Abstract
Lynch syndrome is the most common familial colorectal cancer syndrome. It is linked to germline mutations in one of four DNA mismatch repair (MMR) genes. A comprehensive family history is one important way to identify at-risk individuals. The elucidation of the molecular genetics of this syndrome has made it possible to screen for the disorder with molecular tests. Microsatellite instability and/or immunohistochemistry followed by germline testing for mutations in MMR genes is now a standard approach for clinically suspected cases. Correctly recognizing Lynch syndrome is essential for the application of appropriate screening and surveillance measures. Close surveillance and risk-reducing operations can decrease cancer-related mortality. In addition, counseling is an important component of the management of any family with Lynch syndrome.
Keywords: Lynch syndrome, Mismatch repair gene, Microsatellite instability, Immunohistochemistry, Hereditary nonpolyposis colon cancer, Colon cancer
INTRODUCTION
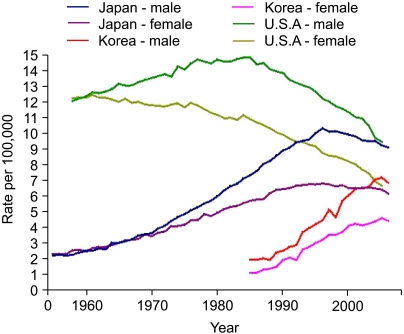
Colorectal cancer is the second leading cause of cancer-related death in the United States, with approximately 140,000 cases diagnosed and 50,000 deaths annually. Recently, an increasing incidence has also been observed in many Asian countries. In Korea, there were 19,570 new cases diagnosed in 2006, making it the 3rd most common cancer diagnosed. That year, there were 6,277 deaths attributable to colon cancer, making it the 4th leading cause of cancer-related deaths in Korea (Fig. 1).
Fig. 1.
Age-standardized mortalities from colon cancer in USA, Japan and Korea. The Age-standardized mortalities from colon cancer in the USA show decreases since the mid 1980's in both sexes. In Japan, there have also been decreases in mortality from colon cancer since the mid 1990's. However, rates are continuing to increase in South Korea (Base line data from Ref. 42).
The majority of colorectal cancer cases are sporadic, occurring in individuals without any known familial predisposition. Approximately 10-30% of all cases occur in the context of a family history, but most of the predisposing genetic factors have not yet been identified. Highly penetrant inherited colorectal cancer syndromes such as familial adenomatous polyposis (FAP), MYH-associated polyposis (MAP), and Lynch syndrome/hereditary nonpolyposis colon cancer (HNPCC) are less common, but account for as many as 5% of all colorectal cancer cases.
These syndromes can be divided into "polyposis"and "nonpolyposis" syndromes (Fig. 2). The polyposis syndromes can be broadly classified as adenomatous polyposis syndromes, hamartomatous polyposis syndromes, and the hyperplastic polyposis syndrome. Familial adenomatous polyposis (FAP) is an autosomal dominantly inherited disorder due to germline mutations in the APC gene. It is characterized by the emergence of hundreds to thousands of colorectal adenomatous polyps during the second or third decade of life. The incidence of FAP is between one in 5,000 to 7,500 individuals, and it manifests equally in both sexes. Left untreated, colorectal adenocarcinoma will develop in 100% of cases by age 50 years, or approximately 10-15 years after the initial appearance of polyposis. In addition to colorectal cancer, there is a strong predilection for other malignancies, including duodenal, periampullary, and thyroid cancer.
Fig. 2.
Inherited syndromes with an increased risk of colorectal cancer.
CHRPE, congenital hypertrophy of the retinal pigmented epithelium.
MAP is the first inherited colon cancer syndrome described with an autosomal recessive pattern of inheritance; biallelic mutations in the mutY homolog (MUTYH or MYH) gene are associated with the early development of multiple adenomatous polyps. Although large population-based studies are lacking, it is estimated that about 1 in 2,500 to 10,000 individuals have biallelic MUTYH mutations, and the lifetime risk of colorectal cancer is estimated to be 80%.1 In European cohorts, 22% to 29% of individuals with more than 10 adenomas and no germline APC mutation were found to carry biallelic MUTYH mutations.
The hamartomatous polyposis syndromes are less common. Peutz-Jeghers syndrome (PJS) is an extremely rare (1 in 150,000 persons) autosomal dominant hamartomatous polyposis syndrome that carries a 93% cumulative lifetime risk of any malignancy and a 39% lifetime risk of colon cancer. Juvenile polyposis syndrome (JPS) is characterized by the development of multiple juvenile polyps in the GI tract. JPS is also inherited in an autosomal dominant pattern. Although affected individuals often present with symptoms of JPS at an average age of 9.5 years, juvenile polyps can occur at any age.
