Abstract
I/R injury is a major deleterious factor of successful kidney transplantation (KTx). Carbon monoxide (CO) is an endogenous gaseous regulatory molecule, and exogenously delivered CO in low concentrations provides potent cytoprotection. This study evaluated efficacies of CO exposure to excised kidney grafts to inhibit I/R injury in the pig KTx model. Porcine kidneys were stored for 48 hrs in control UW or UW supplemented with CO (CO-UW) and autotransplanted in a 14-day follow-up study. In the control UW group, animal survival was 80% (4/5) with peak serum creatinine levels of 12.0±5.1 mg/dl. CO-UW showed potent protection, and peak creatinine levels were reduced to 6.9±1.4 mg/dl with 100% (5/5) survival without any noticeable adverse event or abnormal COHb value. Control grafts at 14d showed significant tubular damages, focal fibrotic changes, and numerous infiltrates. The CO-UW group showed significantly less severe histopathological changes with less TGF-β and p-Smad3 expression. Grafts in CO-UW also showed significantly lower early mRNA levels for proinflammatory cytokines and less lipid peroxidation. CO in UW provides significant protection against renal I/R injury in the porcine KTx model. Ex vivo exposure of kidney grafts to CO during cold storage may therefore be a safe strategy to reduce I/R injury.
INTRODUCTION
Ischemia/reperfusion injury (I/R) of the kidney graft has been considered one of the major deleterious factors of successful kidney transplantation (KTx). In the immediate posttransplant period, it increases risks of delayed and primary-non-function of transplanted grafts and complicates posttransplant recipient management. In addition, I/R injury has been identified as a key risk factor in predisposing earlier development of chronic allograft nephropathy and short graft life by accelerating alloantigen specific immune reactions (1–2). Particularly in the current organ shortage era with an increasing use of ECD kidneys, graft injury due to cold preservation and warm reperfusion can be augmented and significantly impacts renal allograft function. Thus, effective strategies to inhibit initial renal I/R injury would improve short- and long-term outcomes of KTx.
Carbon monoxide (CO) is commonly viewed as a poison in high concentrations due to its ability to bind to hemoglobin and interfere with oxygen delivery. However, mammalian cells endogenously generate CO primarily via the catalysis of heme by heme oxygenases (HO) (3), and endogenously generated CO via the constitutive HO has been shown to serve as a key mechanism to maintain the integrity of the physiological function of organs (4–6). Further, numerous studies have shown that exogenously delivered CO at low concentrations can provide potent cytoprotection (7–9).
In rodent studies, we have shown that in vivo kidney recipient treatment with low doses of inhaled CO or ex vivo treatment of excised kidney grafts with CO during cold preservation in CO-containing UW solution (CO-UW) prevents renal I/R injury, improves graft function, and prolongs recipient survival after syngenic KTx (10–12). Although in vivo CO inhalation could be clinically applicable, ex vivo application of CO in cold storage solution has two significant potential advantages: 1) the strategy decreases the concerns of adverse effects possibly induced during in vivo CO inhalation treatment, and 2) during ex vivo exposure to kidney grafts in cold preservation, CO could function effectively by binding to crucial heme proteins in excised bloodless kidney grafts without interference by hemoglobin.
Based on our encouraging results in rodent experiments, we conducted in this study the preclinical large animal experiment to evaluate the efficacy, clinical applicability, and safety of ex vivo CO delivery method to kidney grafts during cold storage in ameliorating renal I/R injury associating with KTx.
MATERIALS AND METHODS
CO delivery to UW solution and CO concentration
UW solution (Viaspan®, Du Pont, Wilmington, DW) was bubbled with compressed CO gas for 5 mins in a fume hood (CO-UW) before use. The solubility of CO in UW solution and tissue CO contents were determined by TRI lyzer (Taiyo, Osaka, Japan) using the method of gas chromatography as previously described (13).
