Abstract
An undifferentiated (embryonal) liver sarcoma (ULS) originates from a primitive mesenchymal cell, with a predilection for childhood and very rare occurrence in adults. We report a case of a ULS that was incidentally found in a 53-year-old female. Our case was initially interpreted as a large hydatid cyst, which was later suspected to be a neoplastic lesion because its size was increasing and a solid portion was newly detected after shrinkage of the cyst following drainage. The patient underwent successful right hepatic lobectomy with complete resection, and is currently disease-free without adjuvant therapy. Although it is difficult to diagnose a hepatic cyst as a ULS due to its rare occurrence in adulthood and lack of specific findings, its possibility should be considered, especially when its size is increasing, because early diagnosis and curative resection are necessary for a favorable outcome.
Keywords: Embryonal sarcoma, Undifferentiated sarcoma, Liver cyst, Hydatid cyst, Adult
INTRODUCTION
An undifferentiated (embryonal) liver sarcoma (ULS), originating from a hepatic mesenchymal cell, is mainly a malignancy in children and rare in adulthood.1 In the age over 50, only about 10 cases have been reported in the literature.2 The prognosis of ULS is known to be poor due to delayed diagnosis caused by the lack of initial symptoms, rapid tumor growth, and early invasion to adjacent tissue. However, the outcome of treatment has recently improved with the development of new modalities such as primary surgical resection followed by chemotherapy. The typical radiologic finding is a large, septated mass with combined cystic and solid portions. Such findings, however, can also be seen in other liver diseases, among which a hydatid cyst was described as a uni- or multilocular cystic lesion with septation on ultrasonography and computed tomography (CT). Therefore, a careful approach may be needed for the differential diagnosis of a cystic liver mass. We herein report the case of ULS that resembled a hydatid cyst in a 53-year-old female.
CASE REPORT
A 53-year-old female was referred to our hospital in October 2008 for an atypical hepatic cyst detected incidentally on ultrasonography during a regular check-up. Her past medical and family histories were unremarkable. She was asymptomatic and there were no abnormal findings on physical examination. The patient had a normal complete blood count, blood chemistry, and chest radiography. Hepatitis viral markers including HBsAg and Anti-HCV were negative. Serum alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) levels were normal. She had no travel history in the past 5 years.
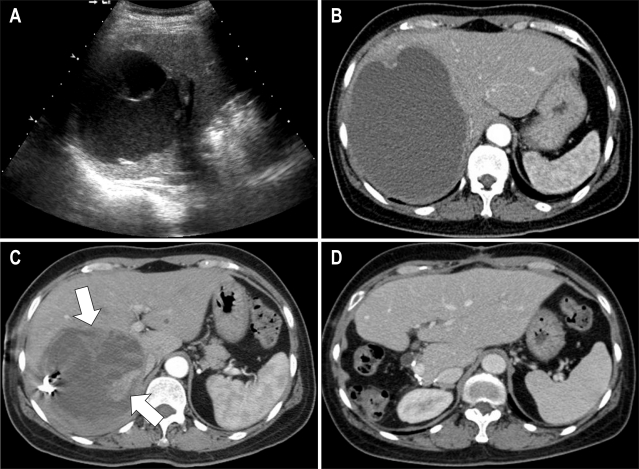
Abdominal ultrasonography showed an 8 cm cystic lesion with septation and echogenic material in the right lobe of the liver, which raised the suspicion of a hydatid cyst (Fig. 1A). CT scan performed 2 months later revealed a hepatic cyst with fine internal septum and slightly thickened peripheral wall with an increased diameter (from 8 to 13 cm) compared to the previous ultrasonography (Fig. 1B). Percutaneous drainage of the hepatic cyst was carefully performed because of its rapid growth and accompanying abdominal discomfort. The drained fluid was bloody, of which bacterial culture and cytology were negative. On follow-up CT taken 2 months later, the size of the cyst decreased from 13 to 7 cm, but a solid lesion mimicking granulation tissue was newly detected on the medial side of the cystic wall, which showed enhancement during the arterial phase (Fig. 1C). A neoplastic cyst rather than simple cyst was suggested at this time, and we therefore decided to proceed with surgical resection.
Fig. 1.
