Abstract
Turner's syndrome is a genetic disorder of the sex chromosomes (e.g., 45,X or 45,X/46,XX) that manifests as various congenital anomalies. Despite its numerous extragonadal manifestations and frequent accompanying abnormalities in liver function tests, liver cirrhosis associated with Turner's syndrome has not been reported in Korea. Moreover, pulmonary arteriovenous malformations (PAVMs) have rarely been reported in association with liver cirrhosis, but there have been no reports of PAVMs occurring in cryptogenic liver cirrhosis associated with Turner's syndrome. We report a case of PAVM that occurred in cryptogenic liver cirrhosis associated with Turner's syndrome.
Keywords: Liver cirrhosis, Turner's syndrome, Pulmonary arteriovenous malformation
INTRODUCTION
Cirrhosis of the liver (LC) is a chronic process involving continuous liver parenchymal destruction and fibrosis with regenerating nodules.1,2 Although most causes of LC can be identified, the cause in 10-20% of such patients cannot be elucidated, and are thus termed cryptogenic LC.1-3
Turner's syndrome is a genetic disorder of the sex chromosomes (e.g., 45,X or 45,X/46,XX). Despite the numerous extragonadal manifestations, including many forms of congenital cardiac anomalies4,5 and frequent association with abnormal liver function tests,6-10 there have been only a few reports worldwide and no reports in Korea regarding the association between LC and Turner's syndrome.11-14
Pulmonary arteriovenous malformations (PAVMs) are relatively rare, but the most common anomaly involving the pulmonary vascular tree. Greater than 80% of PAVMs are congenital and the remainder is acquired by various medical conditions.15 Although the rare correlation between LC and PAVM is well-known, there have been no reports of PAVMs occurring in cryptogenic LC associated with Turner's syndrome. We report a PAVM that occurred in cryptogenic liver cirrhosis associated with Turner's syndrome with a review of the literature.
CASE REPORT
A 37-year-old woman was admitted to Korea University Guro Hospital for evaluation and treatment of a cough, dyspnea, and two episodes of unexplained syncope. Several years before admission to our hospital, the patient had been admitted to another hospital for intractable variceal bleeding. The patient subsequently underwent splenectomy with a splenorenal shunt and liver biopsy, and was then diagnosed with liver cirrhosis based on the pathologic findings. The patient had no other illnesses and no remarkable family medical history. She denied any history of drug or alcohol abuse, unusual sexual habits, or transfusions.
On admission, her vital signs were within normal limits, and she was mentally alert, despite previous history of hepatic encephalopathy. Her height and weight were 140 cm and 38.4 kg. She had a short neck that was not webbed, and had no goiter. Her chest examination revealed a shielded chest, and on auscultation no murmurs were heard, but coarse breath sounds were heard in the right lower chest region. Mild distension and shifting dullness was noted on abdominal examination. Her extremities revealed no peripheral edema or finger clubbing, her arms exhibited cubitus valgus, and her 4th fingers and toes were abnormally short. Her external genitalia showed infantile characteristics. The results of a neurologic examination were normal.
