Abstract
Objective
To determine the effects of switching from lopinavir/ritonavir (LPV/r) to atazanavir/ritonavir (ATV/r) on muscle glucose uptake, glucose homeostasis, lipids, and body composition.
Methods
Fifteen HIV-infected men and women on a regimen containing LPV/r and with evidence of hyperinsulinemia and/or dyslipidemia were randomized to continue LPV/r or to switch to ATV/r (ATV 300mg and ritonavir 100mg daily) for six months. The primary endpoint was change in thigh muscle glucose uptake as measured by positron emission tomography (PET). Secondary endpoints included abdominal visceral adipose tissue, fasting lipids and safety parameters. The difference over time between treatment groups (treatment effect of ATV/r relative to LPV/r) was determined by repeated measures ANCOVA.
Results
After six months, anterior thigh muscle glucose uptake increased significantly (treatment effect +18.2 ± 5.9 μmol/kg/min, ATV/r vs. LPV/r, p=0.035), and visceral adipose tissue (VAT) area decreased significantly in subjects who switched to ATV/r (treatment effect -31 ± 11cm2, ATV/r vs. LPV/r, p=0.047). Switching to ATV/r significantly decreased triglyceride (treatment effect -182 ± 64 mg/dL, ATV/r vs. LPV/r, p=0.02) and total cholesterol (treatment effect -23 ± 8 mg/dL, ATV/r vs. LPV/r, p=0.01), whereas HDL and LDL did not change significantly. Fasting glucose also decreased significantly following switch to ATV/r (treatment effect -15 ± 4 mg/dL, ATV/r vs. LPV/r, p=0.002).
Conclusions
Switching from LPV/r to ATV/r significantly increases glucose uptake by muscle, decreases abdominal visceral adipose tissue, improves lipid parameters and decreases fasting glucose over 6 months.
Keywords: HIV, lipodystrophy, atazanavir, lopinavir, glucose, intra-abdominal fat, lipids
Introduction
HIV-infected patients receiving highly active antiretroviral therapy often demonstrate changes in fat, characterized by central adiposity and/or loss of fat in the extremities and face, as well as dyslipidemia, insulin resistance, and increased cardiometabolic risk. A number of these abnormalities may be associated with protease inhibitor (PI) therapy. In vitro studies have demonstrated that PIs reduce glucose uptake via direct binding to the GLUT4 transporter protein [1]. GLUT4 inhibition occurs in a dose dependent manner and is evident within the range of the therapeutic concentrations achieved in HIV-infection [1, 2]. Both ritonavir (RTV) and lopinavir (LPV) are among the more potent inhibitors of GLUT4 [3, 4]. Atazanavir (ATV), a relatively newer PI, does not affect insulin stimulated glucose uptake in vitro [3-5], but this has not been previously assessed in vitro with RTV boosting. In addition, a sensitive measure of in vivo glucose uptake, Positron Emission Tomography (PET), has not previously been used for in vivo assessments. Many PIs, including RTV and LPV, also impair adipocyte differentiation [5-9], likely by preventing maturation and nuclear localization of sterol-regulator-element-binding-protein-1 (SREBP1) [10, 11]. In contrast, unboosted ATV does not have a significant in vitro effect on adipocyte differentiation [5, 6].
Taken together, in vitro studies suggest that ATV should have relatively less effect on insulin sensitivity and lipid metabolism than other PIs. Indeed, a study in healthy, non-HIV infected males has confirmed that short-term treatment with ATV does not alter insulin stimulated glucose uptake or glycogen storage rate, whereas LPV/r decreases both of these parameters [12]. Similarly, in HIV-infected subjects receiving antiretroviral therapy, switching the PI component to unboosted ATV decreased 2-hour glucose level following oral glucose tolerance test (OGTT) and significantly improved lipid parameters [13].
There is little evidence to date regarding the effects of boosted ATV (ATV/r) on glucose and lipids. In healthy, HIV-negative subjects, LPV/r decreased insulin stimulated glucose uptake to a significantly greater degree than ATV/r, but 2-hour glucose following OGTT increased following both LPV/r and ATV/r [14]. The current study compares the effects of LPV/r and ATV/r on body composition, lipids, insulin stimulated glucose uptake, and tissue-specific glucose uptake as measured by Positron Emission Tomography (PET) in HIV-infected individuals over a relatively long period of 6 months.
Methods
Subject Selection
Sixteen men and women with HIV-infection were recruited through informational mailings to HIV-care providers, postings in HIV community organizations, newspaper advertisements, and the MGH HIV database. Recruitment began in March, 2006, and the last patient was randomized in May, 2008. Written, informed consent was obtained from each subject in accordance with the Massachusetts General Hospital (MGH) and the Massachusetts Institute of Technology (MIT) institutional review boards. The study was approved by the MGH Institutional Review Board and by the MIT Committee on the Use of Humans as Experimental Subjects.
Inclusion criteria included age 18-65y, previously diagnosed HIV infection, stable antiretroviral (ARV) regimen including LPV/r for at least six months prior to study entry, and one or more of the following metabolic complications: fasting insulin ≥ 15 μU/mL, total cholesterol ≥ 200 mg/dL, triglycerides ≥ 150 mg/dL, or treatment with lipid lowering medication. Exclusion criteria included hemoglobin < 11.0 g/dL; pregnancy; history of diabetes mellitus or current therapy with an anti-diabetic agent; therapy with glucocorticoid, growth hormone, or other anabolic agents in the 3 months prior to study entry; contraindication to ATV/r use, including use of proton pump inhibitors, lovastatin, or simvastatin; and new or serious opportunistic infection. In addition, subjects were enrolled only if their HIV-care providers agreed to their participation.