Individuals with the hyperplastic polyposis syndrome exhibit numerous (>20) hyperplastic polyps distributed throughout the colon, and 25% to 35% of these cases also exhibit synchronous colorectal cancers. There is an increased risk of proximal colon cancers. Large hyperplastic polyps (>1 cm) are frequently observed on the right side of the colon.
The most prevalent familial colorectal cancer syndrome is Lynch syndrome, previously known as HNPCC.
Patients with Lynch syndrome do not present clinically with polyposis, but with only a few adenomas as in the cases with sporadic colorectal cancers. Lynch syndrome accounts for close to 3% of the colon cancer burden.2 It is inherited in an autosomal dominant pattern. With appropriate screening, an estimated 12,000 individuals could be diagnosed with Lynch syndrome on a yearly basis in the United States.2 Early recognition is essential in order to institute the necessary surveillance measures that can reduce colon cancer mortality. Because of the absence of an overt polyposis phenotype, Lynch syndrome can be the most challenging hereditary colorectal syndrome to recognize. This review focuses on the key clinical features of Lynch syndrome.
LYNCH SYNDROME GENETICS
Germline mutations in one of several DNA mismatch repair (MMR) genes are responsible for Lynch syndrome. The DNA mismatch repair genes recognize and correct small sequence errors that may occur during DNA replication. Mutations in both copies of a mismatch repair gene result in aberrant stretches of tandemly repeated mono- or dinucleotide sequences known as microsatellites. This is designated microsatellite instability (MSI), and leads to a "mutator phenotype" within the cell. While most DNA microsatellite sequences are located within intronic sequences, selected genes have DNA microsatellite sequences within their coding sequences. When these errors occur in critical growth-regulatory genes, such as transforming growth factor-β receptor type II, insulin-like growth factor II receptor or activin receptor type 2, dysregulated cell proliferation and, ultimately, carcinogenesis may ensue. Other genes that are targets of MSI include cell cycle regulators (E2F4), regulators of apoptosis (BAX), and some of the MMR genes themselves (MSH3 and MSH6).
The four most commonly mutated DNA mismatch repair genes are MLH1, MSH2, MSH6, or PMS2 (Fig. 3). Approximately 70% of mutations are found in MLH1 and MSH2. The MSH6 and PMS2 genes are less commonly involved, and mutations in MSH6 and PMS2 each account for 15% of all known mutations. Mutations in these genes are typically truncating, leading to a premature stop codon and lack of normal protein function. MMR genes lose their function like the way of inactivation of tumor suppressors, so colon cancer arises when the second wild-type allele is mutated somatically in the colonic epithelium. This can occur by deletion (loss of heterozygosity), point mutation, or CpG island methylation. Hypermethylation is seen primarily in the promoter of the MLH1 gene.
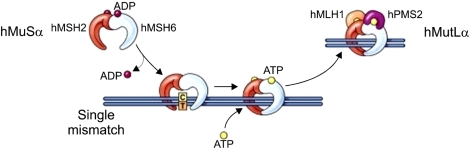
Fig. 3.
Schematic view of DNA Mismatch-repair pathway.43 The heterodimer of hMSH2 and hMSH6 (also known as hMutSα) recognizes single nucleotide mispairs and binds to DNA as a sliding clamp. The heterodimer of hMLH1 and hPMS2 (also known as hMutLα) then binds to hMutSα to guide an exonuclease to remove several bases from the newly synthesized DNA strand, with subsequent re-synthesis of DNA with the correct base pairing. When the MutS complexes bind DNA, they exchange ADP for ATP. For the MMR proteins to be released from DNA, ATP is hydrolyzed to ADP (Reprinted from Gastroenterology 2008;136:1083, with permission).