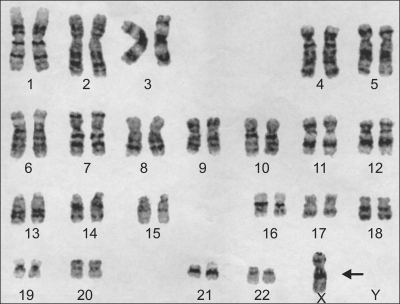
A basic laboratory assessment was performed. The white blood cell count was 8,900/mm3, the hemoglobin was 8.9 g/dL, and platelet count was 160,000/mm3. The results of the liver function tests were as follows: aspartate aminotransferase (AST) 44 IU/L; alanine aminotransferase (ALT) 22 IU/L; γ-glutamyl transpeptidase (γ-GTP) 43 IU/L; alkaline phosphatase (ALP) 142 IU/L; total bilirubin 1.59 mg/dL; direct bilirubin 0.91 mg/dL; total protein 6.38 g/dL; and albumin 2.26 g/dL. The prothrombin time was 66%. An attempt was made to define the etiology of the patient's liver cirrhosis. Chromosomal analysis revealed Turner's syndrome with a genotype of 45,X (Fig. 1). Serologic studies for hepatits B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), and hepatits C antibody (anti-HCV) were all negative. The serum iron level was 101 ug/dL, the TIBC was 266 ug/dL, the ferritin was 4.9 ng/mL, the copper was 108 ug/dL, and the ceruloplasmin was 22 mg/dL. The type and activity of α1 anti-trypsin was normal. The anti-nuclear antibody (FANA), anti-smooth muscle antibody, and anti-mitochondrial antibody titers were all negative. The results of arterial blood gas analysis in room air were as follows: pH, 7.527; CO2, 19.4 mm Hg; O2, 83 mm Hg; and O2, saturation 97%.
Fig. 1.
Chromosomal study of the patient. There is no Y chromosome and only one X chromosome (arrow), which is compatible with 45,X Turner's syndrome.
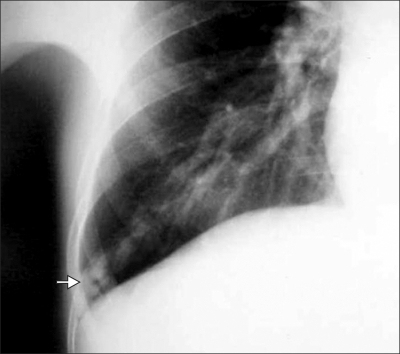
Her chest radiograph revealed prominent peripheral vascular markings on the right lower lung region (Fig. 2). On abdominal sonography, the findings were compatible with liver cirrhosis with a coarse liver echo and a moderate amount of ascites. She was decompensated liver cirrhosis with Child-Pugh calss C. A 12-lead conventional and ambulatory electrocardiogram showed inverted T waves in the precordial leads and no significant arrhythmias. A 2-D echocardiogram revealed no wall motion abnormalities, moderate aortic regurgitation, pulmonary hypertension (85 mm Hg), and an ejection fraction of 60%.
Fig. 2.
X-ray findings of the right lower lung. Prominent peripheral vascular marking with a feeding vessel is visible in the costophrenic angle of the right lower lobe (arrow).
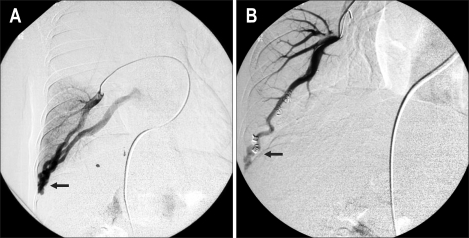
These findings suggested the possibility of pulmonary vascular disease. A pulmonary angiography was thus performed. An arteriovenous malformation of the pulmonary vasculature in the right lower lung region was identified, and was successfully treated with coil embolization (Fig. 3).
Fig. 3.
Pulmonary angiography. (A) A dilated peripheral vessel accompanying direct communication between the pulmonary artery and vein is visible (arrow). (B) This communication disappear after coil embolization (arrow).
The patient's symptoms were relieved, and she was subsequently discharged uneventfully. She had been followed up regularly until her death due to sepsis from spontaneous bacterial peritonitis.