Study Design
This was a six month, randomized, non-blinded comparison of continuing therapy with LPV/r vs. switching to ATV/r. All assessments were performed after a 12-hour overnight fast. After a screening visit to determine eligibility, participants underwent baseline testing, including single-slice computed tomography (CT) scan of the abdomen and mid-thigh, PET scan of the thigh with 2-deoxy-[18F]fluoro-D-glucose (FDG) infusion conducted during a euglycemic hyperinsulinemic clamp, dual energy X-ray absorptiometry (DEXA) scan, anthropometrics, and fasting laboratory evaluation. Four-day food records were collected, and a Modifiable Activity Questionnaire was administered.
Following the baseline evaluation, subjects were randomized in a 1:1 ratio to continue their current antiretroviral regimen including LPV/r or to switch from LPV/r to ATV/r (300mg atazanavir and 100mg ritonavir once daily). No other component of the antiretroviral regimen was changed during the study. The randomization list was prepared by the MGH pharmacy and kept by a third party who provided the treatment assignment to the study investigator at the end of each subject's baseline visit. Randomization was not blinded to patients, primary HIV care providers, or investigators in order to facilitate clinical monitoring, including the likely increase in bilirubin in those assigned to ATV/r.
Subjects returned for safety visits 2 weeks, 1 month, 2 months, and 4 months following randomization. At the 1 month visit, subjects also underwent 2-hour euglycemic hyperinsulinemic clamp. Six months following randomization, subjects returned for an evaluation identical to their baseline assessment.
CT of Thigh and Abdomen at L4
CT scans were performed with a LightSpeed 16-slice CT scanner (General Electric, Milwaukee, WI) as previously described [15, 16].
Hyperinsulinemic Euglycemic Clamp
Following a 12-hour fast, subjects received an infusion of 40 mU × m2 × min–1 regular insulin following a priming dose of 200 mU × m2 × min–1 given over 2 min. A variable infusion of 20% dextrose maintained plasma glucose concentrations at the euglycemic value of 5 mmol/l (90 mg/dl). Blood glucose was determined every 5 minutes using a B-Glucose Analyzer (Hemocue, Lake Forest, CA). Insulin samples were collected every 20 minutes. At baseline and 6 month visits, the clamp procedure continued until the PET scan was complete. At the 1 month visit, the duration of the clamp was 120 minutes.
FDG-PET of mid-thigh
Subjects were placed in a supine position and had placement of a venous catheter for infusion and a catheter in the radial artery for blood sampling. The midthigh region was centered in the camera's field using a reference mark corresponding to the single-slice CT scan. Euglycemic hyperinsulinemic clamp was started as described above. Once the subject's glucose had achieved steady state, transmission images of 10 min duration were acquired with a rotating 68Ge pin source. Following transmission, approximately 10 mCi of FDG were injected intravenously over 1.5 min, and sequential PET images were acquired. Arterial blood samples were obtained every 15s for 3 min, every 30s for 2 min, every 1 min for 5 min, every 5 min for 55 min, and at 75 and 90 min. Arterial FDG plasma radioactivity was measured using a calibrated Baird Atomic well counter.
Body Composition Analysis
Whole body dual-energy X-ray absorptiometry (DEXA) was performed using a Hologic QDR-4500 densitometer (Hologic, Bedford, MA) to determine total body and regional fat mass. Body fat was also measured using a Bioelectrical Impedance Analyzer (RJL Systems, Clinton Township, MI).
Assays
Assays were performed at the MIT Clinical Research Center core laboratory and the Harvard CTSA Core Lab. Serum insulin was measured using either radioimmunoassay (RIA; Siemens Medical Solutions Diagnostics, Deerfield, IL; intra-assay and interassay coefficients of variation from 3.1 to 9.3% and from 4.9 to 10.0%, respectively) or chemiluminescence immunoassay (Ultra-sensitive Beckman Access-2 Chemiluminescence platform; Beckman Coulter, Chaska, MN; sensitivity 0.03 IU/mL, precision 3-5.6%). The correlation between the two assays was r = 0.99, p<0.0001. HIV viral load (Ultrasensitive Roche Amplicor v1.5, lower limit of detection 50 copies/mL), CD4 count, serum glucose, lipids, and chemistries were measured using standard methodologies.
Calculations
Regional FDG glucose uptake
A General Electric/Scanditronix PC4096 15-slice whole body tomograph was used to produce sequential images over the thigh muscle over 90 min. Images were reconstructed using a filtered back-projection algorithm, and projection data were corrected for nonuniformity of detector response, dead time, random coincidences, and scattered radiation. Images were corrected for attenuation by a 68Ge transmission source. Four to six irregular-shaped regions of interest (ROIs) were placed, taking particular caution to exclude bone and major vascular structures. A three-compartment kinetic model was used to estimate glucose kinetics [17, 18]. A nonlinear least squares fitting procedure was used to determine the rate constants (k1, k2, k3, k4) using the arterial input function and the composite tissue time-activity curves. FDG metabolic rate (K) was calculated according to the equation K = (k1k3)/(k2 + k3) [17, 18]. Muscle glucose uptake was obtained by multiplying K by the plasma glucose concentration and then dividing by the lumped constant value of 1.2 [18-20].
Insulin-stimulated glucose disposal
The method of DeFronzo et al [21] was used to determine insulin-stimulated glucose disposal (M) for the interval between 100-120 minutes. M was indexed to fat-free mass (M/LBM, μmol·kg FFM–1·min–1) and corrected for insulin (M/I).