Animals
Large white outbred pigs weighing 20–30 kg body weight (Wally Whippo, Enon Valley, PA) were housed individually in the Division of Laboratory Animal Resources at the University of Pittsburgh and fed with a standard piglet diet and tap water ad libitum. All animal care procedures were in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996). All procedures in this study were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
KTx and experimental design
a) Survival experiment
To evaluate the efficacy of CO-UW in inhibiting renal I/R injury and improving renal graft function, 14-day follow-up experiment was conducted using the kidney autotransplantation model with techniques as previously described (14). Briefly, the pigs were sedated with ketamin (20 mg/kg im) and fully anesthetized with isoflurane with endotracheal tube and positive-pressure ventilation under ECG and pulse oxymeter monitoring. With a midline incision, the left renal vascular pedicle and ureter were atraumatically isolated. After heparin sodium was administered (100 U/kg, iv), the left kidney was removed and immediately cold-flushed with control UW preservation solutions and stored at 4 °C. Then, the kidney grafts were brought into a fume hood and subsequently perfused with 150 ml of control UW (n=5) or CO-UW (n=5) via the renal artery and preserved in respective solution in the glass container for a total of 48 hrs at 4 °C. To prepare CO-UW, compressed CO gas with 5–10% concentrations were selected based on previous studies (11, 15–16). After 48 hrs, the left kidney graft was autotransplanted into the right abdominal side of the animal after right nephrectomy. The renal artery and vein were anastomosed to the aorta and inferior vena cava in an end-to-side fashion. The graft ureter was anastomosed to the bladder.
The cytoprotective effect of CO-UW was studied by analyzing 14-day animal survival, diuresis, serum creatinine and BUN levels and graft histopathology. For histological analysis, a kidney graft wedge sample was obtained at sacrifice on POD14. Blood samples were taken before surgery, every day after KTx for the first 7 days and every other day thereafter for serum creatinine, urea, and electrolytes. Spot urine samples were also obtained early morning to determine urine creatinine and protein levels.
b) Acute phase experiment
To evaluate the efficacy of CO-UW during early posttransplant period, KTx experiments with 48 hrs cold storage were conducted with a follow-up period of 3 hrs. The animals were tranquilized and anesthetized as described above. After donor heparinization (300 IU/kg, iv), both kidneys were isolated and flushed with 1000 mL control or CO-UW solution via the catheter placed in the abdominal aorta. The kidneys were excised and separated in the back table. One kidney was stored in the control (n=3) and the other was with CO-UW solution (n=3) for 48 hrs at 4 °C. The recipient pigs were similarly anesthetized and monitored by means of an ECG, pulse oximeter, and arterial blood pressure via the catheter in the carotid artery. Orthotopic KTx was performed by anastomosing the graft renal artery/vein to the infrarenal aorta/inferior vena cava in an end-to-side fashion. Both native kidneys were removed at the time of reperfusion. Recipient’s hemodynamic condition was controlled to maintain mean arterial pressure at 80 mmHg for the adequate perfusion of kidney grafts with fluid infusion and dopamine (3–5 μg/kg/min). At 3 hrs, recipient animals were sacrificed and grafts samples were obtained for RT-PCR and malondialdehyde (MDA) assays.
Carboxyhemoglobin (COHb) levels
Heparinized arterial blood sample (0.2 ml) was taken during surgery, and blood COHb levels were measured using OSM3 Hemoximeter (Radiometer, Copenhagen, Denmark).
Routine and immuno-histopathology
Graft tissues were fixed in formalin, paraffin-embedded, cut into 6 μm sections, and stained with H&E. The tubular or glomerular morphological changes were blindly reviewed (e.g. tubular necrosis, intratubular cell detachment, brush border integrity, hemorrhage, cellular infiltrates).
Grafts were also embedded in OCT, and 6 μm cryosections were stained with anti-CD3 (Dako, Carpinteria, CA) and anti-αSMA antibodies (Sigma, St. Louis, MO). Sections were also stained with naphthol AS-D chloroacetate esterase staining kit (Sigma Diagnostics) for polymorphonuclear leukocytes (PMN). Positively stained cells were blindly counted in 5 high power fields (x 200) per each section. Interstitial fibrosis was determined by staining with Masson’s Trichrome, and positively stained areas in 5 high power fields (x 100) per each section were quantified by NIH Image software (17).
mRNA isolation and real-Time RT-PCR
Total RNA was extracted from the kidney tissues using the TRIzol reagent (Invitrogen, Carlsbad, CA), and RNA content was measured using 260/280 UV spectrophotometry. mRNA expression was quantified by SYBR Green two-step, real-time RT-PCR for IL-1β, IL-6, IL-18, IFN-γ, TNF-α and β-actin using the previously published primers (18–19). Thermal cycling conditions were 10 mins at 95°C to activate the Amplitaq Gold DNA polymerase, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min on an ABI PRISM 7000 Sequence Detection System (PE Applied Biosystems). The expression of each gene was normalized to β-actin mRNA content and calculated using comparative Ct methods (20).