DISCUSSION
With a phenotypic incidence of 1 in 2,500 live births, Turner's syndrome is the most common monosomal chromosomal anomaly which occurs in women. The main genotypic expression is loss or deletion of chromosome X, most commonly in the form of monosomy X (45,X). Mosaicism occurs, most commonly in the form of 45,X/46,XX. The clinical manifestations of Turner's syndrome are variable, such as primary amenorrhea, sexual infantilism, short stature, and other extragonadal anomalies, including many forms of congenital cardiac anomalies.4,5 Up to 80% of patients with Turner's syndrome have liver enzyme abnormalities due to hormone therapy or obesity, but the elevation of AST, ALT, r-GTP, and ALP generally do not reflect overt hepatic disorders and may be reversible.6-10 Although overt hepatic disorders in Turner's syndrome are very rare, Turner's syndrome and cryptogenic LC may have some linkage. Gravholt et al.6 reported a four-fold risk of LC in patients with Turner's syndrome and several case reports of cryptogenic LC associated with Turner's syndrome have been published.11-14 Although congenital vascular abnormalities in Turner's syndrome have been reported to be the pathogenesis of this unusual relationship,15 the exact mechanism is largely unknown.
The patient described herein had been diagnosed with LC through liver biopsy at another hospital. On the current admission, she was evaluated thoroughly in an effort to find a cause for her liver disease. Her laboratory findings for possible viral hepatitis, hemochromatosis, Wilson's disease, α1 anti-trypsin deficiency, and autoimmune disease were unrevealing. She had no history of alcohol or drug abuse, and no history of hormone replacement therapy, and had no evidence of DM or obesity. Failure to find an etiology for the patient's LC prompted a diagnosis of LC of cryptogenic origin associated with Turner's syndrome.
PAVMs are the most common anomalies of the pulmonary vascular tree. Since the first reported case in 1897, >500 cases have been reported. More than 80% of PAVMs are congenital, and of these, 47-80% are associated with Osler-Weber-Rendu disease. The causes of acquired PAVMs include chest trauma or surgery, long-standing hepatic cirrhosis, metastatic carcinoma, mitral stenosis, infections, and amyloidosis.16 In the case of LC, pulmonary arteriovenous abnormalities in liver disease were first described during a postmortem examination in 1966.17 Thereafter, numerous cases of pulmonary vascular dilatation associated with LC, the so-called hepatopulmonary syndrome (HPS), have been reported. The structural basis of HPS includes precapillary or capillary pulmonary vascular dilatations (type I) and discrete direct arteriovenous communications (type II).
Type II HPS is quite rare compared with type I HPS. Our case had type II HPS, which is a true anatomic PAVM. The angiographic distinction between these two types of vascular dilatations is of utmost importance since true PAVMs may be occluded with intra-arterial embolization.18
Turner's syndrome is associated with various cardiac and vascular anomalies4,5 and angiodysplasia or arteriovenous malformations within the gastrointestinal tract was reported in Turner's syndrome.19,20 Recently, Ostberg et al.21 proposed that women with Turner's syndrome have a fundamental arterial wall defect which may be due to genetic factors or estrogen deficiency. Therefore, PAVMs associated with Turner's syndrome should be considered and cannot be completely ruled out. A PAVM associated solely with Turner's syndrome has not yet been reported. Therefore, although PAVMs in LC are quite rare, the PAVM in the present case may have been associated with LC rather than Turner's syndrome.
The relationship among PAVMs, Turner's syndrome, and LC was not clear-cut in our patient. With all other possible etiologies ruled out, and in light of previous reports linking Turner's syndrome with LC5,11-14 and PAVMs with LC,4,17 we concluded that our patient had a PAVM that occurred in cryptogenic LC associated with Turner's syndrome. This case is of unique interest as it is the first reported case of a PAVM that occurred in cryptogenic LC associated with Turner's syndrome.
