Abstract
Reactivation of hepatitis B virus (HBV) replication is a frequent phenomenon in patients receiving immunosuppressants or chemotherapy. It was recently reported that regional therapy, such as transarterial chemotherapy (TAC) or radiotherapy, can also induce HBV reactivation in patients with hepatocellular carcinoma (HCC), and this can be prevented by preemptive lamivudine treatment. We report an unusual case of fatal hepatitis caused by reactivation of the tyrosine-methionine-aspartate-aspartate (YMDD) lamivudine-resistant strain in a 51-year-old male patient with HCC who was receiving preemptive lamivudine therapy. This patient received combined helical tomotherapy and TAC for the treatment of HCC with pulmonary metastasis. HBV reactivation and hepatitis exacerbation occurred after 2 months of therapy, but preemptive antiviral therapy was continued. Laboratory tests showed that the serum HBV DNA level had increased by more than 10,000-fold and a severe elevation of the aminotransferase level to 1,060 U/L. Although adefovir was added to lamivudine immediately after detecting the YMDD mutants, the patient eventually died of hepatic failure. Our experience suggests that for preemptive therapy, the use of potent antiviral drugs with a low risk of drug resistance as well as close viral monitoring are important for chronic HBV carriers undergoing intensive anticancer therapy.
Keywords: Hepatocellular carcinoma, Viral reactivation, Hepatic failure, Radiotherapy, Transarterial chemotherapy
INTRODUCTION
Hepatitis B virus (HBV) reactivation is a well-known complication in cancer patients who receive cytotoxic chemotherapy. In many cases, the diagnosis of hepatocellular carcinoma (HCC) is made at a more advanced stage, precluding curative treatments such as resection or transplantation. Alternatively, transarterial chemotherapy (TAC) and/or radiotherapy that potentially alter host immunity are often used in treating HCC patients. Recently, several studies have shown that TAC and/or radiotherapy may play some role in inducing viral reactivation in patients with HBV-related HCC,1-3 and lamivudine is useful in reducing the risk of hepatic complications associated with HBV reactivation during therapy.3-5 The current practice guidelines recommend that prophylactic antiviral therapy should be initiated before the start of anticancer chemotherapy for all chronic HBV carriers.6,7
Lamivudine is the most well-studied drug used in prophylaxis against the risk of HBV reactivation during chemotherapy. One of the major drawbacks of its use is the emergence of resistant mutants in the tyrosine-methionine-aspartate-aspartate (YMDD) motif of HBV.8-10 Because the existence of the YMDD mutants may reduce the benefit obtained with lamivudine, but usually not negate it within the initial period of therapy, fatal liver failure associated with the mutants is not commonly observed.11-13 In fact, no case of fatal reactivation hepatitis caused by YMDD mutants has ever been documented in HCC patients on preemptive lamivudine therapy.
In this paper, we report the unusual case of a deceased patient with HBV-related HCC who, despite preemptive lamivudine therapy, developed fulminant hepatic failure resulting from HBV reactivation related to YMDD mutants after radiotherapy and TAC for the treatment of HCC.
CASE REPORT
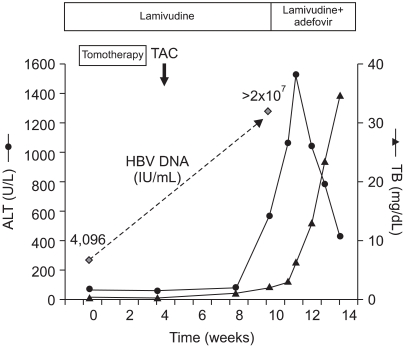
In January 2007, a 51-year-old male patient was referred to our liver unit for the further treatment of HCC. He was diagnosed with HBV-related HCC in March 2005, and 8 cycles of TAC had been performed in another hospital. Pulmonary metastasis was detected 15 months after the diagnosis of HCC, and progressed despite repeated cycles of systemic chemotherapy. As an antiviral therapy, lamivudine was started preemptively on clinical indications in October 2005, when there was no evidence of HBV reactivation. At the time of initial visit to our hospital, laboratory findings showed the following values: albumin, 3.8 g/dL (normal, 3.8-5.1 g/dL); alanine aminotransferase (ALT), 77 U/L (normal, 5-40 U/L); aspartate aminotransferase (AST), 68 U/L (normal, 8-40 U/L); total bilirubin (TB), 0.7 mg/dL (normal, 0.2-1.2 mg/dL); prothrombin time (PT) INR, 0.97; and creatinine, 0.6 mg/dL (normal, 0.6-1.3 mg/dL). Viral markers of HBV showed a positive hepatitis B e antigen (HBeAg), a negative antibody to HBeAg, and the serum HBV DNA level of 4,096 IU/mL. Alpha fetoprotein (AFP) was 244,090 ng/mL. Multidetector computed tomography (MDCT) revealed large viable tumors at segment 4 and a daughter nodule at segment 5 with left portal vein tumor thrombosis (Fig. 1). Helical tomotherapy was done for simultaneous treatment of an intrahepatic mass and metastatic pulmonary nodules, with a total dose of 45 Gy in 10 fractions. Two weeks after tomotherapy, TAC using epirubicin 50 mg and cisplatin 60 mg was performed. At that time, his condition was good and all liver function tests were within the normal ranges, except a mild elevation in the ALT level (65 U/L). On 13 March 2007, about 6 weeks after the last cycle of TAC, he was admitted again for another cycle of TAC. He complained of general weakness and poor