Abstract
Background/Aims
The CYP2C19 polymorphism plays an important role in the metabolism of various proton-pump inhibitors. Several trials have produced conflicting data on eradication rates of Helicobacter pylori (H. pylori) among CYP2C19 genotypes. We investigated whether the CYP2C19 genotype affects the eradication rate of H. pylori by direct comparing the effects of lansoprazole- and rabeprazole-based triple therapies.
Methods
A total of 492 patients infected with H. pylori was randomly treated with either 30 mg of lansoprazole or 20 mg of rabeprazole plus 500 mg of clarithromycin and 1,000 mg of amoxicillin twice daily for 1 week. CYP2C19 genotype status was determined by a PCR-restriction-fragment-length polymorphism method. After 7 to 8 weeks, H. pylori status was evaluated by a C13-urea breath test.
Results
Four hundred and sixty-three patients were analyzed, and the eradication rate was 75.2% in a per-protocol analysis. Eradication rates for the lansoprazole regimen (n=234) were 73.8%, 80.7%, and 85.4% in the homozygous extensive (HomEM), heterozygous extensive (HetEM), and poor metabolizers (PM) groups, respectively (p=0.303). In the case of the rabeprazole regimen (n=229), the eradication rates were 68.6%, 73.0%, and 71.9% in the HomEM, HetEM, and PM groups, respectively (p=0.795).
Conclusions
The efficacies of triple therapies that include lansoprazole or rabeprazole are not affected by CYP2C19 genetic polymorphisms.
Keywords: Helicobacter pylori, CYP2C19, Proton pump inhibitor, Lansoprazole, Rabeprazole
INTRODUCTION
Eradication of Helicobacter pylori (H. pylori) infection is recommended to the patients with peptic ulcer disease, gastric mucosa associated lymphoid tissue (MALT) lymphoma, atrophic gastritis, and after gastric cancer resection.1-4 Current regimens for the eradication of H. pylori consist of a proton pump inhibitor (PPI) and one or two of antibiotic agents. At present, there are five different PPIs on the market: omeparazole, esomeprazole, lansoprazole, pantoprazole and rabeprazole. These are mainly metabolized by the cytochrome P450 (CYP) system except rabeprazole. The principal enzyme implicated in the metabolism of PPIs is CYP2C19.5-7 The phenotype of CYP2C19 has been classified into three groups; the rapid extensive metabolizer (EM) group, the intermediate metabolizer (IM) group and the poor metabolizer group. Each group corresponds to the homozygous extensive metabolizer (HomEM), heterozygous extensive metabolizer (HetEM) and poor metabolizer (PM) by combination of wild or mutant alleles.8 Rabeprazole, however, is mainly metabolized via a non-enzymatic reduction and CYP2C19 is partly involved in the metabolism of rabeprazole.9 Several trials have been published concerning the effect of the CYP2C19 genotype on eradication of H. pylori by various PPIs-based therapies.10-15 However, there are few data available on the direct comparison of eradication rate of rabeprazole with other PPIs considering CYP2C19 polymorphism. With the previously mentioned backgrounds in mind, we aimed to know the influence of CYP2C19 on eradication of H. pylori with rabeprazole or lansoprazole.
MATERIALS AND METHODS
1. Study population
A total of 492 patients was enrolled and randomized prospectively from May 2006 to September 2008 at Asan Medical Center which is the tertiary hospital in the Seoul, Korea. The indications for H. pylori eradication of subjects were peptic ulcer disease, post-endoscopic mucosal resection of gastric cancer or adenoma, MALT lymphoma, non ulcer dyspepsia and gastric polyp. Patients were excluded if they had previously undergone gastrectomy; were pregnant or breastfeeding had an allergy to penicillin; had used a PPI, H2 receptor antagonist, adrenocortical steroids, antiobiotics or non-steroidal anti-inflammatory drugs within the month preceding the study; or had concomitant severe medical illness. The study was conducted under the provisions of the Declaration of Helsinki and approved by the institutional review board of Asan Medical Center. The objective of the study was explained to all patients prior to study, and written informed consent was obtained from each patient.
2. H. pylori eradication: regimen and determination
13C-urea breath test (UBiT, Otsuka, Japan), anti-H. pylori IgG Immulite test (Diagnostic Products Co., Los Angeles, CA, USA) or Wright-Giemsa staining from endoscopic biopsy specimen were performed to assess H. pylori infection status. The difference of 13CO2 value equal to or exceed 2.5‰ was considered to positive urea breath test and the cut-off value of anti-H. pylori IgG Immulite test was 1.1 U/mL according to the manufacturer's recommendations. The patient who was positive in any tests was determined as H. pylori infection.
