Abstract
Gastrointestinal invasive aspergillosis is often reported as part of a disseminated infection, and rarely as an isolated organ infection. Isolated invasive Aspergillus colitis is very rare, being observed only in patients with hematological malignancy and neutropenia. We encountered an unusual case of isolated invasive Aspergillus colitis presenting with hematochezia in a nonneutropenic patient with colon cancer. Fungal hyphae with surrounding inflammatory cells and mucosal necrosis were observed during the histological examination of a biopsy sample obtained at endoscopy. This case indicates that isolated invasive Aspergillus colitis may develop in a variable context of immunosuppression.
Keywords: Aspergillosis, Colitis, Colon neoplasms
INTRODUCTION
Invasive aspergillosis is a major life-threatening infection in immunocompromised patients who are receiving chemotherapy or immunosuppressive therapy, including high-dosage corticosteroids.1 With the advent of more potent chemotherapeutic and immunosuppressive agents, the incidence of invasive aspergillosis has increased.2 Although the lung is the most common site of infection in invasive aspergillosis, the involvement of other organs, such as the central nervous system, paranasal sinuses, heart, bones, joints, eyes, skin, kidneys, and gastrointestinal tract, has been reported infrequently, as a single-organ infection or as a part of disseminated infection.1 To date, invasive Aspergillus colitis without disseminated aspergillosis has not been reported in a nonneutropenic patient without a hematological malignancy.
Here, we report a unique case of isolated invasive Aspergillus colitis in a patient without any predisposing factors for immunosuppression, except for colon cancer and a short course of corticosteroids.
CASE REPORT
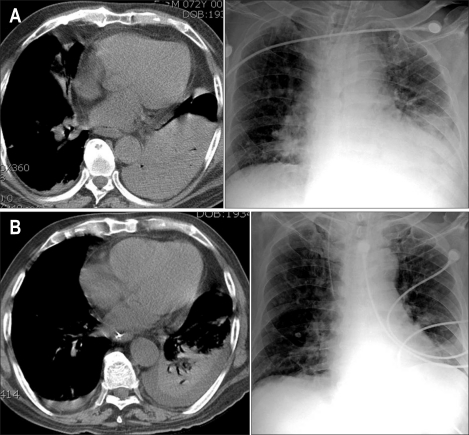
A 72-year-old man was admitted to the intensive care unit for community-acquired pneumonia. He had a history of hypertension, bronchial asthma, and diabetes mellitus and visited the outpatient clinic regularly. There had been no recent steroid therapy for bronchial asthma and no recent changes in hypoglycemic agents. Initial arterial blood gases, complete blood cell counts and blood chemistry results were as follows: pH, 7.413; pCO2, 29.8 mmHg; pO2, 48.2 mmHg; bicarbonate, 20.6 mmol/L; white blood cell count, 15.2 × 109/L; hemoglobin, 15.8 g/dL; platelet count, 148 × 109/L; blood urea nitrogen, 28 mg/dL; creatinine, 1.8 mg/dL; blood glucose, 157 mg/dL. Chest computed tomography (CT) showed a dense consolidation in the left lower lobe (Fig. 1A). He was intubated and placed on mechanical ventilation, and empirical antibiotic therapy was started. Gram-positive cocci were observed in the sputum smear and identified as Streptococcus pneumoniae. Corticosteroids were administered intravenously (methylprednisolone) for an acute asthmatic attack associated with the pneumonia as follows; 1 mg/kg three times a day (two days), 1 mg/kg twice a day (one day), and 1 mg/kg once a day (5 days). Thereafter, 10 mg of oral prednisone was continued. His pneumonia improved (Fig. 1B) and the endotracheal tube was removed on hospital day (HD) 13.
Fig. 1.
Prominent pneumonic consolidation in the left lower lung in radiologic images of the chest (A). Its extent decreases in the follow-up images obtained on hospital day 18 (B).