oral intake. Biochemical measures included a markedly elevation of aminotransferase levels (AST/ALT, 697/564 U/L), PT INR 1.16, and TB 2.2 mg/dL. Follow-up MDCT (Fig. 2) showed the large HCC without a significant interval change in tumor extent, but accompanied with central necrosis, probably due to the radiation effect and partially lipiodol-uptaken tumor areas. Left portal vein thrombosis had slightly regressed. The AFP level decreased to 54,730 ng/mL. We assessed the tumor response as stable disease state. He had never discontinued taking lamivudine, and denied both alcohol consumption and taking herbal medicines. Laboratory tests on admission day 3 showed a further elevation of the ALT level (up to 1,060 U/L), and a marked increase in HBV DNA levels from 4,096 to >2×107 IU/mL. HBV DNA gene sequencing identified the substitution of isoleucine for methionine at residue 204 (M204I) in the YMDD motif, and then, adefovir dipivoxil (10 mg/day) was immediately added to lamivudine on 16 March 2007. Despite adefovir rescue, the aminotransferase levels were persistently high, and bilirubin levels continued to increase up to 38 mg/dL (Fig. 3). His liver function gradually worsened along with the development of ascites and encephalopathy. Unfortunately, he died from hepatic failure on day 40 of admission, despite the best supportive management.
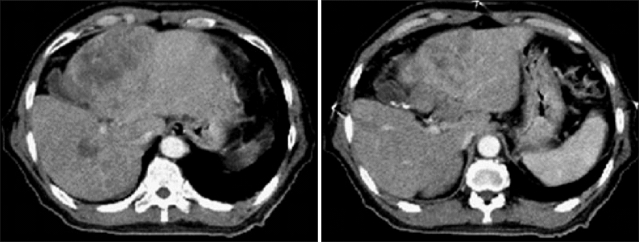
Fig. 1.
Multidetector CT images obtained at the initial visit in our hospital. The large tumor at segment 4 and a daughter nodule at segment 5 of the liver are shown. The left portal vein is filled with low-density material suspected to be tumor thrombi.
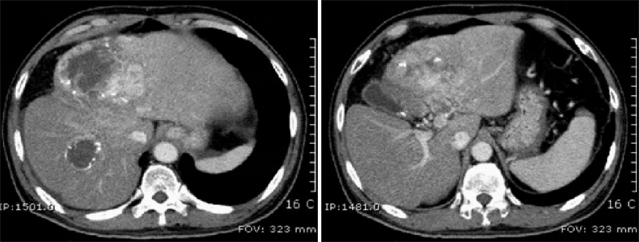
Fig. 2.
Multidetector CT images obtained 8 weeks after tomotherapy. The main mass does not differ from that seen at the initial visit. A daughter nodule at segment 5 shows an area of central necrosis, probably reflecting a radiation effect. The left portal vein thrombosis have slightly regressed.
Fig. 3.
Clinical course of the patient before and after hepatitis B virus (HBV) reactivation.
ALT, alanine aminotransferase; TB, total bilirubin.
DISCUSSION
With the recent introduction of more intensive anticancer therapy protocols and HBV DNA testing with a high sensitivity, reactivation of HBV replication is becoming a more frequent phenomenon in current oncologic practice. Therefore, physicians' awareness of this potentially life-threatening event and better understanding of the clinical parameters related to HBV reactivation during anticancer therapy are of great importance.
Our patient developed severe hepatitis due to HBV reactivation after one session of radiotherapy and TAC. Virologic tests checked at 8-weekly intervals showed a greater than 10,000-fold increase in serum HBV DNA level that eventually resulted in liver failure. This is probably one of the most significant viral increases that have ever occurred in reactivation hepatitis cases.1-3,14 Although it is unclear whether radiotherapy or TAC are responsible for such a significant viral reactivation, there is emerging evidence that HBV reactivation is often noted in HCC patients undergoing either chemotherapy or radiotherapy.1,2,14 Apart from viral factors, the intensity of anticancer therapy that induces host immunosuppression is also known to be another risk factor for HBV reactivation.15 In this respect, our combined chemo-radiotherapy might be a more intensive regimen that can adversely affect the patient immunity. One can speculate that both immunosuppression-enhanced viral replication during chemotherapy and a bystander mechanism related to cytokines such as interleukin-6 released during radiotherapy may have played an additive role in inducing such a severe reactivation in this particular case.15,16
It is worth noting again that our patient was on antiviral therapy, when viral reactivation occurred. Recently, many reports have shown evidence that preemptive lamivudine therapy, which can prevent the viral replication, reduces liver-related morbidity and mortality during cytotoxic chemotherapy.3-5 Despite lamivudine preemptive therapy, however, our patient had significant viral reactivation, and finally died. Adefovir rescue immediately added to lamivudine failed to reverse hepatic failure. Given the medical history of continued lamivudine for more than one year and the detectable HBV DNA level of 4,096 IU/mL before chemo-radiotherapy, the patient might have already harbored the YMDD variant at the time of referral to our hospital. In retrospect, it is possible that more meticulous examination of serum HBV DNA levels and YMDD variants, and timely antiviral rescue could have avoided such hepatic failure. Although a fatal liver failure is a rare event during preemptive lamivudine and rescue therapy, viral replication may be more augmented under intensive therapy that induces strongly immunosuppression of patients, sometimes leading to fatality, as in this case. Overall, this suggests that the use of more potent antiviral drugs and close monitoring are essential against the potential hepatic morbidity associated with drug resistance for patients receiving intensive cytotoxic therapy.