The patients were randomly allocated into two groups: lansoprazole group (LAC) and rabeprazole group (RAC). They were received PPI based triple therapy with amoxicillin 1,000 mg, clarithromycin 500 mg and PPI (lansoprazole 30 mg or rabeprazole 20 mg according to groups) twice daily for 7 days. Compliance was assessed by counting the tablets left over after therapy. The presence of H. pylori was reevaluated based on the results of 13C-urea breath test performed at 7-8 weeks after the end of eradication therapy.
3. CYP2C19 genotyping
For CYP2C19 genotype analysis, polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) using allele-specific primer was performed. Peripheral blood leukocytes of patients were obtained before eradication treatment. Determination of wild type (wt), mutant type 1 (m1) or mutant type 2 (m2) was made by confirmation of base exchange of G681A and G636A on exon 5 and exon 4 of CYP2C19, respectively. Patients were classified into the following three groups: HomEM, wt/wt; HetEM, wt/m1 and wt/m2; PM, m1/m1, m2/m2 and m1/m2.
4. Statistical analysis
The chi-square test was used for categorical variables (gender ratio, reasons of eradication, frequency of CYP2C19 genotype and eradication rate) and one-way analysis of variance was used for continuous variables (age). p-values lower than 0.05 were considered to indicate statistical significance. Statistical analyses were performed using SPSS software (version 14.0; SPSS Inc., Chicago, IL, USA).
RESULTS
1. Demographic and clinical characteristics
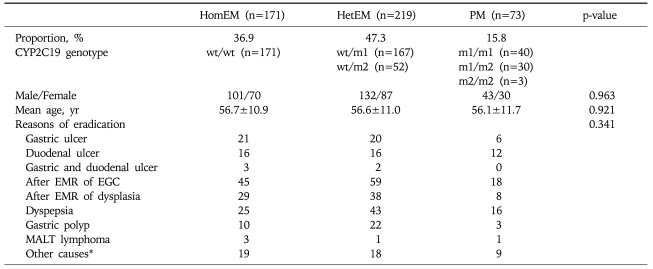
In a total of 492 randomized patients, internal application of eradication regimen over five days and CYP2C19 genotype analysis were performed successfully in 463 patients. Nineteen patients could not take eradication regimen due to side-effect and 10 patients did not perform genotype analysis (Fig. 1). In these 463 analyzable patients, the mean age was 57 years (range, 20 to 83) and male patients were 59.6%. Peptic ulcer (21%), post-endoscopic mucosal resection (43%), and dyspepsia (18%) were common causes of eradication. According to CYP2C19 genotype analyses, HomEM, HetEM and PM were 36.9%, 47.3% and 15.8% respectively. Demographic and clinical characteristics according to each group were described in Table 1. There was no significant difference in characteristics according to each group.
Fig. 1.
Diagram presenting randomization and compliance of study.
Table 1.
Demographic and Clinical Characteristics according to CYP2C19 Genotype
HomEM, homozygous extensive metabolizer; HetEM, heterozygous extensive metabolizer; PM, poor metabollizer; EMR, endoscopic mucosal resection; EGC, early gastric cancer; MALT, mucosa associated lymphoid tissue; wt, wild type; m1, CYP2C19 mutation in exon 5; m2, CYP2C19 mutation in exon 4.
*Other causes include iron deficiency anemia or family history of gastric cancer.
2. Eradication rates of lansoprazole and rabeprazole group according to CYP2C19 status
Overall eradication rates were 75.2% (348/463) by per-protocol analysis and 70.7% (348/492) by intention to treat analysis. Two hundred thirty four and 229 patients were analyzed in LAC and RAC, respectively. HomEM, HetEM and PM of lansoparazole group were 85 (36.3%), 108 (46.2%) and 41 (17.5%), respectively. Those of rabeprazole group were 86 (37.6%), 111 (48.5%) and 32 (14.0%), respectively. There were no differences of proportion of HomEM, HetEM and PM between two eradication group. Detailed proportions according to genotype were showed in Table 2.
Table 2.
Frequencies of CYP2C19 Genotypes in the Eradication Groups
HomEM, homozygous extensive metabolizer; HetEM, heterozygous extensive metabolizer; PM, poor metabollizer; wt, wild type; m1, CYP2C19 mutation in exon 5; m2, CYP2C19 mutation in exon 4.
