Abstract
Cures for advanced hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) are rare and difficult. We report a case of pathologically confirmed complete remission of HCC induced by hepatic arterial infusion chemotherapy (HAIC). A 45-year-old male patient had a massive HCC in the right lobe of the liver and tumor thrombus in the right and main portal veins. He achieved a partial response after two cycles of HAIC with 5-fluorouracil (750 mg/m2) and cisplatin (25 mg/m2). After the completion of six cycles he received a curative partial hepatectomy, and histopathology revealed complete necrosis without any viable tumor cell. He was in good health at a 4-month follow-up. These results suggest that this regimen is a promising therapeutic modality for the treatment of advanced HCC with PVTT.
Keywords: Hepatocellular carcinoma, Portal vein tumor thrombosis, Chemotherapy, 5-fluorouracil, Complete remission
INTRODUCTION
The prognosis of patients with advanced hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) is very poor, with the median survival less than six months.1 Therapeutic options for these patients are very limited and they are generally treated with hepatic arterial infusion chemotherapy (HAIC).2-5 Although HAIC has been reported as an effective therapeutic modality for advanced HCC, cures for advanced HCC with PVTT are rare and difficult.
In the review of published literatures with English language, some cases of complete remission (CR) by HAIC in patients with advanced HCC have been reported.2-5 However, most of them were radiological remission and not confirmed pathologically.
In this paper, we report a case of advanced HCC patients with main PVTT who had achieved pathologically confirmed CR by HAIC with high-dose 5-fluorouracil (5-FU) and cisplatin.
CASE REPORT
A 45-year-old man was admitted to Yeungnam University Hospital due to liver mass found by health screening. He was diagnosed as chronic hepatitis B three years ago but did not any further evaluation or treatment. Abdominal computed tomography (CT) and angiography showed a 10.0×10.5 cm sized mass in the right lobe of liver and tumor thrombus in right and main portal vein (Fig. 1A, B). Laboratory data were as follows: platelet count 235 K/uL, total bilirubin 1.0 mg/dL, aspartate aminotransferase (AST) 41 U/L, alanine aminotransferase (ALT) 14 U/L, albumin 4.4 g/dL, prothrombin time 100%, α-fetoprotein (AFP) 5,845 ng/mL, protein induced by vitamin K absence or antagonist II (PIVKA-II) >2,000 U/L, HBeAg positive, HBeAb negative, hepatitis B virus (HBV) DNA 4.5×106 copies/mL.
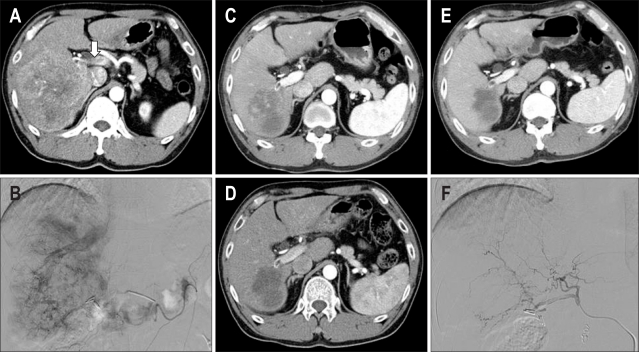
Fig. 1.
CT and hepatic arteriogram. (A, B) CT and angiogram showing a massive hepatocellular carcinoma in the right lobe of the liver and invasion of the main portal vein (arrow). (C, D) After the second and third cycles, the tumor mass is markedly decreased. (E, F) After the completion of six cycles, his serum α-fetoprotein level decreased to 2.8 ng/mL (from 5,845 ng/mL before treatment).
A port was inserted for hepatic arterial infusion chemotherapy. He was treated with 5-FU and cisplatin. The 5-FU 750 mg/m2 was diluted with 5% dextrose water and 200 mL was administered over two hours using a portable infusion pump from day 1 to day 4. Cisplatin 25 mg/m2 was diluted with normal saline and 200 mL was given over one hour using an intraarterial catheter from day 1 to day 4. The chemotherapy was repeated every four weeks.
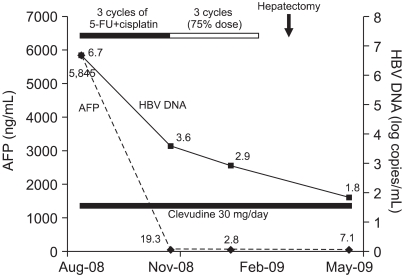
He also received antiviral therapy with clevudine 30 mg per day orally for the treatment of chronic hepatitis B. After 3 cycles of chemotherapy, CT showed a partial response and AFP decreased to 19 ng/mL (Fig. 1C, D). Although the dose was reduced to 75% of full dose because of mild bone marrow suppression since the 4th cycle, he was very tolerable (Fig. 2). After the completion of 6th cycle, his serum AFP was normalized to 2.8 ng/mL and he received a curative partial hepatectomy. The histopathology revealed complete necrosis without any viable tumor cell (Fig. 3). He is under follow-up with good health at 4 months after surgery.