Statistics
The primary endpoint was insulin-stimulated thigh muscle glucose uptake measured by PET. Initial sample size of N=16 was calculated to provide 80% power to detect a 30% change in muscle glucose uptake between groups, using a standard deviation of 19μmol/kg/min [22]. Baseline characteristics in the two groups were compared using Student's t-test for normally distributed variables and Wilcoxon Rank Sum test for non-normally distributed variables. For variables measured only at baseline and six months (PET, CT, DEXA, dietary intake, and physical activity data), Student's t-test was used to compare changes from baseline and determine the treatment effect (net difference over time between the ATVr vs. LPV/r groups) over 6 months. For all other variables, repeated measures ANCOVA, controlling for baseline values, was used to assess the treatment effect of the randomization over 6 months (net difference over time between the ATV/r vs. LPV/r groups). All available data were used. In all Student's t-test calculations, unequal variances were assumed. SAS was used for repeated measures analysis. All other statistical analyses were performed using SAS JMP software, version 5.0.1.2 (SAS Institute, Cary, NC). Statistical significance was defined as p<0.05. Results are mean ± SEM unless otherwise indicated.
Results
Of 16 subjects who enrolled in the study, one subject discontinued prior to the end of his baseline visit and was not randomized or included in the analysis. Following randomization, one subject in the ATV/r group discontinued for personal reasons. Data from 14 subjects (6 ATV/r and 8 LPV/r) were available 1 month after randomization. Following the one-month visit, one subject in the ATV/r group discontinued because of increased viral load following the PI switch, and one subject in the LPV/r group who had low grade viremia at baseline discontinued because subsequent HIV-genotyping showed resistance to LPV, necessitating a change in antiretroviral regimen. Data from 12 subjects (5 ATV/r and 7 LPV/r) were available 6 months after randomization. One person in each treatment group was receiving stable lipid lowering therapy.
Baseline Characteristics
Baseline clinical characteristics are shown in Table 1. Eighty percent of subjects were men, and 73% of subjects had undetectable HIV viral load (<50 copies/mL). Mean duration of HIV-infection was 14 ± 1 years. The components of subjects' antiretroviral regimens are listed in Table 2. On average, subjects had been on their current ARV regimen for 3 ± 0.5 years; all subjects had been taking their current ARVs for at least 9 months. There were no significant differences between treatment groups in baseline use of specific NRTIs, and each subject's NRTI backbone remained the same throughout the study. Mean body mass index (BMI) of the cohort was 29.3±1.9 kg/m2. BMI and body composition measures were not different between the two study groups (Tables 1 and 3). Waist circumference was not different between the groups (104 ± 8 vs. 100 ± 4 cm, p=0.69). Subjects randomized to ATV/r had higher LDL values at baseline (128 ± 8 vs. 94 ± 12 mg/dL, p=0.03) and a trend toward higher total cholesterol levels at baseline (215 ± 12 vs. 182 ± 10 mg/dL, p=0.06). There was also a trend toward lower CD4+ counts in the ATV/r group at baseline (417 ± 70 vs. 623 ± 66, ATV/r vs. LPV/r, p=0.05). There were no statistically significant baseline differences in AST, ALT, bilirubin, muscle glucose uptake measured by PET, or glucose uptake (M/I) during euglycemic clamp (Table 3). In addition, there were no baseline differences in total daily caloric intake (2059±340 vs. 2373±244 kcal/day, ATV/r vs. LPV/r, p=0.47), macronutrient intake, or physical activity as measured by Modifiable Activity Questionnaire (data not shown).
Table 1. Baseline Clinical Characteristics by Treatment Group.
| ATV/r (N=7) | LPV/r (N=8) | p-value* | |
|---|---|---|---|
| Age (y) | 46 ± 3 | 50 ± 2 | 0.24 |
| Sex (M/F) | 5/2 | 7/1 | 0.57 |
| Duration of HIV (y) | 14 ± 2 | 13 ± 2 | 0.90 |
| HIV RNA < 50 c/ML (%) | 71 | 75 | 1.00 |
| BMI (kg/m2) | 29.8 ± 3.4 | 28.9 ± 2.1 | 0.82 |
p-value by Student's t-test for continuous variables and by Fisher's Exact Test for nominal variables (sex, % HIV RNA < 50 c/mL). Results are (mean ± SEM).
Table 2. Baseline Antiretroviral Medications by Treatment Group.
| Baseline ARV Regimen (+LPV/r) | ATV/r (N=7) | LPV/r (N=8) |
|---|---|---|
| Abacavir and Tenofovir DF | 0 | 2 |
| Abacavir and Zidovudine | 1 | 0 |
| Didanosine and Tenofovir DF | 1 | 0 |
| Lamivudine/Zidovudine | 0 | 1 |
| Lamivudine and Nevirapine | 0 | 1 |
| Lamivudine, Tenofovir DF, and Nevirapine | 0 | 1 |
| Saquinavir | 0 | 1 |
| Tenofovir DF | 1 | 0 |
| Tenofovir DF/Emtricitabine | 4 | 2 |
Table 3. Treatment Effect on Body Composition, Metabolic, Immune Function and Safety Parameters.
