Abstract
Malignant fibrous histiocytoma (MFH) is an uncommon soft-tissue sarcoma that occurs primarily in the extremities and rarely involves the retroperitoneum and abdomen. A 63-year-old man was admitted to the emergency room because of epigastric pain. Computed tomography revealed a large heterogeneous enhanced mass originating from the omentum with hemoperitoneum. The patient underwent laparoscopic omental mass excision and hematoma evacuation. Histological examination of the resected tumor revealed MFH. This case was therefore omental MFH presenting with hemoperitoneum.
Keywords: Malignant fibrous histiocytoma, Omentum, Hemoperitoneum
INTRODUCTION
Malignant fibrous histiocytoma (MFH) represents the most common soft tissue sarcoma in adults, involving the extremities and less commonly the trunk, retroperitoneum and other sites.1,2 The prevalence of intra-peritoneal lesion among the all MFH was 5-10%. Although intra-tumoral bleeding of MFH was found in 5%, only a few MFH present as intra-peritoneal bleeding.1,3 We report a rare case of omental MFH presenting with hemoperitoneum, in a 63-year-old men.
CASE REPORT
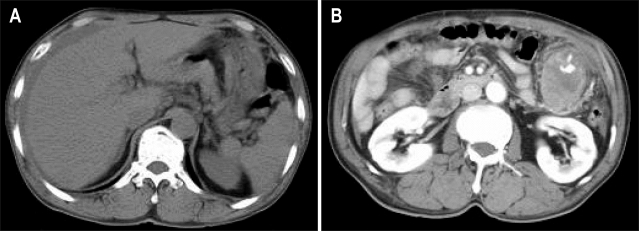
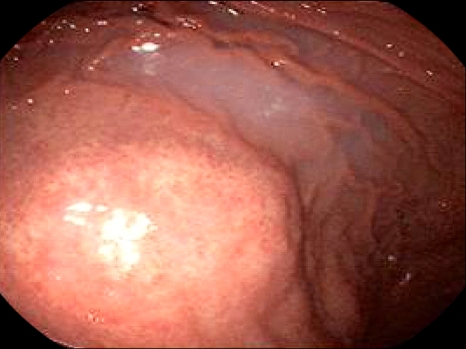
A 63-year-old man was admitted to the emergency department because of epigastric pain for 1 day. The pain was dull and constant. Physical examination revealed a huge, firm palpable mass in the left upper quadrant of abdomen. Blood pressure was 90/60 mm Hg and pulse rate was 96 beats/min. In CBC, hemoglobin was 12 g/dL, WBC was 13.5×103/mm3, CRP was 215 mg/L, Blood chemistry was normal limit. CEA, AFP and CA19-9 were normal limits. Pre-enhanced computed tomography (CT) of the abdomen and pelvis shows a high attenuated fluid density within the peritoneal cavity (Fig. 1A). Post-enhanced CT shows a huge solid mass with enhancing vascular structure in the omentum of left upper quadrant (Fig. 1B). The colonoscopic finding was normal. Esophagogastroduodenoscopy revealed external compression at the greater curvature side of midbody of stomach (Fig. 2).
Fig. 1.
Abdominal CT findings. (A) Pre-enhanced CT revealing the collection of a high-attenuation fluid within the perihepatic space. (B) Postenhanced CT revealing a solid mass of about 5.5 cm with enhanced vascular structure in the left upper quadrant omentum.
Fig. 2.
Esophagogastroduodenoscopy revealed external compression at the greater curvature site of the midbody of the stomach.