MDA assessment
Graft tissue samples were homogenized, and MDA concentration was determined per the manufacturer’s kit direction (Oxidresearch, Portland, OR). MDA was normalized to total protein content as determined by Bradford assay.
Protein isolation and Western blot
Frozen kidney tissues were suspended in RIPA buffer and homogenized. After microcentrifugation at 13,000 rpm for 20 mins at 4 °C, the supernatant was collected, and protein concentration was quantitated. Fifty μg of protein were separated by electrophoresis on 8–15% acrylamide sodium dodecyl surface gels and transferred to nitrocellulose membranes (Scleicher & Schuell, Keene, NH). For the blocking of nonspecific binding, 5% nonfat dry milk or 5% BSA in TBS-Tween was added to the membrane for 1 hr. Membranes were incubated overnight with primary rabbit polyclonal or mouse monoclonal antibodies for TGFβ1 (Santa Cruz, CA), GAPDH (Ambion, Austin, TX), Smad2/3, and phospho-Smad3 (Cell Signaling, Danvers, MA), followed by secondary goat anti-rabbit or anti-mouse antibody (Pierce Chemical, Rockford, IL). The membranes were developed with the SuperSignal detection systems (Pierce Chemical) and exposed to film. The band intensities were measured by NIH Image analysis software.
Statistical analysis
Data are represented as mean ± SD. Comparisons between the groups at different time points were performed using the Student’s t-test, Mann-Whitney U test, or ANOVA using the Statview program (Abacus Concepts, Inc., Berkeley, CA). Differences were considered significant at a p value < 0.05.
RESULTS
Tissue CO concentrations increase in kidney grafts after storing in CO-UW solution
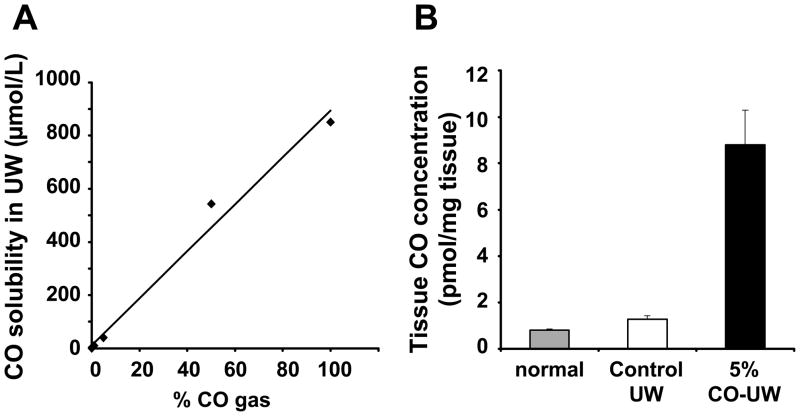
After bubbling gaseous CO into UW solution, CO concentration increased to 10.2 ± 0.2, 40.6 ± 1.6, and 543.5 ± 16.3 μM/L with 1, 5, and 50% CO, respectively, and there was a linear correlation between concentrations of CO gas and soluble levels in UW solution (Fig. 1A).
Figure 1. CO concentration in UW and kidney tissue CO levels.
(A) UW solution was bubbled with 1, 5, 50, and 100% gaseous CO for 5 mins, and CO concentration was determined by TRI lyzer, as described in the Method section. (B) Porcine kidney grafts were stored in control or 5% CO bubbled UW solution for 48 hrs and tissue CO levels were determined by TRI lyzer.
Kidney cortex tissue CO levels in porcine kidney grafts increased to 8.8 ± 1.5 pmol/mg tissue by the end of 48 hrs cold storage in 5% CO-UW, while grafts stored in control UW or normal kidney showed negligible tissue CO levels (Fig. 1B), indicating that CO in UW solution bound to kidney tissue during cold storage period.