Undifferentiated liver sarcoma in a 53-year-old woman. (A) Ultrasonography showes an 8 cm cystic lesion with septation and echogenic material in the right lobe of the liver. (B) CT scan after 2 months reveales a hepatic cyst with a diameter that increases from 8 to 13 cm with slightly thickened periphery. (C) On follow-up CT performed 2 months after drainage, a solid lesion (arrow) mimicking granulation tissue is newly detected on the medial side of the cystic wall. (D) CT scan obtained 6 months after the operation showes no evidence of recurrence.
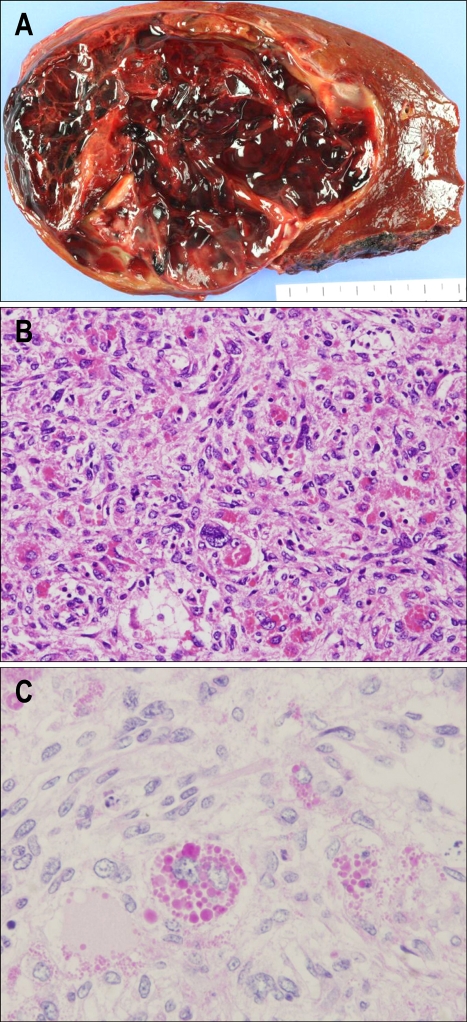
Right hepatic lobectomy was successfully performed. The gross specimen revealed a 1,030 g mass with a well-defined multilobulated cyst filled with blood clots measuring 13×12×8 cm (Fig. 2A). Histopathologic review showed malignant spindle cells with pleomorphic nuclei and frequent mitotic figures (Fig. 2B). Immunohistochemical stain revealed periodic acid-Schiff (PAS) and CD68 were positive (Fig. 2C), which are the markers for cytoplasmic granules in large tumor cells. In addition, CD34, CD31, and factor VIII were positive, which are the markers for vascular endothelial cells, whereas cytokeratin (CK) was negative. Based on these findings, the pathologic diagnosis was ULS. The postoperative period was uneventful. She did not receive adjuvant chemotherapy and there was no recurrence 6 months after resection (Fig. 1D).
Fig. 2.
Gross and microscopic findings. (A) The gross specimen with a well-defined multilobulated cyst filled with blood clots. (B) Atypical spindle cells with pleomorphic nuclei and frequent mitotic figures (H&E stain, ×200). (C) Large stellate cells containing eosinophilic cytoplasmic granules positive for PAS (PAS, ×400).
DISCUSSION
ULS is a rare and highly malignant neoplasm of mesenchymal origin without histologic differentiation. ULS was first documented as a clinicopathologic entity in 1978.1 Before this report, ULS had been referred to by multiple nomenclatures, including mesenchymoma, primary sarcoma of the liver, fibromyxosarcoma, and malignant mesenchymoma.
ULS has a predilection for the pediatric population between the ages of 6 and 10 years. Its incidence in adults over the age of 40 years is exceptionally low;2 only 10 cases of ULS in the sixth decade have been reported. ULS is found more frequently in the right lobe of the liver (59%) than the left lobe (22%), but sometimes simultaneously in bilateral lobes (20%).3
Patients with ULS usually present with abdominal pain or a palpable abdominal mass. Other symptoms include weight loss, anorexia, nausea, vomiting, or jaundice and because of non-specific symptoms, it could be misdiagnosed as acute appendicitis.4 To the best of our knowledge, however, there has been no report regarding ULS that was incidentally found during a regular health check-up as in the case of our patient.
The typical finding of ULS in ultrasonography is a single large, solid echogenic mass with a cystic portion.5 CT findings showed a well-circumscribed huge cystic or solid mass with a few internal septations and a dense peripheral rim corresponding to the fibrous pseudocapsule, which is hypodense in the pre-contrast phase and slightly enhanced in the contrast phase.5,6 Occasionally, it is difficult to differentiate ULS from a hydatid cyst due to the predominant cystic nature with little solid component.6 There were 2 cases in the literature of ULS first interpreted as a hydatid cyst due to similar reasons that were diagnosed in childhood and removed surgically as initial treatment.6,7 In contrast, our case presented in adulthood and was treated with percutaneous drainage for the hydatid cyst, followed by surgical resection due to the suspicion of malignancy.
