. Responses to conflict and cooperation in adolescents with anxiety and mood disorders. J Abnorm Child Psychol. 2007;35:567–577. doi: 10.1007/s10802-007-9113-8. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III. Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, et al. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport. 2003;14:177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick-Kline P, Turk CL, Mennin DS, Hoyt EA, Gallagher CL. Level of emotional awareness as a differentiating variable between individuals with and without generalized anxiety disorder. J Anxiety Disord. 2005;19:557–572. doi: 10.1016/j.janxdis.2004.06.001. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Hornak J, Andrews C, Rolls ET. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008;3:144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J et al (2009) Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci [DOI] [PMC free article] [PubMed]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ongur D, Farabaugh A, Iosifescu DV, Perlis R, Fava M. Tridimensional personality questionnaire factors in major depressive disorder: relationship to anxiety disorder comorbidity and age of onset. Psychother Psychosom. 2005;74:173–178. doi: 10.1159/000084002. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Sirota A, Niaura R, Rauch SL, Brown WA. Neural and endocrine correlates of sadness in women: implications for neural network regulation of HPA activity. J Neuropsychiatry Clin Neurosci. 2004;16:446–455. doi: 10.1176/jnp.16.4.446. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biol Psychiatry. 2004;55:1179–1187. doi: 10.1016/j.biopsych.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Perico CA, Skaf CR, Yamada A, Duran F, Buchpiguel CA, Castro CC, et al. Relationship between regional cerebral blood flow and separate symptom clusters of major depression: a single photon emission computed tomography study using statistical parametric mapping. Neurosci Lett. 2005;384:265–270. doi: 10.1016/j.neulet.2005.04.088. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception. I. The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Herbert BM, Kaufmann C, Auer DP, Schandry R. Interoceptive awareness, anxiety and cardiovascular reactivity to isometric exercise. Int J Psychophysiol. 2007;65:167–173. doi: 10.1016/j.ijpsycho.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Traut-Mattausch E, Schroeder H, Schandry R. Interoceptive awareness mediates the relationship between anxiety and the intensity of unpleasant feelings. J Anxiety Disord. 2007;21:931–943. doi: 10.1016/j.janxdis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Cabanac M. Pleasure, the common currency of emotions. Ann N Y Acad Sci. 2003;1000:293–295. doi: 10.1196/annals.1280.028. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA. The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry. 1997;42:446–452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- Ravindran AV, Smith A, Cameron C, Bhatla R, Cameron I, Georgescu TM, et al. Toward a functional neuroanatomy of dysthymia: a functional magnetic resonance imaging study. J Affect Disord. 2009;119:9–15. doi: 10.1016/j.jad.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Reiss S. Trait anxiety: it’s not what you think it is. J Anxiety Disord. 1997;11:201–214. doi: 10.1016/s0887-6185(97)00006-6. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SK, Viirre ES, Bailey KA, Gerke MA, Harris JP, Stein MB. Randomized placebo-controlled trial of a selective serotonin reuptake inhibitor in the treatment of nondepressed tinnitus subjects. Psychosom Med. 2005;67:981–988. doi: 10.1097/01.psy.0000188479.04891.74. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1212–1225. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Mraovitch S, Granata AR, Anwar M, Reis DJ. A role of insular cortex in cardiovascular function. J Comp Neurol. 1987;257:189–207. doi: 10.1002/cne.902570206. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss: anticipation predict avoidance learning. Psychol Sci. 2008;19:320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22:941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV. Appraisal of the self-schema construct in cognitive models of depression. Psychol Bull. 1988;103:147–162. doi: 10.1037/0033-2909.103.2.147. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP. Cortical activations during judgments about the self and an other person. Neuropsychologia. 2004;42:1168–1177. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Shipley MT. Insular cortex projection to the nucleus of the solitary tract and brainstem visceromotor regions in the mouse. Brain Res Bull. 1982;8:139–148. doi: 10.1016/0361-9230(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc Lond B Biol Sci. 2005;360:797–814. doi: 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Dohnel K, Sodian B, Meinhardt J, Thoermer C, Hajak G. Neural correlates of true and false belief reasoning. Neuroimage. 2007;35:1378–1384. doi: 10.1016/j.neuroimage.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Stein MB. Public health perspectives on generalized anxiety disorder. J Clin Psychiatry. 2004;65(Suppl 13):3–7. [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kessler RC, Kendler KS. Latent class analysis of lifetime depressive symptoms in the national comorbidity survey. Am J Psychiatry. 1998;155:1398–1406. doi: 10.1176/ajp.155.10.1398. [DOI] [PubMed] [Google Scholar]
- Teachman BA, Gregg AP, Woody SR. Implicit associations for fear-relevant stimuli among individuals with snake and spider fears. J Abnorm Psychol. 2001;110:226–235. doi: 10.1037//0021-843x.110.2.226. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Taylor MJ, Cooper Z, Hayhurst H, Paykel ES. Depressive thinking: shifts in construct accessibility or in schematic mental models? J Abnorm Psychol. 1995;104:500–507. doi: 10.1037//0021-843x.104.3.500. [DOI] [PubMed] [Google Scholar]
- Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, et al. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2000;288:159–162. doi: 10.1016/s0304-3940(00)01224-6. [DOI] [PubMed] [Google Scholar]
- Vaitl D. Interoception. Biol Psychol. 1996;42:1–27. doi: 10.1016/0301-0511(95)05144-9. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J, Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. J Physiol. 1995;483:783–795. doi: 10.1113/jphysiol.1995.sp020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Southwick SM, Bremner JD. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull. 2007;40:8–30. [PMC free article] [PubMed] [Google Scholar]
- Wang L, Labar KS, McCarthy G. Mood alters amygdala activation to sad distractors during an attentional task. Biol Psychiatry. 2006;60:1139–1146. doi: 10.1016/j.biopsych.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Gao Y, Chen J, Zhang W, Lin C. False belief reasoning in the brain: an ERP study. Sci China C Life Sci. 2008;51:72–79. doi: 10.1007/s11427-008-0014-z. [DOI] [PubMed] [Google Scholar]
- Watkins PC, Vache K, Verney SP, Muller S, Mathews A. Unconscious mood-congruent memory bias in depression. J Abnorm Psychol. 1996;105:34–41. doi: 10.1037//0021-843x.105.1.34. [DOI] [PubMed] [Google Scholar]
- Wertz AE, German TC. Belief-desire reasoning in the explanation of behavior: do actions speak louder than words? Cognition. 2007;105:184–194. doi: 10.1016/j.cognition.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Res Brain Res Rev. 2003;43:224–230. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- Williams JM, Healy D, Teasdale JD, White W, Paykel ES. Dysfunctional attitudes and vulnerability to persistent depression. Psychol Med. 1990;20:375–381. doi: 10.1017/s0033291700017694. [DOI] [PubMed] [Google Scholar]
- Williams NL, Shahar G, Riskind JH, Joiner TE., Jr The looming maladaptive style predicts shared variance in anxiety disorder symptoms: further support for a cognitive model of vulnerability to anxiety. J Anxiety Disord. 2005;19:157–175. doi: 10.1016/j.janxdis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
- Yoshida W, Ishii S. Resolution of uncertainty in prefrontal cortex. Neuron. 2006;50:781–789. doi: 10.1016/j.neuron.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Young L, Saxe R. The neural basis of belief encoding and integration in moral judgment. Neuroimage. 2008;40:1912–1920. doi: 10.1016/j.neuroimage.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proc Natl Acad Sci USA. 2007;104:8235–8240. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15:983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]