Animals with kidney grafts stored in CO-UW show improved graft function and survival
To explore the effects of ex vivo CO exposure during the cold storage period on renal graft function, we conducted the pig kidney autotransplantation with 48 hrs cold storage in control UW or CO-UW solution. As summarized in Table 1, two groups showed comparable animal body weights, CIT, operation (graft nephrectomy and transplant) times and animals’ general conditions at the end of transplant surgery.
TABLE 1.
Summary of Control and CO-UW Groups
| Control UW n=5 | CO-UW n=5 | |
|---|---|---|
| Body weight (kg) | 27.4 ± 2.3 | 28.2 ± 1.3 |
| Cold ischemic time (hr) | 44.4 ± 0.8 | 44.2 ± 0.3 |
| Operation time | ||
| Nephrectomy (hr) | 1.6 ± 0.1 | 1.8 ± 0.3 |
| Transplantation (hr) | 2.9 ± 0.2 | 3.0 ± 0.5 |
| Implantation time (min) | 46.0 ± 5.9 | 42.2 ± 5.4 |
| General condition at the end of KTx | ||
| Body temperature (oC) | 36.1 ± 0.6 | 36.9 ± 0.3 |
| Pulse oximetry (%) | 99.8 ± 0.4 | 100 ± 0.0 |
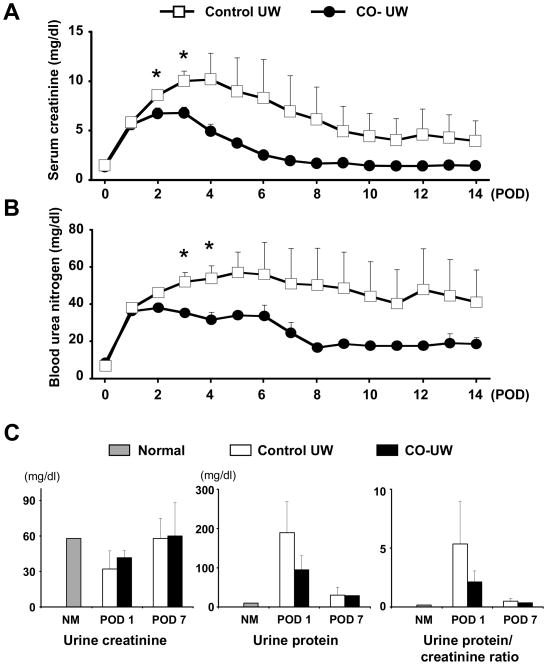
All animals except for one survived for the follow-up period of 14 days. One animal in control UW group died on POD1 due to lung edema secondary to renal graft failure. Serum creatinine and BUN levels sharply increased on POD1 in both groups, and continued to elevate in the control group with the peak creatinine value of 12.0 ± 5.1 mg/dl on POD 4.0 ± 1.4 (Fig. 2A, 2B). In contrast, an increase of creatinine levels in CO-UW group was halted on POD2, and peak creatinine level was 6.9 ± 1.4 mg/dl on POD 2.4 ± 0.5. Serum creatinine and BUN levels were lower in CO-UW than in the control group at all time points and significant difference was achieved on POD2 and 3. CO-UW group showed nearly normal creatinine and BUN values by POD14. In contrast, the control group showed high creatinine and BUN levels even on POD14.
Figure 2. Renal graft function after KTx.
(A) Serum creatinine levels and (B) BUN levels after pig kidney autotransplantation with 48 hrs cold storage in control UW (n=5) or CO-UW solution (n=5). CO-UW group showed lower serum creatinine and BUN levels compared to those in control UW group at all time points. * P<0.05 vs. control UW at the same time point.
(C) Spot urine samples were obtained on POD1-7, and urinary creatinine and protein levels were measured, and urine protein/creatinine ratio was calculated.
