Abstract
Vascular dysfunction associated with Type 2 diabetes is initially manifested in the pre-diabetic condition and continuously expressed as this complex disease progresses to include other cardiovascular complications that collectively increase patient risk to morbidity and mortality. Many factors are known to affect vascular function and this review focuses on the role of adipokines and obesity in this process. Growing evidence suggests that adipose-derived adipokines, such as cytokines, chemokines and hormones, plays a significant role in the regulation of vascular function. Inhibition of vascular reactive oxygen species (ROS) formation and lowering plasma free fatty acid level are all potential therapeutic targets for type 2 diabetes-induced vascular dysfunction. Bariatric surgery is a relatively new and more aggressive treatment for the morbidly obese patient that also results in an instant and obvious improvement of vascular function through as yet unexplained mechanisms. These therapies show great promise for the prevention and cure of diabetes-induced vascular dysfunction.
Keywords: adipokines, bariatric surgery, dyslipidemia, reactive oxidative species
Introduction
American Diabetes Association reported in 2007 that diabetes was the 6th leading cause of death in the United States and 26 million people currently had been diagnosed with this disease. Type 2 diabetes is generally present with other metabolic cardiovascular risk factors including dyslipidemia, hypertension, and visceral obesity and is closely related to various cardiovascular disorders [1]. Multiple factors may be involved in type 2 diabetes-induced vascular dysfunction and identifying the therapeutic targets for impaired vascular function is critical in the management of diabetes-related cardiovascular disease. Dysregulation of adipokine expression, overproduction of reactive oxygen species (ROS) and dyslipidemia contribute to the development of vascular dysfunction in diabetes and are demonstrated to be effective therapeutic targets in diabetes-related vascular complications [2–4]. Bariatric surgery has also been reported to improve vascular function in morbidly obese and type 2 diabetic patients [5]. The present review summarizes the central mechanisms involved in vascular dysfunction in type 2 diabetes and suggests possible strategies to prevent and cure diabetes-associated vascular diseases.
1. Role of adipokines in vascular dysfunction
The increased prevalence of obesity and obesity-related cardiovascular risk factors are closely associated with the rising incidence of cardiovascular diseases and type 2 diabetes [6]. The classical perception of adipose tissue as a passive repository for fatty acids has been replaced by the notion that adipose tissue serves as a highly active metabolic and endocrine organ by regulating lipid and glucose metabolism and producing a large number of cytokines (eg. tumor necrosis factor-α (TNF-α) and Interleukin (IL)-6 (IL-6)), chemokines (eg. IL-8 and monocyte chemoattractant protein-1 (MCP-1)) and hormones (eg. leptin and adiponectin) [2]. Obesity and diabetes-related vascular dysfunction and insulin resistance may well be caused by altered signaling from adipose tissue to blood vessels, as adipokines produced by adipose tissue may affect vascular function and insulin sensitivity [7, 8]. Central adiposity, particularly a greater amount of intra-abdominal or visceral fat, is proposed to contribute to chronic subclinical inflammation, which is linked to vascular dysfunction and atherosclerosis [9]. An important mechanism in the regulation of inflammation by perivascular adipose is macrophage infiltration [8]. Clinical studies suggest that abdominal adipose macrophage infiltration is associated with systemic arterial dysfunction and insulin resistance in obese subjects [10]. Hormones (leptin, adiponectin) mainly produced by adipocytes also actively participate in the regulation of vascular function and insulin sensitivity [11]. Thus, adipose-derived adipokines may play an endocrine, paracrine/autocrine role in regulating vascular function and insulin sensitivity in diabetes.
In the context of the worldwide increase of obesity and its cardiovascular and metabolic complications, perivascular adipose tissue presents an exciting new field of research for obesity-related vascular disease and type 2 diabetes. Future research is needed to 1) identify the novel adipokines that potentially contribute to the pathogenesis of obesity and diabetes, 2) elucidate the paracrine/autocrine role of adipokines in affecting vascular function, 3) assess the effects of life style management (such as calorie restriction and exercise), diet supplement and pharmacological intervention on body weight, adiposity and adipokine expression profile, and, 4) provide direct evidence that the modulation of adverse adipokine expression pattern leads to the improvement of vascular function in diabetic patients. Results from these studies will improve understanding of the physiological function and therapeutic potential of perivascular adipose-derived adipokines in the vascular dysfunction of type 2 diabetes.