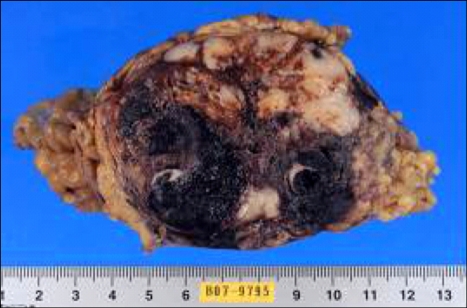
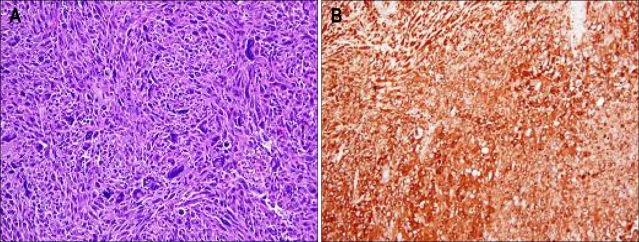
The patient underwent laparoscopic omental mass excision and hematoma evacuation. Gross pathological examination shows a 9×7×7 cm, 179 g oval shape mass with black brownish color (Fig. 3). Pathology of the excised specimen revealed a storiform architectural arrangement and consisted of spindle cells and histiocyte-like cells (Fig. 4A). Immunohistochemical stains were positive for alpha 1-antichymotrypsin, vimentin and negative for c-Kit, CD34, desmin, smooth muscle actin, S-100 protein, panCK, EMA, calretinin (Fig. 4B). The final histopathologic diagnosis was MFH that was originate from omentum. There was no evidence of distant metastasis.
Fig. 3.
Gross pathological examination revealing a 9×7×7-cm oval mass weighing 179 g with a cystic necrotic portion.
Fig. 4.
(A) Histologic examination revealing a storiform architectural arrangement and consisting of spindle cells and histiocyte-like cells (H&E stain, ×200). (B) Tumor cell showing positive immunostaining for alpha 1-antichymotrypsin (×200).
The patient refused further treatment with chemotherapy or radiotherapy. The patients is well condition and no evidence of local recurrence or distant metastasis at 12 months after the operation.
DISCUSSION
MFH is a common soft tissue sarcoma, usually occurring in the seventh decades of life.1 It usually arises 50% in the lower limb, 24% in the upper limbs, 16% in the trunk and 9% in retroperitoneum.2 Primary solid omental tumors are rare, and various including leiomyosarcoma, hemangiocytoma, fibrosarcoma, leiomyoma, liposarcoma, desmoid tumor, fibroma, mesothelioma, fibroma, and myosarcoma.4 Among the primary omental tumors, MFH was reported only three cases in English literature.5-7 But primary omental MFH complicated with hemoperitoneum has not been reported.
When the MFH occurs in the abdomen or retroperitoneum, patient complains of abdominal distension, varicocele and hernia.1 The symptoms indicating aggressive lesion were abdominal fullness, pain, palpable mass, general fatigue or progressive weight loss.8 In the present case, there was no symptom which suggested aggressive lesion, except epigastric pain.
MFH can develop variable degrees of tumor hemorrhage either spontaneously or as a result of response to chemotherapy.1,3 Approximately 5% of MFH cases demonstrate intra-tumoral hemorrhage in a necrotic space that may be confused with hematoma or cystic tumor.9,10 The most of all cases of abdominal MFH with extra-tumoral bleeding were metastatic lesions involved alimentary tract causing gastrointestinal bleeding.11-13 Recently, there was a report of subhepatic peritoneal MFH not only experienced intra-tumoral hemorrhage but also developed tumor rupture with extra-tumoral bleeding into the peritoneal cavity, mimicking ruptured hepatocellular carcinoma.14 Also, there were four reports which presented with hemoperitoneum by primary solid omental tumor,15-18 but primary omental MFH complicated with hemoperitoneum has not been reported. In the present case, large amount of bloody ascites is also associated with intra-tumoral hemorrhage and hyperevascularity of omental MHF.