Recently, gene rearrangements and epimutations have also been reported as etiologies of deficient mismatch repair. A study from Finland showed large genomic rearrangements in the MLH1 and MSH2 genes in 12/41 (29%) patients who failed to demonstrate MLH1 (3/25) or MSH2 (9/16) expression in tumor tissue.3 Germline methylation was first reported by Suter et al.4 in 2 individuals meeting clinical criteria for Lynch syndrome that failed to exhibit MMR gene mutations. One individual had an MLH1 epimutation in a fraction of spermatozoa, suggesting that it may be transmitted among generations. They also reported on another patient who had a hypermethylated MLH1 allele that was maternally transmitted; however, the mother and siblings who shared the same maternal allele were unmethylated at MLH1, suggesting that the epimutation arose as a de novo event in the germline (during oogenesis or in the zygote).5 Finally, a recent report in Chinese and Dutch families revealed heterozygous germline deletions of the last exons of the TACSTD1 gene that lies directly upstream of MSH2. This resulted in methylation of the MSH2 gene promoter and loss of MSH2 gene expression. Therefore, the loss of MSH2 protein can be manifested not only by germline MSH2 mutations, but also by mutations of an adjacent regulatory gene (TACSTD1).6
CLINICAL FEATURES OF LYNCH SYNDROME
Lynch syndrome is inherited in an autosomal dominant manner. Unlike the diffuse polyposis observed in other hereditary colon cancer syndromes, there is a small and finite number (<10) of polyps in patients with Lynch syndrome that are usually located in the right colon, and cannot be differentiated endoscopically from sporadic colon polyps. Polyps can progress to cancer more rapidly (1-3 years)7 when compared to polyps seen in the general population. Recent studies have suggested a median age of diagnosis of colorectal cancers of 61.2 years and a lifetime colorectal cancer risk of 52.2% in women and 68.7% in men.8 Synchronous and metachronous tumors are frequently observed. Histologically, the tumors are characterized by a heavy infiltration of lymphocytes, a medullary growth pattern, or a mucinous or signet ring cell differentiation. 9
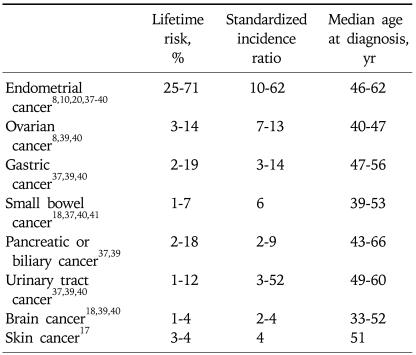
Lynch syndrome is also associated with an increased risk of extra-colonic cancers (Table 1). A unique set of extra-colonic tumors is associated with the Lynch syndrome and includes cancer of the endometrium, ovary, stomach, urinary tract, biliary tract, pancreas, small bowel, brain, and skin. The uterus is the most common extra-colonic site in women. The cumulative lifetime risk of endometrial cancer is equal to or greater than the cumulative risk of CRC.8,10 Women with Lynch syndrome have a 25-71% cumulative lifetime risk of endometrial cancer. It frequently develops in the fifth decade, 10-20 years earlier than sporadic cases. Muir-Torre and Turcot syndromes are unusual variants of Lynch syndrome, and they are associated with cutaneous sebaceous neoplasms and brain tumors (glioblastoma), respectively.
Table 1.
Estimated Overall Lifetime Extra-Colonic Cancer Risks in Lynch Syndrome
Lifetime risks can vary based upon the specific mismatch repair gene mutated and the gender of the mutation carrier.
GENOTYPE/PHENOTYPE CORRELATIONS
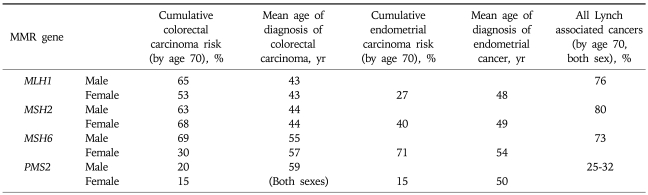
The specific risks of cancer in individuals with Lynch syndrome depends upon which mismatch-repair gene is mutated (Table 2). Choi et al.11 analyzed 32 families from the Canadian familial colorectal cancer registry. Males with MLH1 mutations exhibited a significantly higher CRC risk than females (67% vs 35% by age 70, p=0.02), while the risk was similar in MSH2 carriers (about 54%). The relative risk was constant with age (hazard ratio; between 5.5-5.1 over age 30-70) in MLH1 carriers, while the harzard ratio in MSH2 carriers decreased with age (from 13.1 at age 30 to 5.4 at age 70). Kastrinos et al.12 reported that MLH1 carriers (n=112) had a higher prevalence of colorectal cancer (79% vs 69%, p=0.08) and younger age of diagnosis (42.2 years vs 44.8 years, p=0.03) when compared to MSH2 carriers (n=173). While the prevalence of endometrial cancer in women (68/167, 41%) was similar in both groups (36% vs 44%), other extra-colonic cancers were more frequent in MSH2 carriers compared to MLH1 carriers (24% vs 9%; OR, 3.2; 95% CI, 1.5 to 6.6; p=0.001) and their families (p<0.001). Goecke et al.13 analyzed 988 patients from 281 families with deleterious MSH2 and MLH1 germline mutations (124 MLH1 and 157 MSH2). Interestingly, the rectosigmoid colon was the most prevalent colonic tumor location in both MLH1 and MSH2 carriers (69.8% and 58.9%, respectively). Skin tumors were more frequent in MSH2 carriers (4.2% [33/781] vs 0.8% [5/600], p=0.001). A significantly earlier age of cancer diagnosis was observed in MLH1 mutation carriers compared with MSH2 mutation carriers.14-16 Geary et al.17 observed a clustering of renal and ureteral cancers in families with MSH2 mutations. Bladder cancer was more common in MSH2 families when compared to the general populations (RR=3.6, p=0.001). All patients with bladder cancer had relatives with ureteral cancer, suggesting a clustering of uroepithelial cancer within families. Watson et al.18 also analyzed the risk of Lynch syndrome-associated cancers. While the overall lifetime risk of urologic tract cancers by age of 70 was 8.4% (p<0.02), risks were significantly higher in males from MSH2 families (p<0.0001). Their lifetime risk estimate for urologic tract tumors was nearly 28%. The overall lifetime risk for ovarian cancer was 6.7%, and this was also higher in members of MSH2 families (p<0.006). Chhibber et al.19 examined mismatch repair protein loss in tumors from patients who have Muir-Torre syndrome (MTS). In contrast to other reports, MSH6 was the mismatch repair protein that was most commonly lost (17/41 [41%]), followed by MSH2 (14/41 [34%]) and MLH1 (18/41 [20%]).