| ATV/r | LPV/r | Treatment Effect (ATV/r vs LPV/r) | p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2wk | 1mo | 2mo | 4mo | 6mo | Baseline | 2wk | 1mo | 2mo | 4mo | 6mo | |||
| Body Composition | ||||||||||||||
| Body weight (kg) | 90.0 ± 14.2 | 89.5±14.3 | 93.1 ± 16.2 | 94.2 ± 19.7 | 94.2 ± 18.5 | 92.2 ± 19.0 | 86.3 ± 4.4 | 86.7±5.1 | 86.1 ± 4.5 | 85.8±4.3 | 85.2 ± 4.5 | 85.7 ± 4.5 | -1.2 ± 0.7 | 0.10 |
| DEXA % Body Fat | 26.2 ± 2.1 | - | - | - | - | 23.4± 3.2 | 23.0 ± 2.7 | - | - | - | - | 24.1 ± 3.0 | -3.3 ± 2.3 | 0.23 |
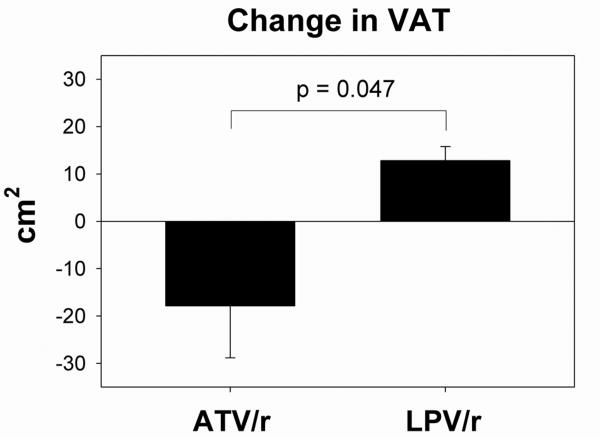
| VAT (cm2) | 116 ± 17 | - | - | - | - | 91 ± 15 | 159 ± 21 | - | - | - | - | 167 ± 23 | -31 ± 11 | 0.047 |
| SAT (cm2) | 322 ± 84 | - | - | - | - | 205 ± 41 | 229 ± 48 | - | - | - | - | 242 ± 51 | -41 ± 33 | 0.29 |
| Thigh SC fat (cm2) | 89 ± 19 | - | - | - | - | 87 ± 23 | 56 ± 11 | - | - | - | - | 64 ± 12 | -15 ± 11 | 0.24 |
| Glucose Parameters | ||||||||||||||
| Thigh muscle glucose uptake by PET (μmol/kg/min) | 13.0 ± 4.9 | - | - | - | - | 26.7 ± 5.7 | 26.7 ± 5.9 | - | - | - | - | 24.4 ± 7.9 | 18.2 ± 5.9 | 0.035 |
| Fasting gluc (mg/dL) | 87 ± 3 | 73 ± 4 | 78 ± 4 | 78 ± 6 | 82 ± 5 | 84 ± 3 | 87 ± 4 | 100 ± 12 | 98 ± 10 | 96 ± 8 | 93 ± 9 | 90 ± 8 | -15 ± 4 | 0.002 |
| M/I LBM (μmol/kg/min per μu/mL insulin × 100) | 60.7 ± 20.1 | 187.5±146.9 | 39.0 ± 7.9 | 105.7 ± 41.9 | 61.7 ± 18.1 | 49.2 ± 8.5 | 94.9 ± 56.2 | 0.12 | ||||||
| Lipid Parameters | ||||||||||||||
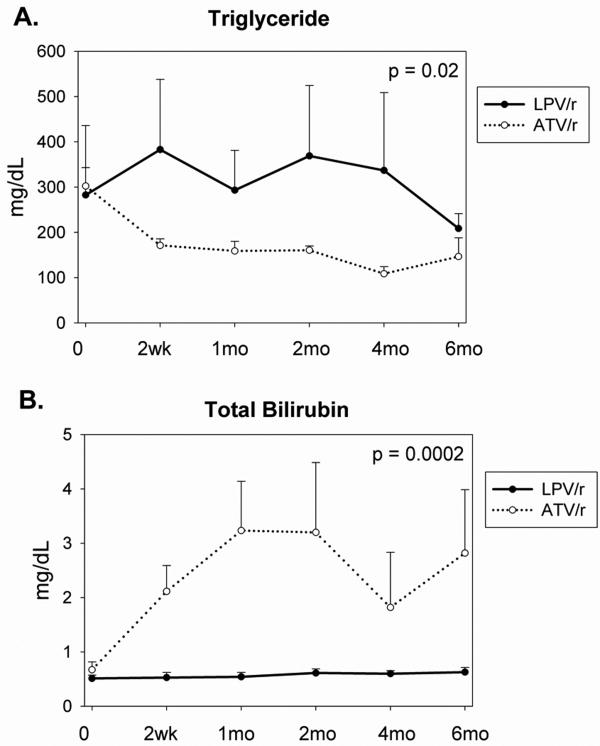
| Triglycerides (mg/dL) | 302 ± 134 | 171 ± 14 | 159 ± 21 | 161 ± 10 | 109 ± 16 | 147 ± 41 | 283 ± 61 | 383±155 | 293 ± 88 | 369±155 | 337 ± 172 | 209 ± 33 | -182 ± 64 | 0.02 |
| Total cholesterol (mg/dL) | 215 ± 12 | 213 ± 18 | 197 ± 18 | 194 ± 12 | 201 ± 16 | 171 ± 10 | 182 ± 10 | 195 ± 17 | 193 ± 11 | 194 ± 14 | 192 ± 14 | 178 ± 6 | -23 ± 8 | 0.01 |
| HDL (mg/dL) | 42 ± 4 | 45 ± 4 | 42 ± 7 | 46 ± 5 | 47 ± 7 | 42 ± 4 | 36 ± 4 | 41 ± 6 | 38 ± 4 | 37 ± 3 | 41 ± 4 | 36 ± 3 | 1 ± 2 | 0.54 |
| LDL (mg/dL) | 128 ± 8 | 133 ± 15 | 126 ± 10 | 124 ± 10 | 130 ± 10 | 106 ± 8 | 94 ± 12 | 92 ± 14 | 104 ± 11 | 106 ± 13 | 100 ± 12 | 103 ± 9 | -5 ± 6 | 0.44 |
| Non-HDL Cholesterol (mg/dL) | 173 ± 9 | 168 ± 17 | 156 ± 15 | 148 ± 12 | 154 ± 13 | 129 ± 6 | 147 ± 10 | 154 ± 16 | 156 ± 12 | 157 ± 13 | 151 ± 15 | 142 ± 6 | -25 ± 8 | 0.007 |
| Immune Function | ||||||||||||||
| CD4 count (th/mm3) | 417 ± 70 | - | 402 ± 88 | 478 ± 92 | 475 ± 135 | 432 ± 86 | 623 ± 66 | - | 593 ± 66 | 617 ± 78 | 734 ± 70 | 688 ± 87 | 12 ± 34 | 0.72 |
| Viral load (Log10 copies/mL) | 0.5 ± 0.3 | - | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.4 | - | 0.6 ± 0.4 | 0.4 ± 0.4 | 0.0 ± 0.0 | 0.8 ± 0.8 | -0.2 ± 0.2 | 0.31 |
| Safety Parameters | ||||||||||||||
| ALT (U/L) | 55 ± 9 | 71 ± 9 | 65 ± 5 | 58 ± 8 | 68 ± 15 | 61 ± 13 | 60 ± 10 | 55 ± 8 | 48 ± 7 | 50 ± 9 | 59 ± 13 | 65 ± 13 | 16 ± 4 | 0.004 |
| AST (U/L) | 43 ± 8 | 40 ± 8 | 44 ± 7 | 36 ± 7 | 61 ± 32 | 39 ± 13 | 38 ± 10 | 31 ± 6 | 33 ± 12 | 29 ± 7 | 42 ± 15 | 42 ± 11 | 7 ± 6 | 0.22 |
| Total bilirubin (mg/dL) | 0.7 ± 0.1 | 2.1 ± 0.5 | 3.2 ± 0.9 | 3.2 ± 1.3 | 1.8 ± 1.0 | 2.8 ± 1.2 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 1.7 ± 0.3 | 0.0002 |