2. Role of ROS in vascular dysfunction
Hyperglycemia impairs not only glucose homeostasis in the various tissues but also vascular function [12]. Increased ROS in type 2 diabetes plays a key role in the pathogenesis of cardiovascular disease, and the enhanced production of superoxideanion (O.−2) radicals in type 2 diabetes results in impaired vascular function through the endothelial and vascular smooth muscle cell. Among multiple intracellular sources for O.−2 radicals (eg, mitochondria, xanthine oxidase, NAD(P)H oxidase, etc), NAD(P)H oxidase is the major source of O.−2 radicals in the vasculature [3, 13, 14] and is involved in the endothelial dysfunction in type 2 diabetes [13, 15, 16]. Another important contributor to vascular ROS generation is uncoupled endothelial nitric oxide synthase (eNOS). In the absence of either L-Arginine or tetrahydrobiopterin (BH4), eNOS is unable to transfer electrons to L-Arginine and this electron reacts with oxygen to form an O.−2 radical [3]. The principal mechanism for O.−2 radicals-mediated endothelial dysfunction is the decreased bioavailability of nitric oxide (NO) through forming peroxynitrite anion (ONOO−) [17]. ONOO− can produce further oxidativedamage to cells because of its inherent potency and stability [3, 13]. Our lab recently reported that the increased signaling of TNF-α and/or advanced glycation end products (AGE)/receptor for AGE (RAGE) (AGE/RAGE) in type 2 diabetes induces endothelial dysfunction through enhanced production of O2.− radicals, which causes an increased formation of ONOO− in coronary arterioles. ROS-induced vascular dysfunction can be ameliorated by treatment of the specific targets. The neutralizing antibody to TNF-α and soluble RAGE restored endothelial function in type 2 diabetic mice by reducing superoxide production and NAD(P)H oxidase activation in coronary microcirculation. Moreover, direct inhibition of NAD(P)H oxidase (source of O.−2 radicals) with apocynin, and/or treatment of O.−2 radicals scavenger, TEMPOL, restores the impaired endothelium-dependent vasodilation in diabetic mice coronary microcirculation [13, 16]. Therefore, TNF-α and/or AGE/RAGE may be novel therapeutic targets for diabetes associated vascular dysfunction in coronary microcirculation [13, 18].
3. Role of dyslipidemia in vascular dysfunction
Elevated plasma free fatty acid level is an independent risk factor for cardiovascular disease in diabetic patients. Endothelial dysfunction could be induced by a direct 4-day lipid infusion in volunteers, which elevated plasma free fatty acids to the levels observed in the metabolic syndrome. This effect was observed in both lean non-diabetic insulin-resistant subjects and healthy volunteers, and independent of insulin-sensitivity [4]. Different types of free fatty acids have differential effects on endothelial function. A higher percentage of serum polyunsaturated fatty acids was associated with better vascular function in type 2 diabetic patients, while saturated fatty acids showed a negative correlation with endothelial function [19]. Similarly, high-fat meals can impair endothelial function within 3~4 hour, while replacement of unsaturated fatty acids with the saturated fatty acids has protective effects on postprandial endothelial dysfunction [20]. Interestingly, endothelial function is preserved in patients with severe hypertriglyceridemia and low functional lipoprotein lipase (LPL) activity, suggesting that triacylglycerol hydrolysis by LPL and release of fatty acids might be critical to postprandial lipaemia induced vascular dysfunction [21, 22]. In agreement with this observation, Shimabukuro et al. demonstrated that postprandial endothelial dysfunction was correlated with increased plasma free fatty acid level but not other parameters, such as triacylglycerol, insulin, glucose, total cholesterol, HDL cholesterol, and adiponectin [23]. Increased plasma free fatty acid level results in intracellular accumulation of diacylglycerol (also known as a potent protein kinase C (PKC) activator) by esterification of free fatty acids, and subsequent PKC activation [24]. Elevated diacylglycerol level and PKC (especially, but not exclusively, PKC-βII isoform) activity in endothelial cells can inhibit Akt phosphorylation induced by insulin or vascular endothelial growth factor (VEGF) and subsequent eNOS expression [25]. PKCβ isoform-selective inhibitor, ruboxistaurin, showed a reduced toxicity compared with general PKC inhibitors, and have therapeutic potential for diabetic microvascular disease and, in particular, diabetic retinopathy [26]. Free fatty acids can also directly affect endothelial function by increasing reactive ROS production and reducing nitric oxide bioavailability within 15 minutes. Interestingly, polyunsaturated fatty acid, generally regarded as a “good” fatty acid, can inhibit endothelial dependent relaxation as well, although to a lesser extent compared with the saturated fatty acids [27]. Collectively, since plasma free fatty acid level is closely correlated with endothelial dysfunction in diabetic patients, dietary control of fat intake is very important in the management of diabetes-induced vascular complication. Although there is abundant evidence suggesting the protective effects of unsaturated fatty acid on vascular dysfunction in contrast to saturated fat, high levels of unsaturated fatty acid also have the potential to induce endothelial dysfunction.
