Abstract
Background/Aims
Vaccination against hepatitis A virus (HAV) is recommended for patients with chronic hepatitis B (CHB), since they are potentially at an increased risk of HAV-related morbidity and mortality. However, little is known about the adherence to these recommendations in the community. This study evaluated the current vaccination status and immunity against HAV among Korean military soldiers with CHB.
Methods
We performed a prospective study of Korean military soldiers from August 2008 to January 2009. We enrolled 96 soldiers with CHB on a consecutive basis. We assessed their vaccination history and the presence of anti-HAV immunoglobulin G (IgG).
Results
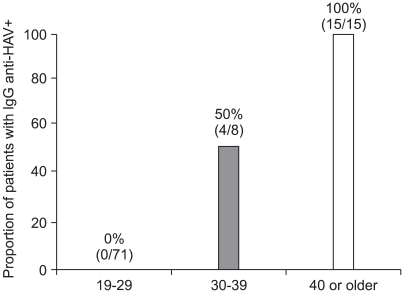
The HAV vaccination rate of the soldiers enrolled in our study was 2% (2 of the 96 soldiers). The seroprevalence rates of anti-HAV IgG among military soldiers without a vaccination history were 0%, 50%, and 100% for those aged 19-29 years (n=71), 30-39 years (n=8), and 40 years or older (n=15), respectively (p<0.001).
Conclusions
The HAV vaccination rate is very low among military soldiers. Public health efforts aimed at raising awareness about HAV vaccination in patients with CHB should be strongly encouraged.
Keywords: Hepatitis A, Vaccination, Military personnel, Seroepidemiologic study
INTRODUCTION
Hepatitis A virus (HAV) superinfection in patients with chronic liver disease is associated with a potentially increased risk of HAV-related morbidity and mortality as compared to that of patients without underlying chronic liver disease.1,2 Thus, for patients with chronic liver disease, HAV vaccination is recommended by the World Health Organization (WHO), the U.S. Centers for Disease Control and Prevention (CDC) and others.3-6
Vaccination against HAV has been deemed unnecessary in Korea, even in high risk groups, since immunity against HAV has been considered almost universal in Korean adults.7-9 However, improvements in the socioeconomic status and general public health measures of Asian countries, including Korea, over the last 20 years has led to a shift in the seroprevalence of hepatitis A.10-13 Recent reports from Korea suggest that Korea is in transition from a higher HAV endemic area to a lower one for HAV infection region, and that a growing number of young Korean adults are susceptible to HAV infection.8 Currently in Korea, HAV vaccination is recommended for patients with chronic liver disease, and who are not immune to HAV.8,10 In spite of the growing need, little is known about adherence to this recommendation in clinical practice.
Korea has a military draft system. Young men in their early 20s are required to fulfill their duty to the army; infection with chronic hepatitis B (CHB) does not allow for exemption. Military personnel are considered to be at higher risk for acquiring HAV than are those in the civilian population.14 Considering the rapid change in the HAV seroepidemiology in Korea, young soldiers with CHB might be at a potentially increased risk for HAV-related morbidity and mortality. Evaluation of their immunity against HAV is urgently needed. Military personnel hail from every part of Korea. Therefore our study can reflect the current adherence to HAV vaccination in military personnel through the country.
In this study, we evaluated current vaccination status against HAV among military soldiers and the susceptibility to HAV infections among soldiers with CHB in the Republic of Korea Army. We also performed an additional retrospective study to ascertain the prognosis of acute hepatitis A infection in recent years.
MATERIALS AND METHODS
1. Current vaccination status and immunity against HAV
From August 2008 to January 2009, each soldier with a history of hepatitis B virus infection who visited the Armed Forces Daejeon Hospital was interviewed and tested for immunity against HAV by testing anti-HAV IgG (Abbott Laboratories, Wiesbaden, Germany). We excluded soldiers negative for hepatitis B virus surface antigen (HBsAg). During the study period, 101 consecutive military soldiers were enrolled, and five soldiers were excluded since they showed negative results for HBsAg. In total, we analyzed 96 military soldiers with CHB on a consecutive basis.