Mean ± SEM for each treatment group at each study visit. For variables measured only at baseline and 6 months, treatment effect and p-value determined by Student's t-test for normally distributed variables. For all other variables, measured at multiple time points, treatment effect equal to the net difference between the treatment groups over time and p-value determined from repeated measures ANCOVA. All baseline comparisons P>0.05 between groups except for CD4, P=0.05 and LDL, P=0.03. VAT is visceral adipose tissue; SAT is subcutaneous adipose tissue. Thigh SC fat determined by cross-sectional CT scan.
Glucose uptake by thigh muscle (PET)
Insulin mediated glucose uptake into anterior thigh muscle increased significantly in subjects who switched to ATV/r compared to those who remained on LPV/r (p=0.035, Table 3).
Glucose Homeostasis
Fasting glucose decreased significantly in the ATV/r vs. the LPV/r group (p=0.002), with a net difference over time between the treatment groups of -15 ± 4 mg/dL in the ATV/r vs. LPV/r group, Table 3. For whole body insulin stimulated glucose disposal as measured during euglycemic, hyperinsulinemic clamp, the treatment effect trended in a direction favoring ATV/r, but the p-value was not significant (p=0.12).
Body Composition
Body weight did not change significantly in either group during the study (Table 3). Subjects who changed to ATV/r demonstrated a significant decrease in VAT (change from baseline -18 ± 11 vs. +13 ± 3 cm2, net difference of -31 ± 11 cm2, ATV/r vs. LPV/r, p=0.047, Figure 2). Mean decrease in VAT from baseline was 15.5% in the ATV/r group, compared to a mean increase of 10.8% in the LPV/r group. No significant effects of switching to ATV/r were demonstrated on abdominal SC fat, thigh SC fat, or percent total body fat (Table 3). Neither total fat, extremity fat, nor leg fat by DEXA changed significantly between the groups (data not shown).
Fig 2.
Change in visceral adipose tissue (VAT) after 6 months in each treatment group. p=0.047 for comparison between groups by Student's t-test.
Lipids
Fasting serum triglyceride decreased significantly in the ATV/r group compared to the LPV/r group, p=0.02, Figure 1A (net difference over time between treatment groups was -182 ± 64 mg/dL, ATV/r vs. LPV/r, Table 3). Similarly, fasting total cholesterol (p=0.01) and non-HDL cholesterol (p=0.007) decreased significantly in the ATV/r group (Table 3). No significant change was observed in HDL or LDL (Table 3).
Fig 1.
Fig 1A: Change in serum triglyceride concentrations in each treatment group during the study. p=0.02 for comparison between groups by repeated measures ANCOVA.
Fig 1B: Change in serum bilirubin concentrations in each treatment group during the study. p=0.0002 for comparison between groups by repeated measures ANCOVA.
Immune Function
Neither CD4 nor viral load changed significantly between treatment groups over 6 months (Table 3).
Diet and Exercise
Four day food records collected at baseline and six month visits showed no significant changes between groups in total caloric intake (change from baseline -322±78 vs. -273±326 kcal/day, ATV/r vs. LPV/r, p=0.89) or intake of carbohydrate, fat, or protein (data not shown). Metabolic equivalents (METs) of leisure-time activity also did not significantly change between groups (data not shown).