One concern about hepatic morbidity due to HBV reactivation in this case was a consideration of radiation-induced liver disease (RILD), which is often encountered after conventional radiotherapy for HCC. Although these two causes are often confounded, this case was differentiated from RILD as a definitive increase in viral load was followed by hepatic morbidity.2,15 In addition, helical tomotherapy which our patient received is a new image-guided intensity-modulated radiotherapy system that permits precisely more high-dose radiation to the target region, while sparing normal tissues.17,18 Thus, the involvement of RILD in this case might have been minimal or almost negligible.
In conclusion, there is the potential for hepatitis due to reactivation of drug resistant strains that possibly develop during preemptive antiviral therapy in chronic HBV carriers receiving cytotoxic therapy. This case suggests that the use of potent antiviral drugs with a low risk of drug resistance and meticulous viral monitoring are quite important for preemptive settings among chronic HBV carriers with advanced liver disease undergoing intensive anticancer therapy.
References
- 1.Jang JW, Choi JY, Bae SH, et al. Transarterial chemo-lipiodolization can reactivate hepatitis B virus replication in patients with hepatocellular carcinoma. J Hepatol. 2004;41:427–435. doi: 10.1016/j.jhep.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:813–819. doi: 10.1016/j.ijrobp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43:233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 4.Lau GK, Yiu HH, Fong DY, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Lim LL, Wai CT, Lee YM, et al. Prophylactic lamivudine prevents hepatitis B reactivation in chemotherapy patients. Aliment Pharmacol Ther. 2002;16:1939–1944. doi: 10.1046/j.1365-2036.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Leung N, Guan R, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25:472–489. doi: 10.1111/j.1478-3231.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 8.Atkins M, Gray DF. Lamivudine resistance in chronic hepatitis B. J Hepatol. 1998;28:169. doi: 10.1016/s0168-8278(98)80219-5. [DOI] [PubMed] [Google Scholar]
- 9.Jardi R, Buti M, Rodriguez-Frias F, et al. Rapid detection of lamivudine-resistant hepatitis B virus polymerase gene variants. J Virol Methods. 1999;83:181–187. doi: 10.1016/s0166-0934(99)00125-1. [DOI] [PubMed] [Google Scholar]
- 10.Liaw YF. Management of patients with chronic hepatitis B. J Gastroenterol Hepatol. 2002;17:406–408. doi: 10.1046/j.1440-1746.2002.02736.x. [DOI] [PubMed] [Google Scholar]
- 11.Honkoop P, Niesters HG, de Man RA, Osterhaus AD, Schalm SW. Lamivudine resistance in immunocompetent chronic hepatitis B. Incidence and patterns. J Hepatol. 1997;26:1393–1395. doi: 10.1016/s0168-8278(97)80476-x. [DOI] [PubMed] [Google Scholar]
- 12.Lai CL, Chien RN, Leung NW, et al. Asia Hepatitis Lamivudine Study Group. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 13.Melegari M, Scaglioni PP, Wands JR. Hepatitis B virus mutants associated with 3TC and famciclovir administration are replication defective. Hepatology. 1998;27:628–633. doi: 10.1002/hep.510270243. [DOI] [PubMed] [Google Scholar]
- 14.Yeo W, Lam KC, Zee B, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15:1661–1666. doi: 10.1093/annonc/mdh430. [DOI] [PubMed] [Google Scholar]
- 15.Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209–220. doi: 10.1002/hep.21051. [DOI] [PubMed] [Google Scholar]
- 16.Chou CH, Chen PJ, Lee PH, Cheng AL, Hsu HC, Cheng JC. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin Cancer Res. 2007;13:851–857. doi: 10.1158/1078-0432.CCR-06-2459. [DOI] [PubMed] [Google Scholar]
- 17.Mackie TR, Balog J, Ruchala K, et al. Tomotherapy. Semin Radiat Oncol. 1999;9:108–117. doi: 10.1016/s1053-4296(99)80058-7. [DOI] [PubMed] [Google Scholar]
- 18.Hong TS, Welsh JS, Ritter MA, et al. Megavoltage computed tomography: an emerging tool for image-guided radiotherapy. Am J Clin Oncol. 2007;30:617–623. doi: 10.1097/COC.0b013e3180546770. [DOI] [PubMed] [Google Scholar]