Table 2.
Cancer Risks Stratified by Specific MMR-Gene Mutations
MSH6 and PMS2 germline mutations are reported less frequently in Lynch syndrome, but they present with specific clinical phenotypes. Hendriks et al.20 reported that the cumulative risk for Lynch associated tumors was significantly lower in MSH6 carriers when compared to MLH1 or MSH2 mutation carriers (p=0.002). Watson et al.18 reported the risk of colorectal cancer in MSH6 carriers in the Dutch HNPCC database. The median age of onset of colorectal cancer in putative mutation carriers was 10 years higher for MSH6 (54 years; 95% CI, 51 to 56) compared to MLH1 and MSH2 carriers (44 years; 95% CI, 43 to 45). MSH6 families also showed a lower incidence of colorectal cancer compared with MLH1 and MSH2 families (p<0.001). However, in about half of these families, at least one family member developed colorectal or endometrial cancer in their fourth decade. Recently, Baglietto et al.21 reported the lifetime risk of colorectal cancers in a large series of 113 MSH6 families to be 22% and 10% (men and women, respectively), of endometrial cancer to be 26% in women, and of any cancer to be 24% and 40% (men and women, respectively) by the age of 70. Compared to the general population, there was a 25 fold increase (HR, 25.5; 95% CI, 16.8 to 38.7; p<0.001) in the risk of endometrial cancers and a six-fold increased incidence of other Lynch associated cancers (HR, 6.0; 95% CI, 3.4 to 10.7; p<0.001) in MSH6 mutation carriers.
PMS2 germline mutations also play a role in Lynch syndrome. The phenotype of PMS2 germline mutations is usually attenuated. They are associated with older age of colorectal cancer (mean age of onset, 59 years), and carriers have a lower risk of Lynch-associated cancers in general. Senter et al.22 performed an analysis of PMS2 mutations using long range PCR and MLPA for 99 probands diagnosed with Lynch syndrome-associated tumors showing isolated loss of PMS2 by immunohistochemistry. Germline PMS2 mutations were detected in 62% of probands. The incidence of colorectal cancer in mutation carriers was 5.2 fold higher and the incidence of endometrial cancer was 7.5 fold higher compared to the general population. The cumulative cancer risk was only 15-20% for colorectal cancer, 15% for endometrial cancer, and 25-32% for any Lynch syndrome-associated cancer by the age of 70 (Table 2).
DIAGNOSIS OF LYNCH SYNDROME
1. Clinical diagnosis
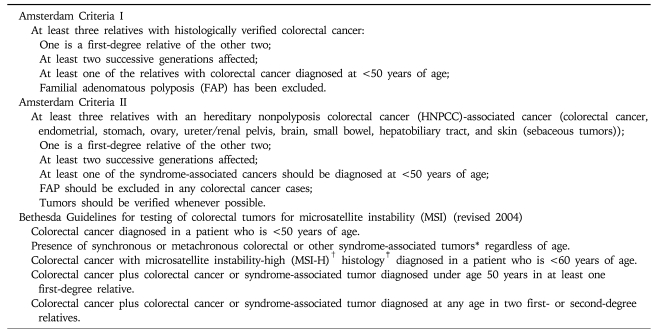
Lynch syndrome can be difficult to recognize, due to the absence of overt polyposis. To establish the diagnosis, every patient with colon cancer should undergo a detailed family history. A family history suggestive of Lynch syndrome is delineated by the Amsterdam II criteria (Table 3). The broader set of Bethesda guidelines was developed to maximize sensitivity but at the cost of reduced specificity (Table 3). However, limiting tumor analysis only to patients who fulfill Bethesda criteria failed to identify 28% of cases of Lynch syndrome.2 Specific features that should raise suspicion of Lynch syndrome are multiple family members affected with colorectal cancer or associated extra-colonic tumors, young age of diagnosis of colon cancer, or multiple Lynch syndrome-associated cancers in a single individual. Currently, fulfillment of Amsterdam criteria is insufficient to establish a definitive diagnosis of Lynch syndrome, and molecular testing is required for this purpose.