Adverse Effects
Total bilirubin increased significantly during the study in the ATV/r relative to the LPV/r group (Table 3, Figure 1B). Two subjects in the ATV/r group had grade 3 elevation (bilirubin 2.6 - 5.0 mg/dL [23]), but bilirubin normalized in one of these subjects by the 2 month visit. Two ATV/r subjects had grade 4 elevation (bilirubin > 5.0 mg/dL [23]); in both cases this elevation persisted throughout the study. No subject developed symptoms or discontinued due to hyperbilirubinemia. Subjects in the ATV/r compared to the LPV/r group also demonstrated a mild but significant increase in serum ALT (p=0.004, Table 3). No subject had ALT elevation of Grade 2 or greater (≥2.6 times ULN [23]) throughout the study. There was no significant difference in AST between treatment groups.
Discussion
Disorders of glucose homeostasis and dyslipidemia have been associated with use of specific PIs and may contribute to increased cardiovascular disease risk in HIV-infected patients receiving chronic HAART[24]. ATV has demonstrated reduced effects on glucose and lipids, but limited studies have been done in which patients have been switched to ATV in the context of continuing other ARVs and maintaining ongoing ritonavir at low boosting doses. In this study, NRTI backbones were similar between treatment groups and did not change during the study, isolating metabolic differences between LPV/r and ATV/r. We show that switching from LPV/r to ATV/r increased glucose uptake in the anterior thigh muscle as measured by PET, decreased triglyceride and total cholesterol, and significantly reduced visceral fat over 6 months. To our knowledge, this is the first study to demonstrate that switching to ATV/r from LPV/r increases glucose uptake into muscle in vivo using PET.
The findings that switching from LPV/r to ATV/r increases glucose uptake into muscle and decreases fasting glucose are consistent with studies showing that LPV and RTV inhibit GLUT4 in vitro [1-4], whereas ATV does not demonstrate an effect on glucose uptake [3, 4]. Whole body glucose disposal by clamp approached, but did not reach significance, with a treatment effect favoring ATV/r vs. LPV/r. Limited sample size and variability may have contributed to this result. In addition, the clamp results might reflect ongoing hepatic insulin resistance in spite of improved muscle-specific glucose uptake, as shown specifically by PET. It is particularly significant that subjects in our study who switched to ATV/r demonstrated increased muscle glucose uptake in spite of continued RTV boosting. The RTV used for boosting, combined with the other ARVs in our subjects' treatment regimens, may have diminished the overall improvement in insulin sensitivity.
Our finding of a beneficial effect on lipids, equal to an improvement of approximately 180 mg/dL in triglyceride and 20 mg/dL in total cholesterol over 6 months following a switch to ATV/r, is consistent with the results of other studies comparing ATV/r to other regimens in both HAART-naïve and treatment experienced populations [25-27]. Low dose ritonavir has itself been shown to contribute to dyslipidemia [28, 29], and thus it is clinically relevant that we have shown a significant reduction in triglyceride levels in the context of ongoing ritonavir therapy.
To our knowledge, the significant decrease in visceral adipose tissue after switch to ATV/r has not been reported previously, and it is of a similar magnitude (-15%) to that observed in other studies with strategies such as GH or GHRH analogues [30-34]. Jemsek et al demonstrated that, in a cohort of antiretroviral-naïve subjects, both ATV and efavirenz increased VAT to a similar degree after 48 weeks of treatment [35]. Likewise, Moyle et al did not demonstrate a difference in VAT or SAT 48 weeks after a switch from other PIs to ATV/r [36]. The cohorts in these studies, however, may have had fewer metabolic abnormalities than our subjects. The eligibility criteria for Moyle's study specified waist circumference >90cm, whereas our study population had a mean waist circumference of 102 ± 4 cm and therefore may have had more visceral adiposity. Similarly, the median BMI in the Jemsek cohort was 23kg/m2, compared to an average BMI in our cohort of 29 kg/m2. Further, the Jemsek cohort was antiretroviral-naïve, whereas our subjects were treatment-experienced and selected on the basis of hyperinsulinemia or hyperlipidemia secondary to HAART. Recent data from Pischon et al [37] demonstrate that increased central adiposity poses a risk for increased mortality, independent of BMI among non-HIV patients. Though similar studies have not been done in the HIV population, reduction in visceral fat is nonetheless likely a useful treatment goal for HIV patients [38]. Moreover, the reduction in visceral fat with switch to ATV/r suggests that LPV/r was contributing to increased visceral adiposity in our patients, as no other element of the ARV regimen was changed.
In terms of safety, we did not see an overall change in immune function among patients completing the study, but one patient was discontinued for an increase in viral load on ATV/r and subsequently achieved virologic suppression after changing to another regimen. Switching to ATV/r from LPV/r should only be considered in those patients who have stable virologic suppression and no contraindications to atazanavir, including hypersensitivity and the use of proton pump inhibitors or certain statins. Moreover, any switch of ARV therapy carries a risk of virologic failure and requires careful, frequent monitoring. For those patients who are virologically stable and are experiencing significant metabolic complications from HAART, however, our data suggest that a switch to ATV/r may ameliorate cardiometabolic risk.
We did see anticipated increases in bilirubin, although none of the patients had clinically significant increases in magnitude to necessitate study discontinuation. There was also a small but statistically significant increase in ALT in the group that switched to ATV/r. No patient developed grade 2-4 ALT elevation in either the LPV/r or ATV/r group, however, and no subject discontinued the study because of ALT elevation. Increase in transaminases after switch to ATV/r has not been reported in larger studies [27, 39], and the finding of increased ALT in our smaller study requires further investigation.