The eradication rates of LAC and RAC were 79.1% and 71.2%, respectively (p=0.050). The eradication rates of HomEM, HetEM and PM were 71.3%, 76.7% and 79.3%, respectively (Table 3). The difference of eradication rate according to CYP2C19 polymorphism was not significant (p=0.311). The eradication rates of LAC or RAC according to the CYP2C19 polymorphism were showed in Table 3. The statistical differences were not shown between LAC and RAC according to each subgroup, that is, HomEM, HetEM and PM.
Table 3.
Eradication Rates in Each Regimen according to CYP2C19 Genetic Polymorphisms
Numbers in parentheses represent the number cured/total number.
HomEM, homozygous extensive metabolizer; HetEM, heterozygous extensive metabolizer; PM, poor metabolizer.
*p-values of each row are derived from the comparisons of each CYP2C19 genetic polymorphism group; †p-values of each column are derived from the comparisons of each lansoprazole and rab.
DISCUSSION
Many other studies that investigate the effect of CYP2C19 polymorphism on H. pylori eradication rate in various PPIs had heterogeneous therapeutic schedules, especially with dose of each PPIs and therapeutic regimen. In addition, most studies have small sample size under 100. Because of these reasons, two meta-analyses16,17 were tried recently but had controversial results. For example, one study concluded that the influence of eradication regimen including lansoparzole was related with CYP2C19 polymorphism but the other did not. Our study was aimed for overcoming confounding factors such as treatment duration, dosage of regimen and antimicrobial resistance with the largest sized single center trial. Moreover we performed direct comparison between lansoprazole and rabeprazole which was known to be unaffected by CYP2C19 genetic polymorphism. Our study showed no statistical differences in the eradication rate of H. pylori according to CYP2C19 genetic polymorphism in LAC. This result is different with Furuta's one. In Furuta's study10 which was designed with lansoprazole or omeprazole, amoxicillin, clarithromycin for 7 days, eradication rate was different according to CYP2C19 genetic polymorphism. This study was designed in largest scale ever (n=130), but individual therapy arms are combined. Our LAC has larger sample size (n=234) and therefore may have more statistical power. Other possible cause is interethnic difference in CYP2C19 polymorphism. Approximately 2-6% of Caucasians and 1% of African-Americans have been identified as PMs, whereas the frequency of PMs in Japanese (19-23%) is much higher.8,18-20 The frequency of PMs in Korean (13%)21 is lower than that in Japanese.
Rabeprazole, as we above mentioned, provides reliable control of gastric acid secretion, with more potent anti-secretory activity than that in other PPIs such as omeprazole and lansoprazole,22 a more rapid rise in intragastric pH,23 and less effect of CYP2C19 on its metabolism.24-28 In Japan, standard rabeprazole dose is 10 mg14 and most Japanese articles uses 10 mg b.i.d. for H. pylori eradication. We used rabeprazole 20 mg as standard dose likewise in North America and Europe.29,30 No differences among CYP2C19 genetic polymorphism in our RAC are compatible with other previous study results.24-28
In the direct comparison of LAC and RAC according to genotypic difference, the eradication rate of LAC in each genotypic polymorphism is statistically similar to that of RAC. The studies about pharmacokinetics and pharmacodynamics of both PPIs are like below; Mean 24-hour pH and percentage time for pH>4 were not significantly different between lansoprazole 30 mg and rabeprazole 20 mg in 72 healthy volunteers.31 The median values of the 24-hour percent time for pH>4 of HomEM were 59% in 20 mg rabeprazole and 56% in 30 mg lansoprazole.32 There were 3 clinical studies25,26,28 that directly compared H. pylori eradication rates of lansoprazole and rabeprazole according to CYP2C19 polymorphism. Although these studies had relatively small sample size and insufficient randomization, they all reported no significant differences of eradication rate between lansoprazole and rabeprazole group according to each CYP2C19 polymorphism. Our large scale study could consolidate the result that lansoprazole as well as rabeprazole were not influenced by CYP2C19 polymorphism.
Our result showed tendency toward higher eradication rates in LAC than RAC in all CYP2C19 subtypes. This tendency might be resulted from co-administration of clarithromycin. Clarithromycin is mainly metabolized by CYP3A4 and potent inhibitor of CYP3A4. Unlike rabeprazole, which is metabolized mainly via nonenzymatic pathway, lansoprazole is metabolized by CYP3A4 as well as CYP2C19. Therefore drug interaction occurs between lansoprazole and clarithromycin more than rabeprazole and clarithromycin. As a result, the plasma concentration of lansoprazole is increased.9,33
The limitation of our study is that there is no consideration of the antibiotics resistance. Although single center trial would control this limitation to some degree, further study needs resistance profile. Another limitation is relative small sample size of study, although our study is the largest study ever. If enough subjects have included, increasing tendency of eradication rate of LAC according to the CYP2C19 polymorphysm (74.1%, 80.6%, and 85.4%) could have been meaningful in statistically. However too many subjects per group (over 200 in our study's situation) would be needed, even in PM.