Urine output was noticed soon after transplant surgery in CO-UW group, while animals in the control group showed urine output on POD1 to 4. In spot urine samples obtained in the early morning on POD1, urinary creatinine levels were decreased in both groups when compared to those during preoperative period; however, urinary creatinine levels recovered by POD7 (Fig. 2C). Urinary protein amounts dramatically increased on POD1 in the control group, but the CO-UW group tended to show less severe proteinuria. Accordingly, renal injury was estimated by calculating urine protein/creatinine ratio (21–22). Lower ratios in the CO-UW group suggested reduced renal injury compared to those in the control group; however, statistically significant differences between the control and CO-UW groups were not achieved (control: 5.3 ± 3.6 vs. CO-UW: 2.1 ± 0.9 on POD1).
CO-UW does not increase blood COHb levels or associate with adverse events
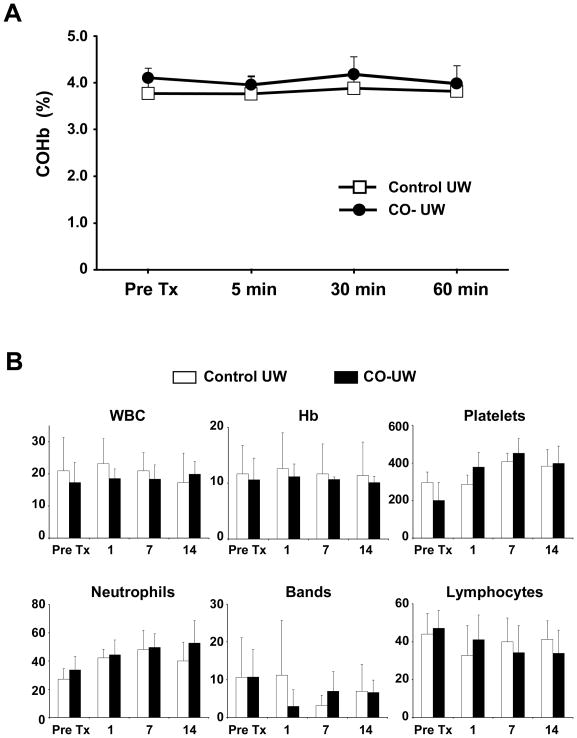
As the major concern in applying CO for treatment is COHb formation and disturbance in oxygen delivery in the body, arterial blood COHb levels were measured during immediate postreperfusion period. There was no significant increase of COHb level after transplanting kidneys preserved in CO-UW (Fig. 3A).
Figure 3. COHb levels and hematological analysis in recipient blood.
(A) Arterial blood samples were obtained at 5, 30, and 60 mins after graft reperfusion and analyzed for COHb levels. (B) Blood samples obtained after transplantation for blood cell counts for WBC (x109/L), Hemoglobin (Hb, g/dl), and platelets (x109/L), as well as leukocyte differentiation, including neutrophils (%), bands (%), lymphocytes (%). n=4 in control group and n=5 in CO-UW group.
Furthermore, there was no significant difference between the two groups in WBC counts, platelet counts, or Hb levels. Leukocyte differentiation in general showed no difference in neutrophils, lymphocytes, or other cell counts in the two groups (Fig. 3B); however, slightly less counts for bands were noticed in CO-UW group on POD1. In addition, we did not observe any adverse event due to CO-UW; hepatic enzymes (e.g. ALT, AST, ALP, bilirubin), electrolytes, glucose, total protein and albumin were similar between groups (data not shown).
Grafts in CO-UW show less severe histopathological changes
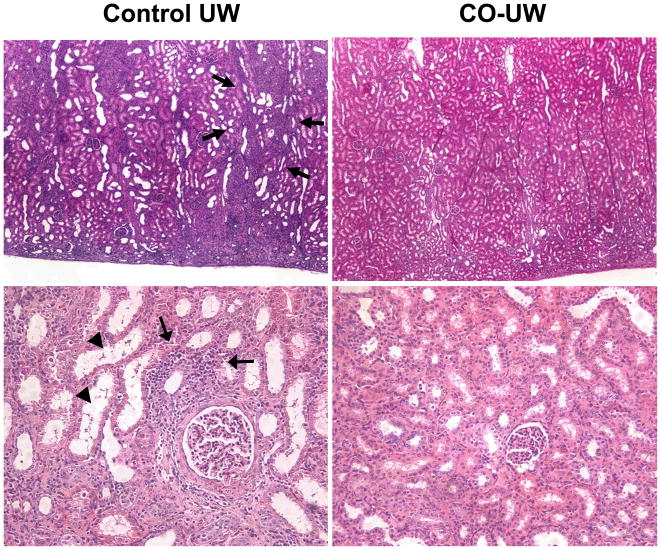
Routine histopathology of graft cortex samples on POD14 revealed variable levels of persisting tubular and glomerular injury, focal interstitial inflammatory infiltration, and focal fibrosis. Severities of focal glomerular necrosis and tubular injury were more prominent in the control-UW than in the CO-UW group (Fig. 4). Changes of interstitial and tubular inflammation were also less in the CO-UW than in the control group; however, these changes were in general minor, and the small number of animals precluded formal grading and statistical analysis.