Our study has a number of limitations. Our sample size was small due to the complexity of the study procedures, and thus we may have been underpowered to detect changes in specific parameters. While it is clear that ATV/r improved glucose uptake into the muscle and overall glucose levels, further studies characterizing hepatic glucose production during clamp will be needed to determine the direct effects of LPV/r switching to ATV/r on hepatic insulin resistance. Moreover, changes in glucose metabolism may have been greater without the use of ritonavir, but we felt that use of boosted atazanavir was more clinically relevant because recommendations for initial treatment of HIV specify PI boosting with low-dose ritonavir [40].
In conclusion, this study demonstrates that switching from LPV/r to ATV/r increases muscle glucose uptake, reduces visceral adiposity, and decreases triglyceride and total cholesterol. Larger studies are needed to confirm whether the metabolic advantages we demonstrated from switching to ATV/r can be sustained longer than 6 months, in a larger population of HIV-infected patients, and whether the increases in glucose trafficking into muscle will eventually translate into improved overall insulin sensitivity. Moreover, it will be important to determine whether changes in visceral fat, glucose and lipids translate into decreased cardiovascular disease risk, large enough to justify the risks associated with such a switch. However, our study at least suggests the potential utility of such a switch, with continued low dose ritonavir boosting, for the HIV patient on long term HAART with hyperinsulinemia, dyslipidemia and increased central adiposity on LPV/r.
Acknowledgments
The authors acknowledge the support of Hang Lee, Ph.D. in the MGH Biostatistical Center the MGH and MIT bionutrition and nursing staffs, the personnel of the PET lab and the patients for their participation in the study.
Funding: Funding was provided by BMS in the form of an investigator initiated research grant and NIH M01-RR-01066 and 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. NIH funding also provided by T32 HD052961-03 trainee support to T.S., K01 AI062435 to G.R., and K24 DK064545-06 to S.G. G.R. has received grant support from Boehringer Ingelheim, Gilead, and Schering-Plough and has been a consultant for Abbott Laboratories. S.G. has received research support from Bristol Myers Squibb and Theratechnologies and served as a consultant for Theratechnologies and Serono, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of Authors:
T. Stanley: recruitment, study procedures, data collection, data analysis, manuscript preparation.
T. Joy: recruitment, study procedures, data collection, manuscript preparation.
C. Hadigan: study design, study procedures, manuscript preparation.
J. Liebau: recruitment, study procedures, data collection.
H. Makimura: study procedures, data collection.
C. Chen: data analysis, manuscript preparation.
B. Thomas: study procedures, data collection, data analysis.
S. Weise: study procedures, data collection, data analysis.
G. Robbins: recruitment, manuscript preparation.
S. Grinspoon: study design, data analysis, manuscript preparation.
ClinTrials Registration: NCT00413153
References
- 1.Hertel J, Struthers H, Horj CB, Hruz PW. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J Biol Chem. 2004;279:55147–55152. doi: 10.1074/jbc.M410826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–20254. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 3.Parker RA, Flint OP, Mulvey R, Elosua C, Wang F, Fenderson W, et al. Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Mol Pharmacol. 2005;67:1909–1919. doi: 10.1124/mol.104.010165. [DOI] [PubMed] [Google Scholar]
- 4.Yan Q, Hruz PW. Direct comparison of the acute in vivo effects of HIV protease inhibitors on peripheral glucose disposal. J Acquir Immune Defic Syndr. 2005;40:398–403. doi: 10.1097/01.qai.0000176654.97392.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim RJ, Wilson CG, Wabitsch M, Lazar MA, Steppan CM. HIV protease inhibitor-specific alterations in human adipocyte differentiation and metabolism. Obesity (Silver Spring) 2006;14:994–1002. doi: 10.1038/oby.2006.114. [DOI] [PubMed] [Google Scholar]
- 6.Jones SP, Waitt C, Sutton R, Back DJ, Pirmohamed M. Effect of atazanavir and ritonavir on the differentiation and adipokine secretion of human subcutaneous and omental preadipocytes. Aids. 2008;22:1293–1298. doi: 10.1097/QAD.0b013e3283021a4f. [DOI] [PubMed] [Google Scholar]
- 7.Pacenti M, Barzon L, Favaretto F, Fincati K, Romano S, Milan G, et al. Microarray analysis during adipogenesis identifies new genes altered by antiretroviral drugs. Aids. 2006;20:1691–1705. doi: 10.1097/01.aids.0000242815.80462.5a. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, MacNaul K, Szalkowski D, Li Z, Berger J, Moller DE. Inhibition of adipocyte differentiation by HIV protease inhibitors. J Clin Endocrinol Metab. 1999;84:4274–4277. doi: 10.1210/jcem.84.11.6234. [DOI] [PubMed] [Google Scholar]
- 9.Vernochet C, Azoulay S, Duval D, Guedj R, Cottrez F, Vidal H, et al. Human immunodeficiency virus protease inhibitors accumulate into cultured human adipocytes and alter expression of adipocytokines. J Biol Chem. 2005;280:2238–2243. doi: 10.1074/jbc.M408687200. [DOI] [PubMed] [Google Scholar]
- 10.Caron M, Auclair M, Vigouroux C, Glorian M, Forest C, Capeau J. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001;50:1378–1388. doi: 10.2337/diabetes.50.6.1378. [DOI] [PubMed] [Google Scholar]
- 11.Bastard JP, Caron M, Vidal H, Jan V, Auclair M, Vigouroux C, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359:1026–1031. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 12.Noor MA, Parker RA, O'Mara E, Grasela DM, Currie A, Hodder SL, et al. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. Aids. 2004;18:2137–2144. doi: 10.1097/00002030-200411050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Guffanti M, Caumo A, Galli L, Bigoloni A, Galli A, Dagba G, et al. Switching to unboosted atazanavir improves glucose tolerance in highly pretreated HIV-1 infected subjects. Eur J Endocrinol. 2007;156:503–509. doi: 10.1530/EJE-06-0648. [DOI] [PubMed] [Google Scholar]