We also assessed the status of underlying liver disease in all participants. We classified liver disease status into the following categories: HBeAg positive CHB, HBeAg negative CHB, inactive HBsAg carrier state.15 Patients were classified as being in an inactive HBsAg carrier state if they met the following criteria; 1) HBsAg+ >6 months, 2) HBeAg-, anti HBe+, 3) Serum HBV DNA <105 copies/mL, and 4) normal serum aminotransferase levels. Patients with ongoing anti-viral therapy with nucleoside analogue were noted and classified into the anti-viral treatment group. We assessed the samples for evidence of liver cirrhosis and hypersplenism, primarily using ultrasonography;16 no cases of cirrhosis were found, likely due to the fact that patients with liver cirrhosis are exempted from military service.
An investigator blind to the result of the anti-HAV IgG test conducted the interviews. Participants were asked the following questions.
Have you heard of HAV infection?
Do you know that HAV infection can be prevented by vaccination?
Did you receive an HAV vaccination?
Where did you grow up?
What is your family income?
The soldier's hometowns were categorized into seven major administrative districts within Korea: Seoul, Incheon, and Gyeonggi province, Gangwon province, Chungcheng area (Daejeon, Chungcheongnam-do, and Chungchengbuk-do), Gyeongsang area (Daegu, Ulsan, Gyeongsangnam-do, and Gyeongsangbuk-do), Jeolla area (Gwangju, Jeollanam-do, and Jeollabuk-do), and Jeju Island. In this study, the soldiers were from Seoul (n=16), Gyeonggi province (n=12), Gangwon province (n=3), Chungcheng area (n=15), Gyeongsang area (n=26), Jeolla area (n=22), and Jeju Island (n=2).
There are many different views about what level of family income constitutes middle class. The World Health Organization suggested that a family income between 50-150% (60-150% in European Union) of the country's median family income is middle class. According to the data from the Korea National Statistical Office, the median value of family income in 2007 was about 2,390,000 won/month. Therefore, we arbitrarily categorized family income into three groups (low income group: <2,000,000 won/month, intermediate income group: 2,000,000-4,000,000 won/month, high income group: >4,000,000 won/month).
2. Retrospective study to ascertain the trend of acute hepatitis A in recent years
The Defense Medical Information System (DEMIS) was reviewed to identify those individuals who had abnormal liver function tests results and who were admitted to the Armed Forces Capital Hospital from January 2000 to December 2008. Among these designated persons, those who were confirmed to have acute hepatitis A according to history, clinical features, laboratory data and serologic data (IgM antibody to HAV) were included and analyzed. Patients who had abnormal liver function tests results secondary to trauma, cholecystitis, cholangitis, liver abscess, sepsis, rhabdomyolysis, heat stroke, acute hepatitis B, acute hepatitis C, toxic liver disease or exacerbation of chronic hepatitis were excluded. Severe acute hepatitis A was defined when prothrombin time decreased below 40%.17
This study was reviewed and permitted by the ROK (Republic of Korea) Army security division and was carried out in accordance with the Helsinki Declaration.18,19
3. Statistical analysis
Statistical analysis was performed using the Fisher's exact test to compare discrete variables, and t-test or Mann-Whitney test for numeric variables, as appropriate. To identify the factors associated with severe hepatitis A, multiple logistic regression analysis was performed using the variables with p values of <0.100 on the univariate analysis. More generous p value criteria were used to include characteristics with a marginal association. Analyses were performed using SPSS (ver. 16.0; SPSS Inc., Chicago, IL, USA). p<0.05 was considered to be significant.