Table 3.
Amsterdam I and II Criteria and Bethesda Guidelines
*Syndrome-associated tumors include colorectal, endometrial, stomach, ovarian, pancreas, ureter or renal pelvis, biliary tract, and brain (usually glioblastoma as seen in Turcot syndrome) tumors, sebaceous gland adenomas and keratoacanthomas in Muir-Torre syndrome, and carcinoma of the small bowel; †MSI-H, microsatellite instability-high in tumors refers to changes in two or more of the five National Cancer Institute-recommended panels of microsatellite markers; ‡Presence of tumor infiltrating lymphocytes, Crohn disease-like lymphocyte reaction, mucinous/signet-ring differentiation, or medullary growth pattern.
2. Genetic and molecular diagnosis
There are two types of molecular tests available in the workup of Lynch syndrome: tumor testing and blood genetic testing. Tumor testing includes MSI analysis and immunohistochemistry (IHC). Paraffin-embedded tissue can be used for this purpose. The original Bethesda guidelines proposed a panel of five microsatellite markers for the uniform analysis of MSI in Lynch Syndrome. These included two mononucletide (BAT-25 and BAT-26) and three dinucleotide (D5S346, D2S123, and D17S250) repeats. Differences in the fragment size of tumor-derived DNA versus DNA from normal tissue are scored, with high levels of instability defined as ≥2/5 loci, or >30% of loci unstable.
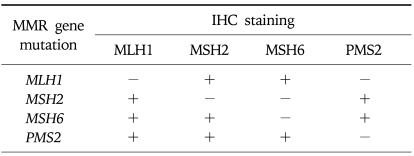
An adjunctive approach to MSI testing is immunohistochemical staining for MMR proteins (Table 4). Specific staining is performed on tumor tissue for each of the 4 mismatch repair proteins. Mutations in MMR genes typically lead to truncated nonfunctional proteins that do not stain by IHC. If a tumor exhibits MSI or abnormal IHC staining, genetic testing using peripheral blood DNA is the next step. An abnormal immunostain can guide the choice of genes analyzed (Table 4).23,24 Advantages of IHC testing compared with MSI are that it is less expensive, can easily be done at most pathology departments, and can directly indicate the specific gene that is mutated. Both IHC and MSI tests are somewhat complementary and can be integrated into a genetic testing algorithm (Fig. 4). For example, mutations in MSH6 tend to result in weaker or no MSI in tumors (<30% of microsatellite markers unstable25), so they could be missed by MSI testing but would be detectable by IHC.
Table 4.
IHC Findings Associated with MLH1, MSH2, MSH6, and PMS2 Mutations
+, nuclear staining present; -, nuclear staining absent.
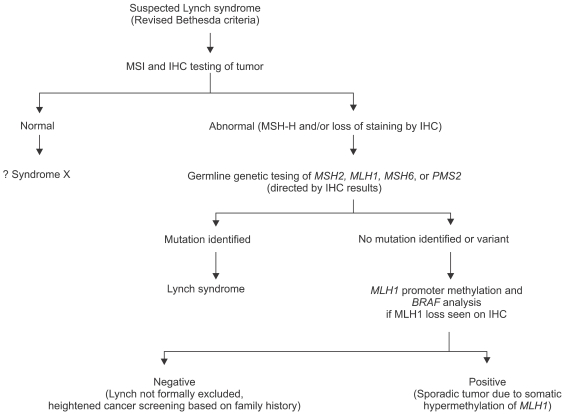
Fig. 4.
Diagnostic algorithm for Lynch syndrome using genetic testing.
Up to 15% of sporadic colon cancers are also associated with microsatellite instability, usually due to somatic hypermethylation of the MLH1 promoter.26,27 These cases can be distinguished from Lynch syndrome by the presence of mutations in the BRAF gene in the tumor.28,29
Family history can provide important clues to the presence of the Lynch syndrome. However, in certain cases, a small family size, insufficient or incomplete information about relatives, a physician's unfamiliarity with Lynch syndrome, or reduced penetrance of a germline mutation in a MMR gene may obscure the diagnosis. For these reasons, widespread MSI/IHC testing of all colon tumors has been proposed. For families in which tumor tissue is not available, direct germline testing is an option. If the result is positive for a pathogenic mutation, other family members can then be tested. At-risk relatives with a true negative test result do not need intensive cancer screening.