4. Role of body weight control in vascular dysfunction
Several methods have been introduced for treating obese and type 2 diabetic patients such as diet, exercise, behavior modification, liposuction and medication [14, 28, 29]. There is, as yet, no consensus as to which method is the best for managing body weight and blood glucose level in type 2 diabetes. Exercise and diets have shown beneficial effects on body weight and as therapies for diabetes and vascular dysfunction; however, liposuction has not shown positive effects on the latter [29].
Recently, bariatric surgery was introduced as a newer and more aggressive treatment for the morbidly obese and diabetic patient; treatment dramatically improved type 2 diabetic conditions, resulting in normal blood glucose and allowed stoppage of diabetes-related medicine [30–32]. More importantly, endothelium-dependent vasodilation function is enhanced after gastric bypass surgery in morbidly obese patients with type 2 diabetes. Flow-mediated dilation of brachial artery [5] and bradykinin-mediated venodilation [33] are improved after surgery. However, the mechanism by which gastric bypass surgery improves endothelial function in morbidly obese and diabetic patients has not been clearly elucidated yet. Vazquez et al. suggested that an improved endothelial function after surgery was mainly due to alterations of NO production and eNOS activity [33]. This suggestion can be supported by the studies investigating the role of ghrelin in vascular function in the morbidly obese patients. Ghrelin level is lower in the individuals with obesity-associated metabolic syndrome, including obesity and type 2 diabetes, [34, 35] but gastric bypass surgery significantly increases the ghrelin level in the severely obese individuals [36, 37]. Ghrelin improves endothelial function in metabolic syndrome through increased eNOS expression and activity [34, 38] suggesting increased level of ghrelin after bariatric surgery may play a key role in improving endothelial function. However, no direct evidence has been presented yet; addressing mechanisms for how bariatric surgery ameliorates vascular dysfunction in obese and diabetic patients may provide promising therapeutic targets for type 2 diabetes-induced vascular dysfunction.
Concluding Remarks
Most investigations to elucidate therapeutic targets for the management of type 2 diabetes have focused on how to control insulin sensitivity and maintain glucose homeostasis. The present review identifies direct molecular targets for the treatment of type 2 diabetes and suggests they should be included in the quest to prevent and cure diabetes-induced vascular dysfunction. The therapeutic targets proposed in the review are based on in vivo and in vitro studies and, thus, the physiological relevance of these ideas is confirmed. We believe this work will lead to new therapies for treating this insidious disease.
Figure 1.