RESULTS
1. Baseline characteristics of military soldiers with CHB
The median age of soldiers with CHB was 21 years (range, 19 to 55), and all soldiers were male. Seventy-three (76%) soldiers were aged 19-29, eight (8%) soldiers were aged 30-39, and 15 (16%) soldiers were more than 40 years of age. HBsAg was positive in all soldiers. Of the 96 soldiers, 34 (36%) reported that they did not know whether any members of their families were infected with CHB. Of the 62 soldiers who knew their family histories, 55 (89%) reported that they had a family member with hepatitis B virus infection, and 45 of 55 (73%) reported that their mothers were infected with hepatitis B virus. Twelve were receiving antiviral therapy (13%), 18 (19%) were classified as HBeAg negative CHB, 43 (45%) were classified as HBeAg positive CHB and 23 (24%) were classified as being in an inactive HBsAg carrier state.
2. Awareness and vaccination status against HAV in soldiers with CHB
Even though soldiers with CHB are at an potentially increased risk for HAV-related morbidity and mortality, awareness and vaccination rates against HAV in military soldiers with CHB were low (Fig. 1). Only about half of the participants were aware of HAV, only 38% (37 of 96) knew a vaccine for HAV is available, and only two of the 96 soldiers (2%) had received the vaccination. IgG anti-HAV was positive in both patients (aged 19 and 21) with a history of HAV vaccination. Even among soldiers who were informed of HAV (n=55), 33% did not know that HAV is preventable through vaccination.
Fig. 1.
Awareness and vaccination rates against hepatitis A virus (HAV) among military soldiers with chronic hepatitis B.
3. Seroprevalence of HAV
The seroprevalence of IgG anti-HAV in military personnel without vaccination history was 20% (19 of 94). The seroprevalences of IgG anti-HAV were 0%, 50%, and 100% for those aged between 19-29 years (n=71), 30-39 years (n=8), and 40 years or older (n=15), respectively (Fig. 2, p<0.001). The seroprevalence of HAV did not differ by geographic area (p=0.213), and family income was not associated with the seroprevalence of HAV (p=0.508).
Fig. 2.
The seroprevalence of hepatitis A virus (HAV) according to specific age groups in patients without a vaccination history.
4. Acute hepatitis A in recent years
From January 2000 through December 2008, 134 cases of acute hepatitis A were entered into our database. The occurrence of acute hepatitis A showed an increasing trend: 17 cases from 2000 to 2002, 40 cases from 2003 to 2005, and 77 cases from 2006 to 2008. There was no case which caused death or required liver transplantation and every patient recovered with only supportive care.
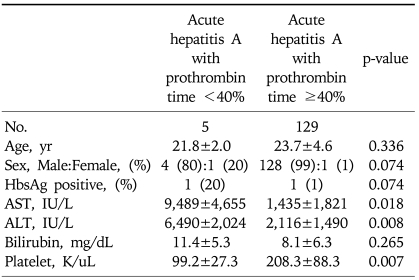
Analysis of laboratory findings disclosed that the nadir prothrombin time independently correlated with peak serum alanine aminotransferase (ALT) level (regression coefficient=-0.634, p<0.001) and lowest platelet count (regression coefficient=0.211, p=0.003), but was not associated with serum aspartate aminotransferase (AST) level or age. There were 5 cases (3.8%) with severe acute hepatitis A, defined by prothrombin time of less than 40%. By univariate analysis, peak ALT, peak AST, and lowest platelet level were factors associated with severe hepatitis (Table 1). However, in this study, we found that there was no independent predictor of severe hepatitis according to multivariate analysis; peak ALT (p=0.325), peak AST (p=0.440), and lowest platelet level (p=0.487). The presence of HBsAg was not an independent factor for acquiring severe hepatitis A in this study. Nevertheless, in two patients with chronic hepatitis B, one patient (50%) presented with severe acute hepatitis A.
Table 1.
Clinical Parameters Associated with Severe Acute Hepatitis A
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
DISCUSSION
In this study, we found that (i) the current vaccination rate against HAV was very low among military soldiers with CHB, (ii) young military soldiers with CHB were susceptible to HAV, and (iii) the number of acute hepatitis A patients requiring hospitalization among military soldiers in Korea showed an increasing trend in recent years. Kang et al.20 reported 11 outbreaks of acute hepatitis A among young Korean soldiers from January 2000 through December 2004. Lee et al.21 also reported an outbreak of acute hepatitis A in the army involving 67 cases of symptomatic cases. These findings suggest that a strategy for minimizing this vaccine-preventable disease in the military is urgently needed.