In our patient, the rationale for drainage was a rapid increment in size from 8 to 13 cm in 2 months, producing mass effect with abdominal discomfort. In the past, percutaneous drainage of a hepatic hydatid cyst was considered inappropriate because it might cause anaphylatic shock from rupture of the cyst and dissemination into the peritoneal cavity.8 In the last 20 years, however, percutaneous catheterization was demonstrated to be a safe, effective, and reliable method of treatment of a hepatic hydatid cyst with a rare occurrence of the complications mentioned above.9-13 Candidates for this procedure include patients with a prominent cyst with hypoechogenic components without calcification, active infection, and biliary rupture.11 Our case satisfied these conditions, and percutaneous drainage was safely performed.
Notably, the significant solid portion was not detected on the initial imaging study but was revealed after drainage of the fluid within the cystic mass. The reason for the delayed detection was probably that the large amount of fluid exerted volume effects to flatten the solid lesion in the periphery, which became pronounced only after shrinkage of the cystic portion. At the moment, a neoplastic cyst was suggested rather than a hydatid cyst, and the operation was performed thereafter. Therefore, it is necessary to consider the possibility of hidden malignancy when a large cystic lesion with septation that is increasing in size is present in the liver.
In the gross finding, ULS consists of solid, gray-white tumor tissue with red and yellow areas of hemorrhage and necrosis.2 Histologic findings reveal undifferentiated mesenchymal cells with hyperchromatic and variable sized nuclei and a fibrotic pseudocapsule that invades to adjacent liver parenchyma. Generally in ULS, immunohistochemical and special stains show positivity for CD68, α-1-antitrypsin (AAT), α-1-antichymotrypsin (AACT), and vimentin.14 PAS can be positive when there are eosinophilic granules in large stellate cells.15 In a few case studies, positivity to S-100 protein CK and neuron-specific enolase (NSE) has also been reported. Immunostaining for factor VIII is mostly negative within the tumor cell, but can be positive when endothelial cells are abundantly proliferated. In addition, the entrapped biliary epithelium may express strong positivity for CK.16 Our case revealed positivity for PAS and CD68 expressed in large tumor cells and for CD34, CD31, and factor VIII expressed in vascular endothelial cells. On the other hand, it was negative for CK and borderline for AACT. Collectively, we could interpret our case as a cystic mass compatible with ULS that did not have biliary elements but endothelial elements.
It has been known that the clinical outcome of ULS is poor.1 Although operation is the standard treatment for resectable ULS, there is to date no standard care for advanced cases. In the case of unresectable ULS, neoadjuvant treatment followed by radical resection have been suggested for better prognosis.17 For an advanced case with extrahepatic extension and portal vein occlusion, it was reported that curative resection was possible after aggressive treatment with a multimodality strategy including neoadjuvant chemotherapy and radiotherapy, which enabled the anticipation of long-term disease-free survival.18 In addition, better survival was reported with intensive adjuvant therapy, even when there was residual tumor after radical resection.19 In our case, the patient did not receive additional chemotherapy or radiotherapy because of complete resection. Currently, she is being followed up at the outpatient clinic without recurrence 6 months after resection.
In conclusion, we hereby report a case of a 53-year-old female with ULS that was incidentally found during a health check-up. The cystic lesion was initially suspected as a hydatid cyst, which was later suspected for malignancy because it showed a rapid increment in size and solid portion was detected after drainage of the cyst. Although it is difficult to diagnose a hepatic cyst as ULS due to its rare occurrence in adulthood and lack of specific findings, we should at least suspect the possibility, especially when its size is increasing, because early diagnosis and curative resection are necessary for a favorable outcome.