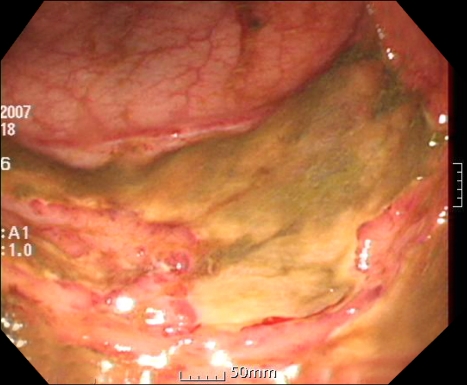
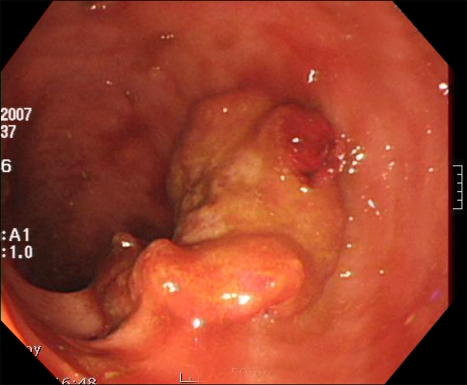
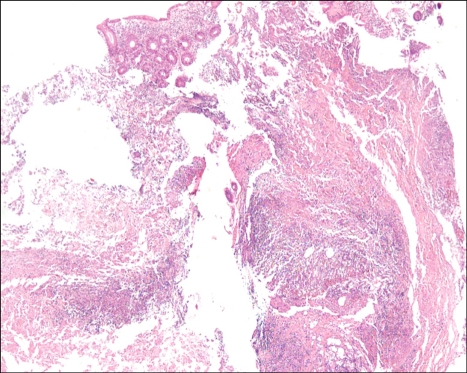
During his recovery, a small amount of hematochezia developed, beginning on HD 5. It was not hemodynamically significant state and there was no evidence of upper gastrointestinal bleeding from the nasogastric tube irrigation. Sigmoidoscopy was performed on HD 12 due to recurrent hematochezia. The scope was advanced to the junction between the sigmoid colon and descending colon. Multiple shallow geographic ulcers with yellow exudates at their bases were seen in the sigmoid colon and the distal descending colon (Fig. 2). Since the ulcers had accompanying edematous change and venous dilatation in the sigmoido-descending junctional area, especially in the old aged, hemodynamically unstable patient, ischemic colitis was suggested. In addition, a 2.5×2.5-cm ulcerofungating tumor was found in the sigmoid colon and it was located roughly 5 cm distal from the ulcer (Fig. 3). The tumor was biopsied, and the histological diagnosis was compatible with moderately differentiated adenocarcinoma. Colon cancer in co-existence with ischemic colitis was diagnosed at first. The ulcers were thought to be bleeding focuses and supportive care was started. Follow-up sigmoidoscopy was performed on HD 17 because of persistent hematochezia. There was no overall change and the ulcers were biopsied. While waiting for the histological diagnosis to be confirmed, empirical intravenous ganciclovir (5 mg/kg/day) was administered. We could not rule out the cytomegalovirus colitis in the patient receiving steroid therapy, even without objective evidence of cytomegalovirus infection. The histological examination revealed a piece of mucosa admixed with necrotic tissue that contained inflammatory cells (Fig. 4). The necrotic tissue contained many fungal hyphae (Fig. 5), i.e., acute-angle dichotomously branching septate hyphae, consistent with Aspergillus. Abdominal CT scan did not show any remarkable findings other than eccentric wall thickening in the distal sigmoid colon, which was compatible with colon cancer. Invasive aspergillosis of other organs was not seen in chest CT, abdominal CT, and serial simple radiographs. On HD 19, Amphotericin B deoxycholate (1 mg/kg/day) was administrated. However, on HD 22, it was changed to liposomal amphotericin B (3 mg/kg/day), because of the febrile reaction to amphotericin B deoxycholate. The hematochezia disappeared after 13 days of antifungal treatment. However, central venous catheter-related, methicillin-resistant Staphylococcus aureus bacteremia developed on HD 22. The bacteremia persisted for 13 days, despite vancomycin therapy. The patient was reintubated and placed on a ventilator on HD 30. He died on HD 37 of newly developed ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Autopsy was not done in the patient.
Fig. 2.
A shallow geographic ulcer is apparent at the junction between the sigmoid colon and distal descending colon.
Fig. 3.
An ulcerofungating tumor is seen in the sigmoid colon.
Fig. 4.
A piece of mucosa with ulcer debris containing inflammatory cells and necrotic tissue (H&E stain, ×40).
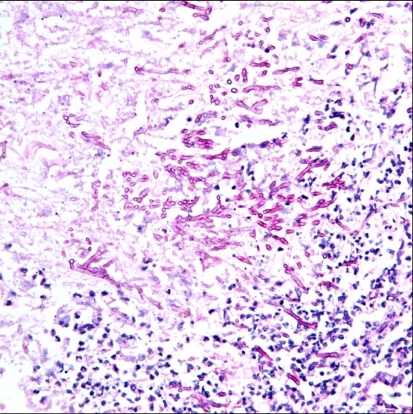
Fig. 5.
Acute-angled branching, septate hyphae of Aspergillus (PAS stain, ×400).
DISCUSSION
Invasive aspergillosis primarily involves the lungs, with subsequent dissemination to other organs, such as central nervous system, heart, skin, liver, kidneys and gastrointestinal tract. Therefore, gastrointestinal invasive aspergillosis is invariably described in the context of disseminated infection.3 A recent review reported the frequency of gastrointestinal invasive aspergillosis among cases of invasive aspergillosis as approximately 17% based on several autopsy series and 2% based on retrospective studies, and that it was almost always accompanied by invasive pulmonary aspergillosis.3
Isolated gastrointestinal invasive aspergillosis is rare. There have been several reports of isolated invasive Aspergillus stomatitis and enteritis in the English language literature.3-5 Characteristically, all of these patients had hematological malignancies, received chemotherapy and had experienced neutropenia just before the invasive aspergillosis developed. Although Aspergillus spores may be ingested, the gastrointestinal tract is not thought to be a common portal of entry, since it is an unfavorable environment for the growth of Aspergillus.4 However, mucosal ulceration after chemotherapy and neutropenia in patients with hematological malignancies may be a predisposing condition for invasive aspergillosis.3 This patient is unique in that he had no hematological malignancy and experienced no episode of neutropenia around the time that the invasive aspergillosis developed. The potential risk factors for invasive aspergillosis may be colon cancer and a short course of steroids. However, we could not explain the association with the risk factors clearly. A few clinical features made the association unlikely, such as spatial separation of the tumor and ulcers, and short interval between steroid use and the onset of hematochezia.