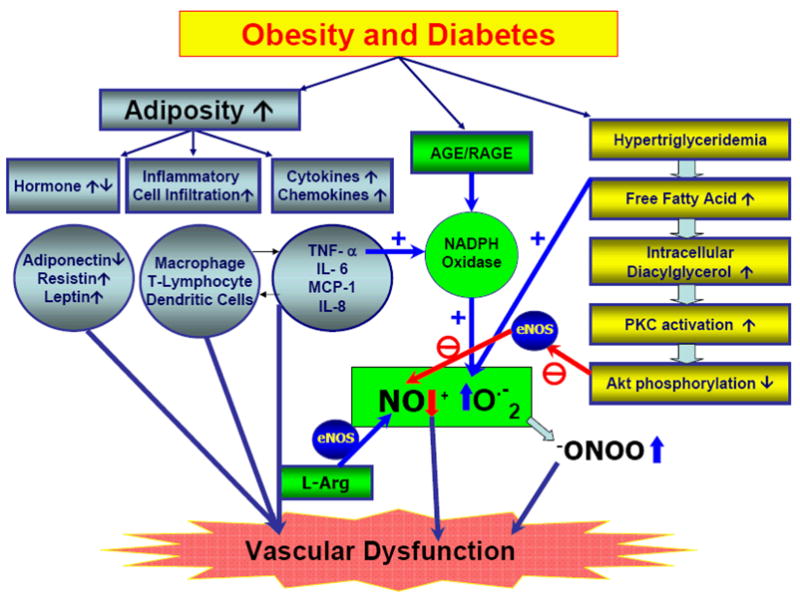
Schematic flow of obesity and type 2 diabetes-induced vascular dysfunction. Type 2 diabetes results in vascular dysfunction through the increase in 1) adiposity (blue), 2) NAPDH oxidase-induced ROS production in vasculature (green), and 3) hyperglyceridemia (yellow). Increased adiposity in obesity and type 2 diabetes is associated with adverse expression pattern of various adipose-derived hormones, inflammatiory cytokines and chemokines, and enhanced adipose inflammatory cell infiltration. Enhanced O.−2 radical production through TNF-α and AGE/RAGE signaling reduces NO bioavailability and results in impairment of vascular function. Diverse processes induced by hypertriglyceridemia lead to the generation of endothelial dysfunction and the onset of vascular diseases via decreased bioavailability of NO.
Acknowledgments
Funding Sources
This study was supported by grants from American Heart Association Scientist Development Grant (110350047A), Pfizer Atorvastatin Research Award (2004-37) and NIH grants (RO1-HL077566 and RO1-HL085119) to Dr. Cuihua Zhang.
References
- 1.Plutzky J, Viberti G, Haffner S. Atherosclerosis in type 2 diabetes mellitus and insulin resistance: mechanistic links and therapeutic targets. Journal of Diabetes and its Complications. 2002;16(6):401–415. doi: 10.1016/s1056-8727(02)00202-7. [DOI] [PubMed] [Google Scholar]
- 2.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 3.Mueller CF, Laude K, McNally JS, et al. Redox Mechanisms in Blood Vessels. Arterioscler Thromb Vasc Biol. 2005;25(2):274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 4.Kashyap SR, Belfort R, Cersosimo E, et al. Chronic low-dose lipid infusion in healthy patients induces markers of endothelial activation independent of its metabolic effects. J Cardiometab Syndr. 2008;3(3):141–146. doi: 10.1111/j.1559-4572.2008.00013.x. [DOI] [PubMed] [Google Scholar]
- 5.Gokce N, Vita JA, McDonnell M, et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. The American Journal of Cardiology. 2005;95(2):266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008 Sep 5; doi: 10.1093/eurheartj/ehn387. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekar B, Boylston WH, Venkatachalam K, et al. Adiponectin Blocks Interleukin-18-mediated Endothelial Cell Death via APPL1-dependent AMP-activated Protein Kinase (AMPK) Activation and IKK/NF-{kappa}B/PTEN Suppression. J Biol Chem. 2008;283(36):24889–24898. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eringa EC, Bakker W, Smulders YM, et al. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation. 2007;14(4–5):389–402. doi: 10.1080/10739680701303584. [DOI] [PubMed] [Google Scholar]
- 9.Wexler DJ, Hu FB, Manson JE, et al. Mediating effects of inflammatory biomarkers on insulin resistance associated with obesity. Obes Res. 2005;13(10):1772–1783. doi: 10.1038/oby.2005.216. [DOI] [PubMed] [Google Scholar]
- 10.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28(9):1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein BJ, Scalia R. Adipokines and vascular disease in diabetes. Curr Diab Rep. 2007;7(1):25–33. doi: 10.1007/s11892-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Belmadani S, Picchi A, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115(2):245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 14.Bruun JM, Helge JW, Richelsen B, et al. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290(5):E961–967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 15.Chinen I, Shimabukuro M, Yamakawa K, et al. Vascular Lipotoxicity: Endothelial Dysfunction via Fatty-Acid-Induced Reactive Oxygen Species Overproduction in Obese Zucker Diabetic Fatty Rats. Endocrinology. 2007;148(1):160–165. doi: 10.1210/en.2006-1132. [DOI] [PubMed] [Google Scholar]