In conclusion, triple therapy for H. pylori eradication using lansoprazole or rabeprazole is not affected by CYP2C19 polymorphisms. Our findings would be helpful to design a clinical strategy according to ethnicity and geographic region.
ACKNOWLEDGEMENTS
None of the authors received any financial support for this study and no conflicts of interest are declared.
References
- 1.NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 2.Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:1–12. doi: 10.1111/j.1440-1746.1998.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 3.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson T, Regardh CG, Dahl-Puustinen ML, Bertilsson L. Slow omeprazole metabolizers are also poor S-mephenytoin hydroxylators. Ther Drug Monit. 1990;12:415–416. doi: 10.1097/00007691-199007000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Andersson T, Regardh CG, Lou YC, Zhang Y, Dahl ML, Bertilsson L. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics. 1992;2:25–31. doi: 10.1097/00008571-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Sohn DR, Kobayashi K, Chiba K, Lee KH, Shin SG, Ishizaki T. Disposition kinetics and metabolism of omeprazole in extensive and poor metabolizers of S-mephenytoin 4'-hydroxylation recruited from an Oriental population. J Pharmacol Exp Ther. 1992;262:1195–1202. [PubMed] [Google Scholar]
- 8.Kubota T, Chiba K, Ishizaki T. Genotyping of S-mephenytoin 4'-hydroxylation in an extended Japanese population. Clin Pharmacol Ther. 1996;60:661–666. doi: 10.1016/S0009-9236(96)90214-3. [DOI] [PubMed] [Google Scholar]
- 9.Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;(13 Suppl 3):27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 10.Furuta T, Shirai N, Takashima M, et al. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001;69:158–168. doi: 10.1067/mcp.2001.113959. [DOI] [PubMed] [Google Scholar]
- 11.Take S, Mizuno M, Ishiki K, et al. Interleukin-1beta genetic polymorphism influences the effect of cytochrome P 2C19 genotype on the cure rate of 1-week triple therapy for Helicobacter pylori infection. Am J Gastroenterol. 2003;98:2403–2408. doi: 10.1111/j.1572-0241.2003.07707.x. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto M, Furuta T, Shirai N, Ikuma M, Hishida A, Ishizaki T. Influences of proinflammatory and anti-inflammatory cytokine polymorphisms on eradication rates of clarithromycin-sensitive strains of Helicobacter pylori by triple therapy. Clin Pharmacol Ther. 2006;80:41–50. doi: 10.1016/j.clpt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007;81:521–528. doi: 10.1038/sj.clpt.6100043. [DOI] [PubMed] [Google Scholar]
- 14.Kuwayama H, Asaka M, Sugiyama T, et al. Rabeprazole-based eradication therapy for Helicobacter pylori: a largescale study in Japan. Aliment Pharmacol Ther. 2007;25:1105–1113. doi: 10.1111/j.1365-2036.2007.03298.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang JC, Yang YF, Uang YS, Lin CJ, Wang TH. Pharmacokinetic-pharmacodynamic analysis of the role of CYP2C19 genotypes in short-term rabeprazole-based triple therapy against Helicobacter pylori. Br J Clin Pharmacol. 2009;67:503–510. doi: 10.1111/j.1365-2125.2009.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. Am J Gastroenterol. 2006;101:1467–1475. doi: 10.1111/j.1572-0241.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhao F, Wang J, Yang Y, et al. Effect of CYP2C19 genetic polymorphisms on the efficacy of proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: a meta-analysis. Helicobacter. 2008;13:532–541. doi: 10.1111/j.1523-5378.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- 18.Kupfer A, Preisig R. Pharmacogenetics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur J Clin Pharmacol. 1984;26:753–759. doi: 10.1007/BF00541938. [DOI] [PubMed] [Google Scholar]
- 19.Wedlund PJ, Aslanian WS, McAllister CB, Wilkinson GR, Branch RA. Mephenytoin hydroxylation deficiency in Caucasians: frequency of a new oxidative drug metabolism polymorphism. Clin Pharmacol Ther. 1984;36:773–780. doi: 10.1038/clpt.1984.256. [DOI] [PubMed] [Google Scholar]