An autopsy study reported that the gross gastrointestinal lesions of invasive aspergillosis included ulcers, mucosal flecks, sloughed mucous membranes, polypoid masses, and segmental lesions.6 Several recently reported cases also had mucosal ulceration and necrosis with necrotic debris. Angioinvasion and transmural necrosis are common histopathological findings.5,7-10 The patient also had several colonic ulcers, as seen endoscopically, and extensive necrosis was evident histologically.
Gastrointestinal invasive aspergillosis commonly presents with bowel ischemia and infarction requiring surgical resection of the bowel, together with clinical manifestations, which include fever, abdominal pain, abdominal tenderness, ileus and gastrointestinal bleeding. The mortality rate is very high, even with surgical resection of the infected bowel.3,5,7-10 The patient had mild hematochezia, which resolved only with antifungal therapy, although the improvement in the ulcers was not confirmed in a follow-up endoscopic examination. Improvement may have occurred because the patient was less profoundly immunocompromised than previously reported patients with invasive gastrointestinal aspergillosis.
Aspergillus was not isolated from the stool, sputum or blood samples taken from the patient. The diagnosis of proven or probable aspergillosis requires positive culture results, with only two exceptions; the histopathological demonstration of hyphae consistent with Aspergillus and the surrogate non-culture-based method in patients with compatible clinical manifestations and host factors.1 Although without positive culture results or non-culture-based methods, the diagnosis of probable aspergillosis is thought to be evident from the histopathological findings in the patient.
Invasive aspergillosis is rarely considered until a pathologic examination reveals the characteristic fungal hyphae.5,7-10 This case shows that isolated invasive Aspergillus colitis may develop in various contexts of immunosuppression, particularly in the absence of hematological malignancy, recent chemotherapy or recent neutropenia. A high level of suspicion is needed for the early diagnosis and therapy of isolated invasive Aspergillus colitis.
References
- 1.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 2.Hori A, Kami M, Kishi Y, Machida U, Matsumura T, Kashima T. Clinical significance of extra-pulmonary involvement of invasive aspergillosis: a retrospective autopsy-based study of 107 patients. J Hosp Infect. 2002;50:175–182. doi: 10.1053/jhin.2001.1170. [DOI] [PubMed] [Google Scholar]
- 3.Eggimann P, Chevrolet JC, Starobinski M, et al. Primary invasive aspergillosis of the digestive tract: report of two cases and review of the literature. Infection. 2006;34:333–338. doi: 10.1007/s15010-006-5660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myoken Y, Sugata T, Kyo T, et al. Invasive Aspergillus stomatitis in patients with acute leukemia: report of 12 cases. Clin Infect Dis. 2001;33:1975–1980. doi: 10.1086/324082. [DOI] [PubMed] [Google Scholar]
- 5.Mohite U, Kell J, Haj MA, et al. Invasive aspergillosis localised to the colon presenting as toxic megacolon. Eur J Haematol. 2007;78:270–273. doi: 10.1111/j.1600-0609.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 6.Prescott RJ, Harris M, Banerjee SS. Fungal infections of the small and large intestine. J Clin Pathol. 1992;45:806–811. doi: 10.1136/jcp.45.9.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tresallet C, Nguyen-Thanh Q, Aubriot-Lorton MH, et al. Small-bowel infarction from disseminated aspergillosis. Dis Colon Rectum. 2004;47:1515–1518. doi: 10.1007/s10350-004-0625-9. [DOI] [PubMed] [Google Scholar]
- 8.Enjoji M, Ohtsukasa S, Nagano H, et al. Localized small-bowel infarction caused by Aspergillus during chemotherapy for acute myeloid leukemia: report of a case. Surg Today. 2008;38:449–452. doi: 10.1007/s00595-007-3639-9. [DOI] [PubMed] [Google Scholar]
- 9.Shah SS, Birnbaum BA, Jacobs JE. Disseminated aspergillosis inciting intestinal ischaemia and obstruction. Br J Radiol. 2001;74:1145–1147. doi: 10.1259/bjr.74.888.741145. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary A, Jain V, Dwivedi RS, Misra S. Invasive aspergillosis causing small bowel infarction in a patient of carcinoma breast undergoing chemotherapy. J Carcinog. 2006;5:18. doi: 10.1186/1477-3163-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]