CT findings of MFH consist of well circumscribed mass with variable hypodense areas owing to hemorrhagic necrosis exhibits peripheral solid enhanced component with intra-lesional hypodense areas of myxoid change, hemorrhage or necrosis.19
MFH is microscopically characterized by areas of spindle cells arranged in a storiform pattern, and pleomorphic areas with haphazardly arranged sheets of fibroblasts and histiocytes. The diagnosis of MFH depends on an accurate differential diagnosis from other sarcomas. The differential diagnosis includes gastrointestinal stromal tumor, fibrosarcoma, leiomyosarcoma, and myxoid sarcoma. MFH frequently expresses vimentin, actin, alpha 1-antichymotrypsin and CD 68, which were useful to differential diagnosis.2 The vimentin and alpha 1-antichymotrypsin were positive in the present case.
The mainstay of the therapy for MFH is completely surgical resection with en block regional lymph node dissection. Complete omentectomy for omental MFH appears to be the most appropriate treatment considering the aggressive biologic behavior of the malignant tumor.20 The efficacy of radiation therapy is well established in the treatment of MFH of extremities,21 but the effect of adjuvant radiotherapy to omental MFH is unclear. Doxorubicin is the first treatment option for unresectable or partially resected soft tissue sarcoma,22,23 but palliative or intensified chemotherapy to MFH have failed to survival benefit.24,25
Most MFH are shown regionally invasive nature, distant metastasis may spread via blood vessel or lymphatics. The prognosis of MFH is poor that the 2-year survival rate of patients with MFH is 60% and the rate of metastases is 42%.1
In conclusion, we report a case of primary omental MFH complicated with hemoperitoneum which was treated by laparoscopic resection. If the patients have omental solid tumor with hemoperitoneum, omental MFH should be considered as a rare differential diagnosis.
References
- 1.Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250–2266. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Kearney MM, Soule EH, Ivins JC. Malifnant fibrous histiocytoma: a retrospective study of 167 cases. Cancer. 1980;45:167–178. doi: 10.1002/1097-0142(19800101)45:1<167::aid-cncr2820450127>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Panicek DM, Casper ES, Brennan MF, Hajdu SI, Heelan RT. Hemorrhage simulating tumor growth in malignant fibrous histiocytoma at MR imaging. Radiology. 1991;181:398–400. doi: 10.1148/radiology.181.2.1656483. [DOI] [PubMed] [Google Scholar]
- 4.Sompayrac SW, Mindelzun RE, Silverman PM, Sze R. The greater omentum. AJR Am J Roentgenol. 1997;168:683–687. doi: 10.2214/ajr.168.3.9057515. [DOI] [PubMed] [Google Scholar]
- 5.Kim OH, Lee KY. Malignant fibrous histiocytoma of primary omental origin in an infant. Pediatr Radiol. 1994;24:285–287. doi: 10.1007/BF02015460. [DOI] [PubMed] [Google Scholar]
- 6.Shimazaki M, Yamada Y, Nagaki M, et al. A case of malignant fibrous histiocytoma with primary site in greater omentum. Nippon Shokakibyo Gakkai Zasshi. 1989;86:275–279. [PubMed] [Google Scholar]
- 7.Schwartz RW, Reames M, McGrath PC, Letton RW, Appleby G, Kenady DE. Primary solid neoplasms of the greater omentum. Surgery. 1991;109:543–549. [PubMed] [Google Scholar]
- 8.Ding GH, Wu MC, Yang JH, et al. Primary hepatic malignant fibrous histiocytoma mimicking cystadenocarcinoma: a case report. Hepatobiliary Pancreat Dis Int. 2006;5:620–623. [PubMed] [Google Scholar]