- 16.Picchi A, Gao X, Belmadani S, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99(1):69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 17.Du X, Edelstein D, Obici S, et al. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116(4):1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Zhang H, Schmidt AM, et al. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2008;295(2):H491–498. doi: 10.1152/ajpheart.00464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perassolo MS, Almeida JC, Steemburgo T, et al. Endothelial dysfunction and serum fatty acid composition in patients with type 2 diabetes mellitus. Metabolism. 2008;57(9):1167–1172. doi: 10.1016/j.metabol.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Jackson KG, Armah CK, Minihane AM. Meal fatty acids and postprandial vascular reactivity. Biochem Soc Trans. 2007;35(Pt 3):451–453. doi: 10.1042/BST0350451. [DOI] [PubMed] [Google Scholar]
- 21.Burdge GC, Calder PC. Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br J Nutr. 2005;93(1):3–9. doi: 10.1079/bjn20041282. [DOI] [PubMed] [Google Scholar]
- 22.Chowienczyk PJ, Watts GF, Wierzbicki AS, et al. Preserved endothelial function in patients with severe hypertriglyceridemia and low functional lipoprotein lipase activity. J Am Coll Cardiol. 1997;29(5):964–968. doi: 10.1016/s0735-1097(97)00033-8. [DOI] [PubMed] [Google Scholar]
- 23.Shimabukuro M, Chinen I, Higa N, et al. Effects of dietary composition on postprandial endothelial function and adiponectin concentrations in healthy humans: a crossover controlled study. Am J Clin Nutr. 2007;86(4):923–928. doi: 10.1093/ajcn/86.4.923. [DOI] [PubMed] [Google Scholar]
- 24.Itani SI, Ruderman NB, Schmieder F, et al. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 25.Naruse K, Rask-Madsen C, Takahara N, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55(3):691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 26.Idris I, Donnelly R. Protein kinase C beta inhibition: A novel therapeutic strategy for diabetic microangiopathy. Diab Vasc Dis Res. 2006;3(3):172–178. doi: 10.3132/dvdr.2006.026. [DOI] [PubMed] [Google Scholar]
- 27.Edirisinghe I, McCormick Hallam K, Kappagoda CT. Effect of fatty acids on endothelium-dependent relaxation in the rabbit aorta. Clin Sci (Lond) 2006;111(2):145–151. doi: 10.1042/CS20060001. [DOI] [PubMed] [Google Scholar]
- 28.Fontana L, Villareal DT, Weiss EP, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293(1):E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 29.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350(25):2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 30.Rubino F. Is Type 2 Diabetes an Operable Intestinal Disease?: A provocative yet reasonable hypothesis. Diabetes Care. 2008;31(Supplement_2):S290–296. doi: 10.2337/dc08-s271. [DOI] [PubMed] [Google Scholar]
- 31.Couzin J. Medicine. Bypassing medicine to treat diabetes. Science. 2008;320(5875):438–440. doi: 10.1126/science.320.5875.438. [DOI] [PubMed] [Google Scholar]
- 32.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. Jama. 2008;299(3):316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez LA, Pazos F, Berrazueta JR, et al. Effects of Changes in Body Weight and Insulin Resistance on Inflammation and Endothelial Function in Morbid Obesity after Bariatric Surgery. J Clin Endocrinol Metab. 2005;90(1):316–322. doi: 10.1210/jc.2003-032059. [DOI] [PubMed] [Google Scholar]
- 34.Tesauro M, Schinzari F, Iantorno M, et al. Ghrelin Improves Endothelial Function in Patients With Metabolic Syndrome. Circulation. 2005;112(19):2986–2992. doi: 10.1161/CIRCULATIONAHA.105.553883. [DOI] [PubMed] [Google Scholar]
- 35.Poykko SM, Kellokoski E, Horkko S, et al. Low Plasma Ghrelin Is Associated With Insulin Resistance, Hypertension, and the Prevalence of Type 2 Diabetes. Diabetes. 2003;52(10):2546–2553. doi: 10.2337/diabetes.52.10.2546. [DOI] [PubMed] [Google Scholar]
- 36.Ybarra J, Bobbioni-Harsch E, Chassot G, et al. Persistent Correlation of Ghrelin Plasma Levels with Body Mass Index Both in Stable Weight Conditions and during Gastric-bypass-induced Weight Loss. Obesity Surgery. 2008 Oct 31; doi: 10.1007/s11695-008-9748-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, et al. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18(11):1424–1429. doi: 10.1007/s11695-008-9560-5. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Jhun BS, Ha CH, et al. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology. 2008;149(8):4183–4192. doi: 10.1210/en.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]