References
- 1.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 2.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395–414. doi: 10.1136/jcp.31.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saunders JB, Walters JR, Davies AP, Paton A. A 20-year prospective study of cirrhosis. Br Med J (Clin Res Ed) 1981;282:263–266. doi: 10.1136/bmj.282.6260.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med J. 2002;78:191–197. doi: 10.1136/pmj.78.918.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saenger P. Turner's syndrome. N Engl J Med. 1996;335:1749–1754. doi: 10.1056/NEJM199612053352307. [DOI] [PubMed] [Google Scholar]
- 6.Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol. 1998;51:147–158. doi: 10.1016/s0895-4356(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 7.Krivosheev AB. Development of liver cirrhosis in a female patient with Shereshevskii-Turner syndrome. Klin Med (Mosk) 1990;68:95–96. [PubMed] [Google Scholar]
- 8.Idilman R, De Maria N, Colantoni A, Kugelmas M, Van Thiel DH. Cirrhosis in Turner's syndrome: case report and literature review. Eur J Gastroenterol Hepatol. 2000;12:707–709. [PubMed] [Google Scholar]
- 9.Friedman E, Theodor E, Austein A, Sack J. Cirrhosis in Turner's syndrome. Harefuah. 1980;98:210–211. [PubMed] [Google Scholar]
- 10.Garavelli L, Donadio A, Banchini G, et al. Liver abnormalities and portal hypertension in Ullrich-Turner syndrome. Am J Med Genet. 1998;80:180–182. doi: 10.1002/(sici)1096-8628(19981102)80:2<180::aid-ajmg18>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Elsheikh M, Hodgson HJ, Wass JA, Conway GS. Hormone replacement therapy may improve hepatic function in women with Turner's syndrome. Clin Endocrinol (Oxf) 2001;55:227–231. doi: 10.1046/j.1365-2265.2001.01321.x. [DOI] [PubMed] [Google Scholar]
- 12.Salerno M, Di Maio S, Gasparini N, Rizzo M, Ferri P, Vajro P. Liver abnormalities in Turner syndrome. Eur J Pediatr. 1999;158:618–623. doi: 10.1007/s004310051163. [DOI] [PubMed] [Google Scholar]
- 13.Gravholt CH. Medical problems of adult Turner's syndrome. Horm Res. 2001;(56 Suppl 1):44–50. doi: 10.1159/000048134. [DOI] [PubMed] [Google Scholar]
- 14.Wemme H, Pohlenz J, Schonberger W. Effect of oestrogen/gestagen replacement therapy on liver enzymes in patients with Ullrich-Turner syndrome. Eur J Pediatr. 1995;154:807–810. doi: 10.1007/BF01959786. [DOI] [PubMed] [Google Scholar]
- 15.Roulot D, Degott C, Chazouilleres O, et al. Vascular involvement of the liver in Turner's syndrome. Hepatology. 2004;39:239–247. doi: 10.1002/hep.20026. [DOI] [PubMed] [Google Scholar]
- 16.Larizza D, Locatelli M, Vitali L, et al. Serum liver enzymes in Turner syndrome. Eur J Pediatr. 2000;159:143–148. doi: 10.1007/s004310050038. [DOI] [PubMed] [Google Scholar]
- 17.Berthelot P, Walker JG, Sherlock S, Reid L. Arterial changes in the lungs in cirrhosis of the liver: lung spider nevi. N Engl J Med. 1966;274:291–298. doi: 10.1056/NEJM196602102740601. [DOI] [PubMed] [Google Scholar]
- 18.Malagari K, Nikita A, Alexopoulou E, et al. Cirrhosis-related intrathoracic disease. Imaging features in 1038 patients. Hepatogastroenterology. 2005;52:558–562. [PubMed] [Google Scholar]
- 19.Reinhart WH, Mordasini C, Staubli M, Scheurer U. Abnormalities of gut vessels in Turner's syndrome. Postgrad Med J. 1983;59:122–124. doi: 10.1136/pgmj.59.688.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalle I, Geboes K. Vascular lesions of the gastrointestinal tract. Acta Gastroenterol Belg. 2002;65:213–219. [PubMed] [Google Scholar]
- 21.Ostberg JE, Donald AE, Halcox JP, Storry C, McCarthy C, Conway GS. Vasculopathy in Turner syndrome: arterial dilatation and intimal thickening without endothelial dysfunction. J Clin Endocrinol Metab. 2005;90:5161–5166. doi: 10.1210/jc.2005-0677. [DOI] [PubMed] [Google Scholar]