Fig. 2.
Clinical courses. Serum α-fetoprotein (AFP) and hepatitis B virus (HBV) DNA are indicated by the dashed and solid lines, respectively.
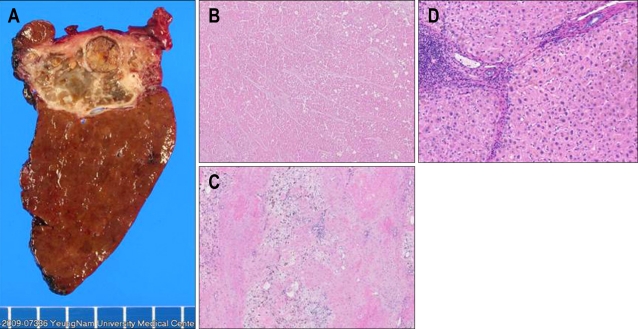
Fig. 3.
Pathology. A curative hepatectomy for segments 6 and 7 is performed. (A) Gross hepatectomy specimen showing necrotic liver mass. (B, C) Microscopic findings of the liver mass showing complete necrosis without any viable tumor cells (H&E stain, ×40). (D) Microscopic finding of the nontumor portion shows cirrhotic change (H&E stain, ×100).
DISCUSSION
The prognosis of patients with HCC and PVTT is extremely poor and therapeutic modalities are limited. The results of transcatheter arterial chemoembolization (TACE) have been unsatisfactory.6 Systemic chemotherapy is rarely effective.7 In addition, radiotherapy alone has limited clinical benefits for advanced HCC, and is usually combined with other modalities.8 Recently, sorafenib, an oral multikinase inhibitor, showed a gain in survival in patients with advanced HCC, but it did not guarantee long-term survival.9
HAIC administered via an injection port delivers repeated arterial infusions of anticancer agents that are mainly exposed to the liver, and systemic adverse effects minimized. The tumor response with HAIC has been reported to be superior to TACE or supportive care.10 Therefore, HAIC might be a reasonable approach to the treatment of patients with advanced HCC.
Although HAIC using low-dose 5-FU and cisplatin has been reported to be a useful treatment regimen, rationales for low-dose 5-FU were not described clearly in the previous studies. Several reasons are speculated. Chemotherapy via hepatic artery can be effective as low-dose than systemic infusion, so regional chemotherapy has a therapeutic benefit in terms of dose of drug. Therefore, low-dose 5-FU might have an effect for HAIC. In addition, rapid infusion of high-dose 5-FU decreases the therapeutic effect by Collin's formula.11 From the review of literatures, response rates of low-dose 5-FU (170-250 mg/m2) were superior to high-dose 5-FU (500-1,000 mg/m2).12-14 However, studies regarding high-dose 5-FU are very limited, and furthermore, randomized controlled trials comparing between high-dose and low-dose 5-FU are lacking.
Urabe et al.3 reported 46.7% of response rate using combination of methotrexate, high-dose 5-FU, cisplatin and subcutaneous IFNα. Based on this report, we have been treating advanced HCC patients with PVTT with high-dose 5-FU, cisplatin with or without subcutaneous IFNα since 2003. As the result, we experienced good tumor response rate (57.1%, 12/21) in patients treated with HAIC with high-dose 5-FU plus cisplatin.15
Cures or pathological CRs for advanced HCCs by a single modality are rare and difficult, even though the current case showed pathological CR in response to treatment. Therefore, a curative surgical resection after tumor regression by HAIC may be a successful therapeutic approach for advanced HCC.
In this case, it was interesting to note that the tumor showed pathologically complete necrosis, even though there was a partial response radiologically. This calls into question the accuracy of the sizing of current CT measurements as the gold standard for response assessment. We predicted that the patient would have a positive outcome with AFP normalization and loss of the vascularity on the CT, even though the tumor size did not decrease after four cycles of chemotherapy.
Response assessments have been based on tumor size measured on the CT. Recently, however, Riaz et al.16 reported the importance of the AFP response as a reliable predictor of the radiological response, progression, and survival. He suggested that the AFP response might be used in patients that have a radiological response or stable disease to further predict outcomes. In addition, if there is no AFP response then the treating physician can consider additional or alternate treatments.