Currently, vaccination against HAV is recommended in Korea for patients with chronic liver disease, who are not already immune to the virus.8,10 Our study showed that despite the current recommendation, the vaccination rate for patients with CHB was very low. The reasons for these low rates are likely to be multifactorial, including patient refusal, poor compliance, lack of knowledge regarding HAV vaccination, or other unidentified reasons. We did not fully assess the reasons for the low vaccination rates in our study, thus the exact cause of low vaccination rates are unknown. However, in our survey, we noted that only 38% of participants knew that there was a vaccination available to prevent HAV. Our survey suggests that public health efforts aimed at raising awareness about HAV vaccination in patients with CHB is needed to overcome the low vaccination rate in the community.
We also noticed that a substantial proportion (78%) of soldiers were susceptible to HAV. Among soldiers younger than 29 years (n=71) and without history of HAV vaccination, none of the soldiers was immune to HAV. Song et al. also reported that the seroprevalences of IgG anti-HAV in patients with chronic liver disease were 0% and 23.1% for those less than 25 (n=14) and those between 26 and 30 years (n=13),7 respectively. Consistent with their report, our data clearly shows that most individuals younger than 30 years are now susceptible to HAV.
Age, birthplace and family income are known factors that are associated with HAV infection.22,23 Song et al.13 noticed a difference in seroprevalence with regard to geographic area, even within the city of Seoul. In our study, we also noticed that age was closely related to HAV seroprevalence. However, neither socioeconomic status nor place of residence was associated with prevalence of HAV infection in our study. Most recent reports from Korea showed an extremely low rate of seroprevalence in young adults,8,9,12,13 which suggests that these factors are no longer useful for predicting HAV infection in young adults.
Since the seroprevalence of anti-HAV is extremely low in individuals younger than age 30, and is unpredictable according to geographic area or family income, and considering that vaccination is recommended to patients with chronic liver disease, a universal vaccination program may be applicable to young individuals with CHB. The need for pre-vaccination antibody testing remains controversial. It may be more cost-effective to offer pre-vaccination antibody testing to populations with a high prevalence of natural immunity to HAV, whereas universal vaccination may be more appropriate in populations with a low prevalence of immunity.24 In addition to seroprevalence data, the cost of serologic testing, the cost of vaccination, and patient compliance information, and the likelihood of occurrence of a clinically significant fatal HAV superinfection should also be determined in order to facilitate the identification of the most efficacious vaccination strategy.8 One effective strategy for young men with CHB might be a vaccination program during military recruiting or during military service. This strategy may provide an effective opportunity for every young man with CHB, since they have to fulfill military duty once in their lifetime.
Our study has limitations because most individuals in this study were healthy young soldiers, and all were male. Also, during the draft, many individuals are exempted from military service for a variety of reasons. This may have caused unintentional selection bias. Lastly, the sample size of this study was relatively small. Thus findings in our study may not be generalized to other setting, and must be confirmed in larger scale studies.
In conclusion, despite recommendations of vaccination against HAV in patients with CHB, we found that the vaccination rates were low in clinical practice, and most patients were susceptible to HAV. Public health efforts aimed at raising awareness about HAV vaccination in patients with CHB should be strongly encouraged.