Hampel et al.2 analyzed 500 tumors with MSI or IHC from an unselected population of colorectal cancers in the U.S., and 113 cases showing MSI or abnormal IHC underwent genetic analysis. Germline mutations were found in 18 probands (MSH2 in 10 cases, MLH1 in 4 cases, MSH6 in 3 cases, PMS2 in 1 case). Only eight patients (44%) were diagnosed under age 50 years, and 13 patients (72%) met the revised Bethesda guidelines. Thus, limiting tumor analysis to patients who fulfill Bethesda criteria would have failed to identify 28% of Lynch syndrome cases. They also reported that the overall prevalence of Lynch syndrome was 2.8% (44 of 1,566 patients) when the results were combined with their 1,066 previously studied patients.
Schofield et al.30 analyzed selected colorectal cancer patients in Western Australia who had been diagnosed before age 60 years. 1,395 patients were screened with MSI with a single BAT-26 marker and MSI was detected in 105/1,344 (7.8%) patients. Loss of MMR protein expression was analyzed in MSI-positive cases by IHC. The estimated proportion of mutation carriers in MSI-positive cases according to their age of diagnosis was 89% (<30 years), 83% (30-39), 68% (40-49) and 17% (50-59). They recommended that MSI should be the initial test for population-based screening of Lynch syndrome, especially in younger colorectal cancer patients, regardless of family history.
Of note, about 40% of the families who meet the Amsterdam criteria do not have a detectable mismatch repair gene mutation or tumors that exhibit MSI. These families are classified as familial colon cancer X. It is hypothesized that familial colon cancer X is an aggregation of low-penetrance CRC susceptibility alleles.
SURVEILLANCE AND MANAGEMENT OF LYNCH SYNDROME
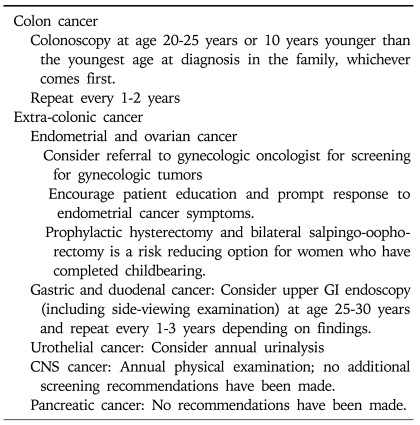
Once the diagnosis of Lynch syndrome has been established or highly suspected by either clinical or molecular criteria, an intensive surveillance program should be initiated. Colonoscopy is recommended rather than flexible sigmoidoscopy because of the predominant proximal location of cancer.31 It should be offered every one or two years, beginning between the age of 20 to 25 years (or age 30 years in patients with MSH6 mutations), or ten years younger than the youngest age at diagnosis in the family, and then repeated every 1 to 2 years.32 Annual colonoscopies are then recommended after the age of 40 years (Table 5).9
Table 5.
Cancer Screening Recommendations for Lynch Syndrome by NCCN (2010)
Endometrial cancer in Lynch syndrome is usually stage I disease, and the overall 5-years survival rate is 88%. It is unclear whether endometrial cancer screening would improve morbidity and mortality for women with Lynch syndrome. Nevertheless, annual transvaginal ultrasound and endometrial aspiration biopsies beginning between age of 25 and 35 can be considered.32 Increased risks of ovarian cancers have also led some to propose annual transvaginal ultrasound with CA-125 beginning at age 30.
Surveillance upper endoscopy can be considered every 2 years starting from age 30 to 35 years, or 5 years earlier than the youngest onset of gastric cancer. According to a German study, only 26% of gastric cancer cases had a family history of gastric cancer, and most were diagnosed after age 35 years.13 However, there are no definitive data that support a benefit for surveillance of gastric cancers in Lynch syndrome.13,33
Because of the increased risk of urinary collecting system cancers, annual renal ultrasound, urinalysis, and urine cytology should be considered in families with a history of urinary tract tumors. Lindor et al.9 recommend annual urinalysis with cytology because of the low cost and non-invasiveness. Watson et al.18 recommend surveillance beginning at age 50. Screening for other Lynch syndrome-associated malignancies is not standardized and most base these decisions upon the specific pattern of tumors occurring within the family.
FAMILY COUNSELING
After an index patient is diagnosed with Lynch syndrome, it is important to offer genetic testing and counseling to relatives at-risk. However, patients may choose not to inform their relatives. Previous studies have estimated that as many as 28% to 40% of at-risk relatives decline genetic testing.
Once a pathogenic mutation has been identified in an affected individual, at-risk relatives can be tested for that same mutation. Then, clinical management can be focused only on at-risk patients who are proven carriers of mutations. Genetic testing should be preceded by formal genetic counseling. The important benefit of genetic testing is to offer risk reduction approaches to MMR mutation carriers.