Another important measure of response is vascularity.17 Loss of vascularity indicates tumor necrosis. Because HCC is a hypervascular tumor, recurrence after local treatment is associated with AFP elevation and the reappearance of a hypervascular mass. Reduction or loss of vascularity after chemotherapy might be an important measure of patient response to treatment.
Therefore, the AFP response and vascularity, as well as tumor size, should be considered when assessing patients for a response to treatment.
Here we reported a case with a pathological CR after HAIC using high-dose 5-FU and cisplatin in advanced HCC with PVTT. The results suggest that this regimen might be a promising therapeutic modality for the treatment of advanced HCC.
References
- 1.Fujii T, Takayasu K, Muramatsu Y, et al. Hepatocellular carcinoma with portal tumor thrombus: analysis of factors determining prognosis. Jpn J Clin Oncol. 1993;23:105–109. [PubMed] [Google Scholar]
- 2.Ando E, Tanaka M, Yamashita F, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 3.Urabe T, Kaneko S, Matsushita E, Unoura M, Kobayashi K. Clinical pilot study of intrahepatic arterial chemotherapy with methotrexate, 5-fluorouracil, cisplatin and subcutaneous interferon-alpha-2b for patients with locally advanced hepatocellular carcinoma. Oncology. 1998;55:39–47. doi: 10.1159/000011833. [DOI] [PubMed] [Google Scholar]
- 4.Takaki-Hamabe S, Yamasaki T, Saeki I, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: is the addition of subcutaneous interferon-alpha-2b beneficial? Hepatol Res. 2009;39:223–230. doi: 10.1111/j.1872-034X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- 5.Obi S, Yoshida H, Toune R, et al. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990–1997. doi: 10.1002/cncr.21832. [DOI] [PubMed] [Google Scholar]
- 6.Okada S. Transcatheter arterial embolization for advanced hepatocellular carcinoma: the controversy continues. Hepatology. 1998;27:1743–1744. doi: 10.1002/hep.510270639. [DOI] [PubMed] [Google Scholar]
- 7.Leung TW, Johnson PJ. Systemic therapy for hepatocellular carcinoma. Semin Oncol. 2001;28:514–520. doi: 10.1016/s0093-7754(01)90144-7. [DOI] [PubMed] [Google Scholar]
- 8.Aebersold DM. Potential and future strategies for radiotherapy in hepatocellular carcinoma. Liver Int. 2009;29:145–146. doi: 10.1111/j.1478-3231.2008.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 10.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol. 1984;2:498–504. doi: 10.1200/JCO.1984.2.5.498. [DOI] [PubMed] [Google Scholar]
- 12.Toyoda H, Nakano S, Kumada T, et al. The efficacy of continuous local arterial infusion of 5-fluorouracil and cisplatin through an implanted reservoir for severe advanced hepatocellular carcinoma. Oncology. 1995;52:295–299. doi: 10.1159/000227477. [DOI] [PubMed] [Google Scholar]
- 13.Hamada A, Yamakado K, Nakatsuka A, Takaki H, Akeboshi M, Takeda K. Hepatic arterial infusion chemotherapy with use of an implanted port system in patients with advanced hepatocellular carcinoma: prognostic factors. J Vasc Interv Radiol. 2004;15:835–841. doi: 10.1097/01.RVI.0000128815.35555.0E. [DOI] [PubMed] [Google Scholar]
- 14.Itamoto T, Nakahara H, Tashiro H, et al. Hepatic arterial infusion of 5-fluorouracil and cisplatin for unresectable or recurrent hepatocellular carcinoma with tumor thrombus of the portal vein. J Surg Oncol. 2002;80:143–148. doi: 10.1002/jso.10116. [DOI] [PubMed] [Google Scholar]
- 15.Eun JR, Lee HJ, Moon HJ, Kim TN, Kim JW, Jang JC. Hepatic arterial infusion chemotherapy using high-dose 5-fluorouracil and cisplatin with or without interferon-a for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis. Scand J Gastroenterol. 2009;44:1477–1486. doi: 10.3109/00365520903367262. [DOI] [PubMed] [Google Scholar]
- 16.Riaz A, Ryu RK, Kulik LM, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734–5742. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 17.Sparchez Z, Radu P, Anton O, Socaciu M, Badea R. Contrast enhanced ultrasound in assessing therapeutic response in ablative treatments of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2009;18:243–248. [PubMed] [Google Scholar]