ACKNOWLEDGEMENTS
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Ministry of National Defense, or the Government of Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Keeffe EB. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol. 1995;90:201–205. [PubMed] [Google Scholar]
- 2.Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 3.Public health control of hepatitis A: memorandum from a WHO meeting. Bull World Health Organ. 1995;73:15–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1996;45:1–30. [PubMed] [Google Scholar]
- 5.Cooksley WG. Consensus statement on the role of hepatitis A vaccination in patients with chronic liver disease. J Viral Hepat. 2000;7(Suppl 1):29–30. doi: 10.1046/j.1365-2893.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 6.Crowcroft NS, Walsh B, Davison KL, Gungabissoon U. Guidelines for the control of hepatitis A virus infection. Commun Dis Public Health. 2001;4:213–227. [PubMed] [Google Scholar]
- 7.Song HJ, Kim TH, Song JH, et al. Emerging need for vaccination against hepatitis A virus in patients with chronic liver disease in Korea. J Korean Med Sci. 2007;22:218–222. doi: 10.3346/jkms.2007.22.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong SH. Current status and vaccine indication for hepatitis A virus infection in Korea. Korean J Gastroenterol. 2008;51:331–337. [PubMed] [Google Scholar]
- 9.Lee D, Cho YA, Park Y, et al. Hepatitis a in Korea: epidemiological shift and call for vaccine strategy. Intervirology. 2008;51:70–74. doi: 10.1159/000127428. [DOI] [PubMed] [Google Scholar]
- 10.Kang JH, Lee KY, Kim CH, Sim D. Changing hepatitis A epidemiology and the need for vaccination in Korea. Asian Pac J Allergy Immunol. 2004;22:237–242. [PubMed] [Google Scholar]
- 11.Park JH. Changes in the seroprevalence of hepatitis A virus antibody in Korea. Korean J Hepatol. 2007;13:1–4. [PubMed] [Google Scholar]
- 12.Sohn YM, Rho HO, Park MS, et al. The changing epidemiology of hepatitis A in children and the consideration of active immunization in Korea. Yonsei Med J. 2000;41:34–39. doi: 10.3349/ymj.2000.41.1.34. [DOI] [PubMed] [Google Scholar]
- 13.Song YB, Lee JH, Choi MS, et al. The age-specific seroprevalence of hepatitis A virus antibody in Korea. Korean J Hepatol. 2007;13:27–33. [PubMed] [Google Scholar]
- 14.Franco E, Giambi C, Ialacci R, Coppola RC, Zanetti AR. Risk groups for hepatitis A virus infection. Vaccine. 2003;21:2224–2233. doi: 10.1016/s0264-410x(03)00137-3. [DOI] [PubMed] [Google Scholar]
- 15.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 16.Kim do Y, Ahn SH, Lee HW, et al. Anti-hepatitis A virus seroprevalence among patients with chronic viral liver disease in Korea. Eur J Gastroenterol Hepatol. 2007;19:923–926. doi: 10.1097/MEG.0b013e3282efa432. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Lee JY, Jang JS, et al. Initial thrombocytopenia as a simple, valuable predictor for clinical manifestation in acute hepatitis A. Scand J Gastroenterol. 2008;43:81–88. doi: 10.1080/00365520701514578. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- 19.Goodyear MD, Krleza-Jeric K, Lemmens T. The Declaration of Helsinki. BMJ. 2007;335:624–625. doi: 10.1136/bmj.39339.610000.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang CI, Choi CM, Park TS, Lee DJ, Oh MD, Choe KW. Incidence and seroprevalence of hepatitis A virus infections among young Korean soldiers. J Korean Med Sci. 2007;22:546–548. doi: 10.3346/jkms.2007.22.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CS, Lee JH, Kwon KS. Outbreak of hepatitis A in Korean military personnel. Jpn J Infect Dis. 2008;61:239–241. [PubMed] [Google Scholar]
- 22.Bell BP, Kruszon-Moran D, Shapiro CN, Lambert SB, McQuillan GM, Margolis HS. Hepatitis A virus infection in the United States: serologic results from the Third National Health and Nutrition Examination Survey. Vaccine. 2005;23:5798–5806. doi: 10.1016/j.vaccine.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 23.Moisseeva AV, Marichev IL, Biloschitchkay NA, et al. Hepatitis A seroprevalence in children and adults in Kiev City, Ukraine. J Viral Hepat. 2008;15(Suppl 2):43–46. doi: 10.1111/j.1365-2893.2008.01028.x. [DOI] [PubMed] [Google Scholar]
- 24.Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1999;48:1–37. [PubMed] [Google Scholar]