- 9.Ko SF, Ng SH, Lin JW, Lee TY. Cystic malignant fibrous histiocytoma of the mesovarium. AJR Am J Roentgenol. 2001;176:549–550. doi: 10.2214/ajr.176.2.1760549a. [DOI] [PubMed] [Google Scholar]
- 10.Huang CC, Ko SF, Ng SH, et al. Cystic malignant fibrous histiocytoma of the gastrocolic ligament. Br J Radiol. 2001;74:651–653. doi: 10.1259/bjr.74.883.740651. [DOI] [PubMed] [Google Scholar]
- 11.Blaauwwiekel EE, Koopal S, Kreeftenberg HG. Metastasized malignant fibrous histiocytoma as a cause of gastrointestinal tract haemorrhage. Scand J Gastroenterol. 1997;32:1272–1274. doi: 10.3109/00365529709028159. [DOI] [PubMed] [Google Scholar]
- 12.Adams HW, Adkins JR, Rehak EM. Malignant fibrous histiocytoma presenting as a bleeding gastric ulcer. Am J Gastroenterol. 1983;78:212–213. [PubMed] [Google Scholar]
- 13.Santoro MJ, Abdulian JD, Chase RL, Griffin RA, Solinger MR, Collen MJ. Malignant fibrous histiocytoma metastatic to the colon presenting as a lower gastrointestinal bleed. Am J Gastroenterol. 1992;87:1051–1053. [PubMed] [Google Scholar]
- 14.Chen HC, Chen CJ, Jeng CM, Yang CM. Malignant fibrous histiocytoma presenting as hemoperitoneum mimicking hepatocellular carcinoma rupture. World J Gastroenterol. 2007;13:6441–6443. doi: 10.3748/wjg.v13.i47.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patriti A, Rondelli F, Gulla N, Donini A. Laparoscopic treatment of a solitary fibrous tumor of the greater omentum presenting as spontaneous haemoperitoneum. Ann Ital Chir. 2006;77:351–353. [PubMed] [Google Scholar]
- 16.Crusco F, Chiodi M, Pugliese F, Mosca S, Fischer MJ, Lupattelli L. Benign omental hemangiopericytoma presenting with hemoperitoneum: radiologic findings. AJR Am J Roentgenol. 2005;184(suppl 3):S67–S69. doi: 10.2214/ajr.184.3_supplement.01840s67. [DOI] [PubMed] [Google Scholar]
- 17.Ritossa C, Ferri M, Destefano I, De Giuli P. Hemoperitoneum caused by cavernous angioma of the omentum. Minerva Chir. 1989;44:907–908. [PubMed] [Google Scholar]
- 18.Ishida H, Ishida J. Primary tumours of the greater omentum. Eur Radiol. 1998;8:1598–1601. doi: 10.1007/s003300050594. [DOI] [PubMed] [Google Scholar]
- 19.Dai J, Shi M, Li G. Computed tomographic features of malignant fibrous histiocytoma. Zhonghua Zhong Liu Za Zhi. 1996;18:140–142. [PubMed] [Google Scholar]
- 20.Aggarwal S, Haldar S, Kumar A, Kumar S, Guleria S, Dawar R. Primary malignant fibrous histiocytoma of the greater omentum. Trop Gastroenterol. 2002;23:193–194. [PubMed] [Google Scholar]
- 21.Peiper M, Zurakowski D, Knoefel WT, Izbicki JR. Malignant fibrous histiocytoma of the extremities and trunk: an institutional review. Surgery. 2004;135:59–66. doi: 10.1016/s0039-6060(03)00325-8. [DOI] [PubMed] [Google Scholar]
- 22.Sleijfer S, Seynaeve C, Verweij J. Using single-agent therapy in adult patients with advanced soft tissue sarcoma can still be considered standard care. Oncologist. 2005;10:833–841. doi: 10.1634/theoncologist.10-10-833. [DOI] [PubMed] [Google Scholar]
- 23.Karavasilis V, Seddon BM, Ashley S, Al-Muderis O, Fisher C, Judson I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112:1585–1591. doi: 10.1002/cncr.23332. [DOI] [PubMed] [Google Scholar]
- 24.Le Cesne A, Judson I, Crowther D, et al. Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: a trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18:2676–2684. doi: 10.1200/JCO.2000.18.14.2676. [DOI] [PubMed] [Google Scholar]
- 25.Edmonson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–1275. doi: 10.1200/JCO.1993.11.7.1269. [DOI] [PubMed] [Google Scholar]