References
- 1.Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:336–348. doi: 10.1002/1097-0142(197807)42:1<336::aid-cncr2820420151>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Psatha EA, Semelka RC, Fordham L, Firat Z, Woosley JT. Undifferentiated (embryonal) sarcoma of the liver (USL): MRI findings including dynamic gadolinium enhancement. Magn Reson Imaging. 2004;22:897–900. doi: 10.1016/j.mri.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Pachera S, Nishio H, Takahashi Y, et al. Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J Hepatobiliary Pancreat Surg. 2008;15:536–544. doi: 10.1007/s00534-007-1265-y. [DOI] [PubMed] [Google Scholar]
- 4.Sakellaridis T, Panagiotou I, Georgantas T, Micros G, Rontogianni D, Antiochos C. Undifferentiated embryonal sarcoma of the liver mimicking acute appendicitis. Case report and review of the literature. World J Surg Oncol. 2006;4:9. doi: 10.1186/1477-7819-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon WK, Kim WS, Kim IO, et al. Undifferentiated embryonal sarcoma of the liver: US and CT findings. Pediatr Radiol. 1994;24:500–503. doi: 10.1007/BF02015012. [DOI] [PubMed] [Google Scholar]
- 6.Joshi SW, Merchant NH, Jambhekar NA. Primary multilocular cystic undifferentiated (embryonal) sarcoma of the liver in childhood resembling hydatid cyst of the liver. Br J Radiol. 1997;70:314–316. doi: 10.1259/bjr.70.831.9166061. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal S, Guleria S, Dinda AK, Kumar L, Tarique S. Embryonal sarcoma of the liver mimicking a hydatid cyst in an adult. Trop Gastroenterol. 2001;22:141–142. [PubMed] [Google Scholar]
- 8.Buetow PC, Buck JL, Pantongrag-Brown L, et al. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology. 1997;203:779–783. doi: 10.1148/radiology.203.3.9169704. [DOI] [PubMed] [Google Scholar]
- 9.Mueller PR, Dawson SL, Ferrucci JT, Jr, Nardi GL. Hepatic echinococcal cyst: successful percutaneous drainage. Radiology. 1985;155:627–628. doi: 10.1148/radiology.155.3.3890001. [DOI] [PubMed] [Google Scholar]
- 10.Akhan O, Ozmen MN, Dincer A, Sayek I, Gocmen A. Liver hydatid disease: long-term results of percutaneous treatment. Radiology. 1996;198:259–264. doi: 10.1148/radiology.198.1.8539390. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Informal Working Group on Echinococcosis. Puncture, aspiration, injection, re-aspiration: an option for the treatment of cystic echinococcosis. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 12.Gabal AM, Khawaja FI, Mohammad GA. Modified PAIR technique for percutaneous treatment of high-risk hydatid cysts. Cardiovasc Intervent Radiol. 2005;28:200–208. doi: 10.1007/pl00021047. [DOI] [PubMed] [Google Scholar]
- 13.Voros D, Katsarelias D, Polymeneas G, et al. Treatment of hydatid liver disease. Surg Infect (Larchmt) 2007;8:621–627. doi: 10.1089/sur.2006.0070. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Liu YP, Geng CZ, Tian ZH, Wu GX, Wang XL. Undifferentiated embryonal sarcoma of liver in an old female: case report and review of the literature. World J Gastroenterol. 2008;14:7267–7270. doi: 10.3748/wjg.14.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker NI, Horn MJ, Strong RW, et al. Undifferentiated (embryonal) sarcoma of the liver. Pathologic findings and long-term survival after complete surgical resection. Cancer. 1992;69:52–59. doi: 10.1002/1097-0142(19920101)69:1<52::aid-cncr2820690111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Lack EE, Schloo BL, Azumi N, Travis WD, Grier HE, Kozakewich HP. Undifferentiated (embryonal) sarcoma of the liver. Clinical and pathologic study of 16 cases with emphasis on immunohistochemical features. Am J Surg Pathol. 1991;15:1–16. [PubMed] [Google Scholar]
- 17.Kim DY, Kim KH, Jung SE, Lee SC, Park KW, Kim WK. Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg. 2002;37:1419–1423. doi: 10.1053/jpsu.2002.35404. [DOI] [PubMed] [Google Scholar]
- 18.McFadden DW, Kelley DJ, Sigmund DA, Barrett WL, Dickson B, Aron BS. Embryonal sarcoma of the liver in an adult treated with preoperative chemotherapy, radiation therapy, and hepatic lobectomy. Cancer. 1992;69:39–44. doi: 10.1002/1097-0142(19920101)69:1<39::aid-cncr2820690109>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Newman KD, Schisgall R, Reaman G, Guzzetta PC. Malignant mesenchymoma of the liver in children. J Pediatr Surg. 1989;24:781–783. doi: 10.1016/s0022-3468(89)80536-6. [DOI] [PubMed] [Google Scholar]