- 14.Noor MA, Flint OP, Maa JF, Parker RA. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. Aids. 2006;20:1813–1821. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 15.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 16.Hadigan C, Kamin D, Liebau J, Mazza S, Barrow S, Torriani M, et al. Depot-specific regulation of glucose uptake and insulin sensitivity in HIV-lipodystrophy. Am J Physiol Endocrinol Metab. 2006;290:E289–298. doi: 10.1152/ajpendo.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley DE, Mintun MA, Watkins SC, Simoneau JA, Jadali F, Fredrickson A, et al. The effect of non-insulin-dependent diabetes mellitus and obesity on glucose transport and phosphorylation in skeletal muscle. J Clin Invest. 1996;97:2705–2713. doi: 10.1172/JCI118724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertoldo A, Cobelli C. Physiological Modeling of Positron Emission Tomograpy Images. In: Carson E, Cobelli C, editors. Modelling Methodology for Physiology and Medicine. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 19.Peltoniemi P, Lonnroth P, Laine H, Oikonen V, Tolvanen T, Gronroos T, et al. Lumped constant for [(18)F]fluorodeoxyglucose in skeletal muscles of obese and nonobese humans. Am J Physiol Endocrinol Metab. 2000;279:E1122–1130. doi: 10.1152/ajpendo.2000.279.5.E1122. [DOI] [PubMed] [Google Scholar]
- 20.Williams KV, Price JC, Kelley DE. Interactions of impaired glucose transport and phosphorylation in skeletal muscle insulin resistance: a dose-response assessment using positron emission tomography. Diabetes. 2001;50:2069–2079. doi: 10.2337/diabetes.50.9.2069. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 22.Virtanen KA, Iozzo P, Hallsten K, Huupponen R, Parkkola R, Janatuinen T, et al. Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes: a positron-emitting tomography study. Diabetes. 2005;54:2720–2726. doi: 10.2337/diabetes.54.9.2720. [DOI] [PubMed] [Google Scholar]
- 23.Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, version 1.0. Dec, 2004. [Google Scholar]
- 24.Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 25.Mobius U, Lubach-Ruitman M, Castro-Frenzel B, Stoll M, Esser S, Voigt E, et al. Switching to atazanavir improves metabolic disorders in antiretroviral-experienced patients with severe hyperlipidemia. J Acquir Immune Defic Syndr. 2005;39:174–180. [PubMed] [Google Scholar]
- 26.Gatell J, Salmon-Ceron D, Lazzarin A, Van Wijngaerden E, Antunes F, Leen C, et al. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: the SWAN Study (AI424-097) 48-week results. Clin Infect Dis. 2007;44:1484–1492. doi: 10.1086/517497. [DOI] [PubMed] [Google Scholar]
- 27.Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 28.Shafran SD, Mashinter LD, Roberts SE. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 2005;6:421–425. doi: 10.1111/j.1468-1293.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 29.Danner SA, Carr A, Leonard JM, Lehman LM, Gudiol F, Gonzales J, et al. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease.European-Australian Collaborative Ritonavir Study Group. N Engl J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 30.Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357:2359–2370. doi: 10.1056/NEJMoa072375. [DOI] [PubMed] [Google Scholar]
- 31.Falutz J, Allas S, Mamputu JC, Potvin D, Kotler D, Somero M, et al. Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. Aids. 2008;22:1719–1728. doi: 10.1097/QAD.0b013e32830a5058. [DOI] [PubMed] [Google Scholar]
- 32.Kotler DP, Muurahainen N, Grunfeld C, Wanke C, Thompson M, Saag M, et al. Effects of growth hormone on abnormal visceral adipose tissue accumulation and dyslipidemia in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;35:239–252. doi: 10.1097/00126334-200403010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Grunfeld C, Thompson M, Brown SJ, Richmond G, Lee D, Muurahainen N, Kotler DP. Recombinant human growth hormone to treat HIV-associated adipose redistribution syndrome: 12 week induction and 24-week maintenance therapy. J Acquir Immune Defic Syndr. 2007;45:286–297. doi: 10.1097/QAI.0b013e3180691145. [DOI] [PubMed] [Google Scholar]
- 34.Lo J, You SM, Canavan B, Liebau J, Beltrani G, Koutkia P, et al. Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation: a randomized controlled trial. Jama. 2008;300:509–519. doi: 10.1001/jama.300.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jemsek JG, Arathoon E, Arlotti M, Perez C, Sosa N, Pokrovskiy V, et al. Body fat and other metabolic effects of atazanavir and efavirenz, each administered in combination with zidovudine plus lamivudine, in antiretroviral-naive HIV-infected patients. Clin Infect Dis. 2006;42:273–280. doi: 10.1086/498505. [DOI] [PubMed] [Google Scholar]
- 36.Moyle G, Girard JM, Andrade J, Salvato P, Bogner J, Hay P, et al. Continuation of BID boosted PI vs switch to once-daily ATV/RTV for the management of lipodystrophy: 48 week primary analysis of the 96 week multicenter, open-label, randomized, prospective ReAL study. XVII International AIDS Conference; Mexico City. 2008. [Google Scholar]
- 37.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 38.Snyder S. Forum for Collaborative HIV Research. Washington, D.C.: George Washington University School of Public Health and Health Services; 2004. Regulatory Considerations for the Treatment of Lipodystrophy. [Google Scholar]
- 39.Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. Aids. 2006;20:711–718. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 40.Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. Jama. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]