It is not certain whether learning that one has a Lynch syndrome mutation is associated with adverse psychosocial outcomes or not. However, Gritz et al.34 demonstrated a greater risk for experiencing both short and long-term distress in patients who have higher levels of baseline mood disturbance, lower quality of life, and lower social support. The counselor, whether genetic counselor or physician, must estimate the individual's emotional state carefully with respect to his or her ability to accept a positive or negative test result.
In families in whom no mutation is found, the physician must make a judgment as to whether Lynch syndrome is sufficiently likely to justify heightened screening, or whether patients should be regarded as having an increased familial risk for colon cancer but not Lynch syndrome.
CONCLUSION
Colorectal cancer can be divided into sporadic and familial (hereditary) cases, and as many as 10-30% of cases have a familial component. However, the genetic basis of most of these cases is unknown. The most common hereditary colon cancer syndrome is Lynch syndrome, which is associated with high lifetime risks of colon and endometrial cancer. Recognition of Lynch syndrome and implementation of appropriate cancer surveillance strategies can reduce mortality from cancer.35,36 Some clues to the diagnosis can be provided by the family history. The diagnostic workup for Lynch syndrome includes tumor testing with IHC and MSI as well as blood germline testing for the 4 genes associated with the disease (MSH2, MLH1, MSH6, PMS2).
References
- 1.Jenkins MA, Croitoru ME, Monga N, et al. Risk of colorectal cancer in monoallelic and biallelic carriers of MYH mutations: a population-based case-family study. Cancer Epidemiol Biomarkers Prev. 2006;15:312–314. doi: 10.1158/1055-9965.EPI-05-0793. [DOI] [PubMed] [Google Scholar]
- 2.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gylling A, Ridanpaa M, Vierimaa O, et al. Large genomic rearrangements and germline epimutations in Lynch syndrome. Int J Cancer. 2009;124:2333–2340. doi: 10.1002/ijc.24230. [DOI] [PubMed] [Google Scholar]
- 4.Suter CM, Martin DI, Ward RL. Germline epimutation of MLH1 in individuals with multiple cancers. Nat Genet. 2004;36:497–501. doi: 10.1038/ng1342. [DOI] [PubMed] [Google Scholar]
- 5.Hitchins M, Williams R, Cheong K, et al. MLH1 germline epimutations as a factor in hereditary nonpolyposis colorectal cancer. Gastroenterology. 2005;129:1392–1399. doi: 10.1053/j.gastro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3[prime] exons of TACSTD1. Nat Genet. 2009;41:112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 7.Rijcken FE, Hollema H, Kleibeuker JH. Proximal adenomas in hereditary non-polyposis colorectal cancer are prone to rapid malignant transformation. Gut. 2002;50:382–386. doi: 10.1136/gut.50.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129:415–421. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 10.Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. Journal of Medical Genetics. 2005;42:491–496. doi: 10.1136/jmg.2004.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YH, Cotterchio M, McKeown-Eyssen G, et al. Penetrance of colorectal cancer among MLH1/MSH2 carriers participating in the colorectal cancer familial registry in Ontario. Hered Cancer Clin Pract. 2009;7:14. doi: 10.1186/1897-4287-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastrinos F, Stoffel EM, Balmana J, Steyerberg EW, Mercado R, Syngal S. Phenotype comparison of MLH1 and MSH2 mutation carriers in a cohort of 1,914 individuals undergoing clinical genetic testing in the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2044–2051. doi: 10.1158/1055-9965.EPI-08-0301. [DOI] [PubMed] [Google Scholar]
- 13.Goecke T, Schulmann K, Engel C, et al. Genotype-phenotype comparison of German MLH1 and MSH2 mutation carriers clinically affected with Lynch syndrome: a report by the German HNPCC Consortium. J Clin Oncol. 2006;24:4285–4292. doi: 10.1200/JCO.2005.03.7333. [DOI] [PubMed] [Google Scholar]
- 14.Kruse R, Rutten A, Lamberti C, et al. Muir-Torre phenotype has a frequency of DNA mismatch-repair-gene mutations similar to that in hereditary nonpolyposis colorectal cancer families defined by the Amsterdam criteria. Am J Hum Genet. 1998;63:63–70. doi: 10.1086/301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangold E, Pagenstecher C, Leister M, et al. A genotype-phenotype correlation in HNPCC: strong predominance of msh2 mutations in 41 patients with Muir-Torre syndrome. J Med Genet. 2004;41:567–572. doi: 10.1136/jmg.2003.012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse R, Lamberti C, Wang Y, et al. Is the mismatch repair deficient type of Muir-Torre syndrome confined to mutations in the hMSH2 gene? Hum Genet. 1996;98:747–750. doi: 10.1007/s004390050298. [DOI] [PubMed] [Google Scholar]
