Abstract
A 32-year-old man presented with epigastric pain. He had a previous episode of acute pancreatitis of undetermined cause 2 years earlier. The patient had taken trimethoprim (80 mg) and sulfamethoxazole (400 mg) twice daily because of acute urethritis 3 days prior to admission. No definite cause of acute pancreatitis could be identified on baseline studies. A thorough history-taking revealed that the patient had an episode of acute pancreatitis while taking trimethoprim (80 mg) and sulfamethoxazole (400 mg) twice daily for 2 weeks for prostatitis prior to the previous admission. Therefore, a cause-and-effect relationship between trimethoprim-sulfamethoxazole (TMP-SMX) and repeated episodes of pancreatitis was highly suggested. The patient was presumably diagnosed as TMP-SMX-induced pancreatitis. The final diagnosis was TMP-SMX-induced pancreatitis. Since drugs are rare causes of acute pancreatitis and the diagnosis of drug-induced pancreatitis is difficult to establish, we report this interesting case along with a review of medical literature.
Keywords: Pancreatitis, Drug, Ttrimethoprim-sulfamethoxazole
INTRODUCTION
Drugs are rare causes of acute pancreatitis (AP). Confirmation of drug-induced pancreatitis (DIP) is difficult to establish.1 Some drugs including valproic acid and didanosine have been shown to cause AP, whereas some drugs have been considered as the cause of AP in cases where no other cause of AP could be found.1 A few cases of AP associated with trimethoprim-sulfamethoxazole (TMP-SMX), and only two cases of recurrent pancreatitis after re-exposure of TMP-SMX have been reported.2-6 We report a case of recurrent AP caused by re-exposure of TMP-SMX and reviewed the medical literature.
CASE REPORT
A 32-year old man presented with epigastric pain, radiating to the back area. The pain was steady and lasted for three days. The patient reported a previous episode of AP two years earlier with no cause identified; computed tomography (CT) and magnetic resonance cholagnio pancreatograpy were performed during the first episode. The patient reported being a social drinker of less than 50 g per week and had no family history of pancreatitis. He developed acute urethritis and had taken cefaclor 250 mg twice and trimethoprim 80 mg/sulfamethoxazole 400 mg twice a day for three days.
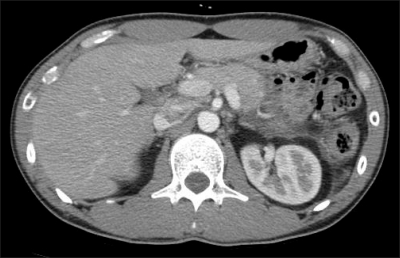
The serum amylase was elevated to 387 IU/L and serum lipase to 1,115 U/L. The liver function tests were normal. The serum calcium level was 9.0 mg/dL and triglyceride level was 32 mg/dL. The abdomen-pelvis CT scan showed pancreatic swelling and peripancreatic infiltration, suggesting acute pancreatitis CT grade C (Fig. 1). There was no evidence of cholelithiasis or gallbladder microlith on ultrasonography. The history revealed that that he had taken trimethoprim 80 mg/sulfamethoxazole 400 mg twice a day for two weeks prior to the previous admission because of prostatitis. A cause-and-effect relationship between TMP-SMX and repeated episodes of pancreatitis was highly suggested as the diagnosis and the patient was presumably diagnosed as a TMP-SMX-induced pancreatitis. The patient discontinued TMP-SMX. After conservative management of six days, the patient was discharged without sequelae.
Fig. 1.
Abdomen-pelvis CT scan. The pancreas is swollen and there is peripancreatic infiltration, which suggested acute pancreatitis at CT grade C.