- 20.Horai Y, Nakano M, Ishizaki T, et al. Metoprolol and mephenytoin oxidation polymorphisms in Far Eastern Oriental subjects: Japanese versus mainland Chinese. Clin Pharmacol Ther. 1989;46:198–207. doi: 10.1038/clpt.1989.126. [DOI] [PubMed] [Google Scholar]
- 21.Sohn DR, Kusaka M, Ishizaki T, et al. Incidence of S-mephenytoin hydroxylation deficiency in a Korean population and the interphenotypic differences in diazepam pharmacokinetics. Clin Pharmacol Ther. 1992;52:160–169. doi: 10.1038/clpt.1992.125. [DOI] [PubMed] [Google Scholar]
- 22.Pantoflickova D, Dorta G, Ravic M, Jornod P, Blum AL. Acid inhibition on the first day of dosing: comparison of four proton pump inhibitors. Aliment Pharmacol Ther. 2003;17:1507–1514. doi: 10.1046/j.1365-2036.2003.01496.x. [DOI] [PubMed] [Google Scholar]
- 23.Saitoh T, Fukushima Y, Otsuka H, et al. Effects of rabeprazole, lansoprazole and omeprazole on intragastric pH in CYP2C19 extensive metabolizers. Aliment Pharmacol Ther. 2002;16:1811–1817. doi: 10.1046/j.1365-2036.2002.01348.x. [DOI] [PubMed] [Google Scholar]
- 24.Dojo M, Azuma T, Saito T, Ohtani M, Muramatsu A, Kuriyama M. Effects of CYP2C19 gene polymorphism on cure rates for Helicobacter pylori infection by triple therapy with proton pump inhibitor (omeprazole or rabeprazole), amoxycillin and clarithromycin in Japan. Dig Liver Dis. 2001;33:671–675. doi: 10.1016/s1590-8658(01)80043-8. [DOI] [PubMed] [Google Scholar]
- 25.Inaba T, Mizuno M, Kawai K, et al. Randomized open trial for comparison of proton pump inhibitors in triple therapy for Helicobacter pylori infection in relation to CYP2C19 genotype. J Gastroenterol Hepatol. 2002;17:748–753. doi: 10.1046/j.1440-1746.2002.02790.x. [DOI] [PubMed] [Google Scholar]
- 26.Kawabata H, Habu Y, Tomioka H, et al. Effect of different proton pump inhibitors, differences in CYP2C19 genotype and antibiotic resistance on the eradication rate of Helicobacter pylori infection by a 1-week regimen of proton pump inhibitor, amoxicillin and clarithromycin. Aliment Pharmacol Ther. 2003;17:259–264. doi: 10.1046/j.1365-2036.2003.01406.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee SB, Park SJ, Ryu JK, et al. Efficacy of triple therapy with rabeprazole for Helicobacter pylori infection in relation to CYP2C19 genotype. Korean J Gastroenterol. 2003;42:468–475. [PubMed] [Google Scholar]
- 28.Miki I, Aoyama N, Sakai T, et al. Impact of clarithromycin resistance and CYP2C19 genetic polymorphism on treatment efficacy of Helicobacter pylori infection with lansoprazole- or rabeprazole-based triple therapy in Japan. Eur J Gastroenterol Hepatol. 2003;15:27–33. doi: 10.1097/00042737-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Hawkey CJ, Atherton JC, Treichel HC, Thjodleifsson B, Ravic M. Safety and efficacy of 7-day rabeprazole- and omeprazole-based triple therapy regimens for the eradication of Helicobacter pylori in patients with documented peptic ulcer disease. Aliment Pharmacol Ther. 2003;17:1065–1074. doi: 10.1046/j.1365-2036.2003.01492.x. [DOI] [PubMed] [Google Scholar]
- 30.Vakil N, Lanza F, Schwartz H, Barth J. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20:99–107. doi: 10.1111/j.1365-2036.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 31.Tolman KG, Taubel J, Warrington S, Chiu YL, Pilmer BL, Pan WJ. Comparison of the effects of single and repeated oral doses of lansoprazole and rabeprazole on ambulatory 24-hour intragastric pH in healthy volunteers. Clin Drug Investig. 2006;26:21–28. doi: 10.2165/00044011-200626010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Shimatani T, Inoue M, Kuroiwa T, et al. Acid-suppressive effects of rabeprazole, omeprazole, and lansoprazole at reduced and standard doses: a crossover comparative study in homozygous extensive metabolizers of cytochrome P450 2C19. Clin Pharmacol Ther. 2006;79:144–152. doi: 10.1016/j.clpt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Miura M, Tada H, Yasui-Furukori N, et al. Effect of clarithromycin on the enantioselective disposition of lansoprazole in relation to CYP2C19 genotypes. Chirality. 2005;17:338–344. doi: 10.1002/chir.20159. [DOI] [PubMed] [Google Scholar]