- 17.Geary J, Sasieni P, Houlston R, et al. Gene-related cancer spectrum in families with hereditary non-polyposis colorectal cancer (HNPCC) Fam Cancer. 2008;7:163–172. doi: 10.1007/s10689-007-9164-6. [DOI] [PubMed] [Google Scholar]
- 18.Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444–449. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Modern Pathology. 2008;21:159–164. doi: 10.1038/modpathol.3800997. [DOI] [PubMed] [Google Scholar]
- 20.Hendriks YM, Wagner A, Morreau H, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127:17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 21.Baglietto L, Lindor NM, Dowty JG, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst. 2010;102:193–201. doi: 10.1093/jnci/djp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419–428. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang DK, Ricciardiello L, Goel A, Chang CL, Boland CR. Steady-state regulation of the human DNA mismatch repair system. J Biol Chem. 2000;275:18424–18431. doi: 10.1074/jbc.M001140200. [DOI] [PubMed] [Google Scholar]
- 24.Chang DK. Diagnostic and therapeutic strategy for the HNPCC patients and their family. Intestinal Research. 2006;4:168–176. [Google Scholar]
- 25.Berends MJ, Wu Y, Sijmons RH, et al. Molecular and clinical characteristics of MSH6 variants: an analysis of 25 index carriers of a germline variant. Am J Hum Genet. 2002;70:26–37. doi: 10.1086/337944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung D, Rustgi A. The hereditary nonpolyposis colorectal cancer syndrome: genetic and clinical implication. Ann Intern Med. 2003;138:560–570. doi: 10.7326/0003-4819-138-7-200304010-00012. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks YM, de Jong AE, Morreau H. Diagnostic approach and management of Lynch Syndrome (Hereditary Nonpolyposis Colorectal Carcinoma): a guide for clinicians. CA Cancer J Clin. 2006;56:213–225. doi: 10.3322/canjclin.56.4.213. [DOI] [PubMed] [Google Scholar]
- 28.Deng G, Bell I, Crawley S, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004;10:191–195. doi: 10.1158/1078-0432.ccr-1118-3. [DOI] [PubMed] [Google Scholar]
- 29.Domingo E, Niessen RC, Oliveira C, et al. BRAF-V600E is not involved in the colorectal tumorigenesis of HNPCC in patients with functional MLH1 and MSH2 genes. Oncogene. 2005;24:3995–3998. doi: 10.1038/sj.onc.1208569. [DOI] [PubMed] [Google Scholar]
- 30.Schofield L, Watson N, Grieu F, et al. Population-based detection of Lynch syndrome in young colorectal cancer patients using microsatellite instability as the initial test. Int J Cancer. 2009;124:1097–1102. doi: 10.1002/ijc.23863. [DOI] [PubMed] [Google Scholar]
- 31.Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome): an updated review. Cancer. 1996;78:1149–1167. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1149::AID-CNCR1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA. 1997;19:915–919. [PubMed] [Google Scholar]
- 33.Renkonen-Sinisalo L, Sipponen P, Aarnio M, et al. No support for endoscopic surveillance for gastric cancer in hereditary non-polyposis colorectal cancer. Scand J Gastroenterol. 2002;37:574–577. doi: 10.1080/00365520252903134. [DOI] [PubMed] [Google Scholar]
- 34.Gritz ER, Peterson SK, Vernon SW, et al. Psychological impact of genetic testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2005;23:1902–1910. doi: 10.1200/JCO.2005.07.102. [DOI] [PubMed] [Google Scholar]
- 35.Järvinen HJ, Mecklin JP, Sistonen P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 1995;108:1405–1411. doi: 10.1016/0016-5085(95)90688-6. [DOI] [PubMed] [Google Scholar]
- 36.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 37.Aarnio M, Mecklin JP, Aaltonen LA, Nystrom-Lahti M, Jarvinen HJ. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995;64:430–433. doi: 10.1002/ijc.2910640613. [DOI] [PubMed] [Google Scholar]
- 38.Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105–110. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 39.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Vasen HF, Stormorken A, Menko FH, et al. MSH2 mutation carriers are at higher risk of cancer than MLH1 mutation carriers: a study of hereditary nonpolyposis colorectal cancer families. J Clin Oncol. 2001;19:4074–4080. doi: 10.1200/JCO.2001.19.20.4074. [DOI] [PubMed] [Google Scholar]
- 41.Schulmann K, Engel C, Propping P, Schmiegel W. Small bowel cancer risk in Lynch syndrome. Gut. 2008;57:1629–1630. doi: 10.1136/gut.2007.140657. [DOI] [PubMed] [Google Scholar]
- 42.International Agency for Research on Cancer (IARC) Lyon, France: IARC; 2009. [cited 2009 Dec 23]. Globocan 2002 [Internet] Available from: http://www-dep.iarc.fr/ [Google Scholar]
- 43.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]