DISCUSSION
Drugs are relatively rare causes of AP, accounting for an estimated 0.1-2%.7 Many drugs have been suspected as causing AP, but the diagnosis of DIP is difficult to confirm.1,7 Some drugs including valproic acid and didanosine have been shown to cause AP, whereas some drugs have been considered as a cause of AP when no other causes of AP have been found.1 Therefore, the true frequency of drug related AP is not known.7
The pathogenic mechanism associated with DIP is not determined yet. A hypersensitivity reaction has been suggested. It tends to occur four to eight weeks after the drug has been started and is not a dose-related phenomenon. On re-challenge with the drug, AP recurs within hours to days.8
The diagnosis of DIP is based on the exclusion of other possible causes of AP. It is important to take a thorough past medical history and drug history. Recently, one report updated the information on DIP and offered simple guidelines using a new classification based on the level of evidence.9 Based on the number of reported cases and re-exposure confirmation, drugs were classified as Class I, II or III drugs. Class I drugs have been associated with at least 20 reported cases of acute pancreatitis and at least 1 case of a positive re-challenge. Class II drugs have been associated with more than 10 reported cases of acute pancreatitis with or without cases with a positive re-challenge. Class III drugs refer to all medications implicated in pancreatitis. TMP-SMX has been classified to class I drug according to these guidelines. A literature search revealed two case reports of acute pancreatitis induced by TMP-SMX exposure. The first report described a case of concurrent TMP-SMX-induced pancreatitis and hepatitis.3 The patient had hepatitis only in the first episode and hepatitis as well as pancreatitis concurrently during the second episode.3 The other case report was recurrent AP that occurred twenty years after the first episode of AP and a causal relationship between the AP and TMP-SMX was demonstrated by recurrent symptoms induced by TMP-SMX re-exposure;6 in this report pancreatitis was not confirmed by imaging studies and the patient was not re-challenged with TMP-SMX for diagnositic purposes.6 Therefore, TMP-SMX might be better classified as a class II drug, rather than class I.
In our case, the first episode of DIP occurred 2 weeks after the exposure to TMP-SMX. On re-exposure with TMP-SMX, the second episode of DIP recurred within three days of drug exposure; discontinuation of TMP-SMX resulted in improvement of the AP. The patient presented with typical abdominal pain and elevated serum amylase and serum lipase as well as imaging (CT scan and ultrasonography) for both episodes of AP. However, the probable cause of pancreatitis could not be determined even after extensive studies during the second episode. The abdomen-pelvis CT and ultrasonography revealed no evidence of cholelithiasis or gallbladder microlith. In addition, other possible causes of acute pancreatitis were excluded. The final diagnosis was TMP-SMX induced acute pancreatitis on two occasions.
There has been no reported case of TMP-SMX-induced acute pancreatitis confirmed by diagnostic re-challenge with TMP-SMX. Re-challenge with TMP-SMX for diagnostic purposes has not been done in this case because of the risk associated with medication exposure. Thorough history-taking revealed the cause-and-effect relationship between TMP-SMX and repeated episodes of pancreatitis. This patient may represent the first case of TMP-SMX-induced pancreatitis, based on recurrent episodes of AP due to repeated exposures to TMP-SMX. In addition, this case illustrates the importance of taking an accurate and thorough medical history.
References
- 1.Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648–661. doi: 10.1016/j.cgh.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Antonow DR. Acute pancreatitis associated with trimethoprim-sulfamethoxazole. Ann Intern Med. 1986;104:363–365. doi: 10.7326/0003-4819-104-3-363. [DOI] [PubMed] [Google Scholar]
- 3.Brett AS, Shaw SV. Simultaneous pancreatitis and hepatitis associated with trimethoprim-sulfamethoxazole. Am J Gastroenterol. 1999;94:267–268. doi: 10.1111/j.1572-0241.1999.00812.x. [DOI] [PubMed] [Google Scholar]
- 4.Alberti-Flor JJ, Hernandez ME, Ferrer JP, Howell S, Jeffers L. Fulminant liver failure and pancreatitis associated with the use of sulfamethoxazole-trimethoprim. Am J Gastroenterol. 1989;84:1577–1579. [PubMed] [Google Scholar]
- 5.Bartels RH, van der Spek JA, Oosten HR. Acute pancreatitis due to sulfamethoxazole-trimethoprim. South Med J. 1992;85:1006–1007. doi: 10.1097/00007611-199210000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Versleijen MW, Naber AH, Riksen NP, Wanten GJ, Debruyne FM. Recurrent pancreatitis after trimethoprim-sulfamethoxazole rechallenge. Neth J Med. 2005;63:275–277. [PubMed] [Google Scholar]
- 7.Balani AR, Grendell JH. Drug-induced pancreatitis: incidence, management and prevention. Drug Saf. 2008;31:823–837. doi: 10.2165/00002018-200831100-00002. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg WM. Acute pancreatitis. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger & Fordtran's gastrointestinal and liver disease. 8th ed. Volume 1. Philadelphia: Saunders Elsevier; 2006. p. 1247. [Google Scholar]
- 9.Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;39:709–716. doi: 10.1097/01.mcg.0000173929.60115.b4. [DOI] [PubMed] [Google Scholar]