Figure 4. Graft routine histopathology.
Kidney grafts were obtained on POD14 and H&E stained sections were reviewed for graft damage. Grafts in control UW group showed persisting tubular injury, such as tubular dilatation (arrow heads), as well as focal interstitial inflammatory infiltrates (arrows), while grafts in CO-UW group had less severe changes. Glomerular injury, such as focal glomerular necrosis and dilatation of Bowman’s capsule, was also less severe in CO-UW than control UW group. Representative images are shown. Original magnification x40 (upper) and x200 (lower)
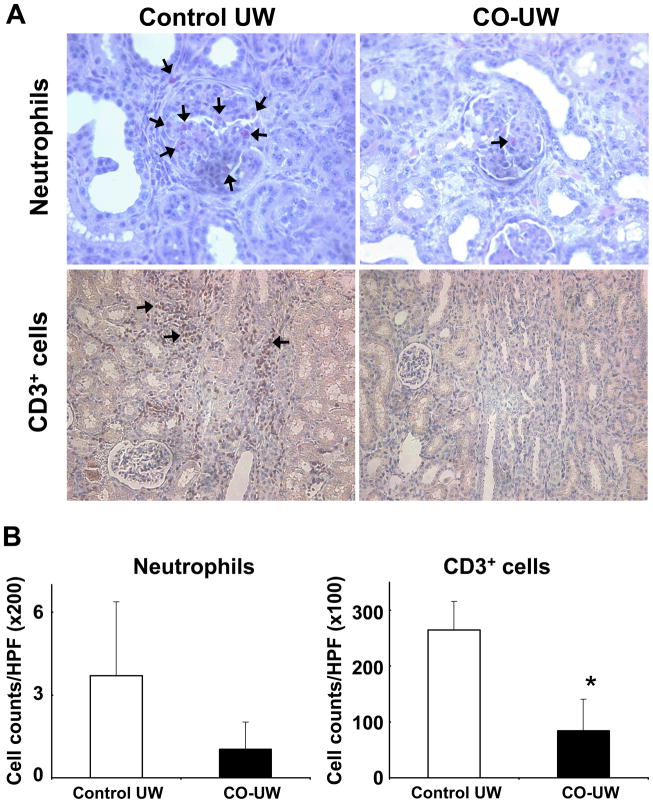
Further analysis of graft infiltrates with naphthol AS-D chloroacetate esterase staining revealed the presence of many neutrophils in the glomeruli of kidney grafts in the control UW group. Neutrophils were also found among focal infiltrates. In contrast, CO-UW preserved grafts showed less numbers of neutrophils. In addition, numerous CD3+ T cells were present in the infiltrates in the control UW group, but the frequency of CD3+ T cells was significantly less in the CO-UW group (Fig. 5A, B).
Figure 5. Immunohistochemistry for graft infiltrates.
Kidney grafts obtained POD14 were stained for neutrophils and CD3+ T cells. More frequent neutrophils (red, arrows) were found in the glomeruli of grafts in control UW group. Among infiltrates, abundant CD3+ T cells (brown, arrows) and neutrophils were detected in grafts in control UW group. CO-UW group showed significantly less number of neutrophils and T cells. (A) Representative images are shown. Original magnification x400 (neutrophils) and x200 (CD3+ T cells). (B) Positively stained cells were counted and shown as the number per microscopic field (x200: neutrophils, x100: CD3+ T cells). n=4 in control group and n=5 in CO-UW group. * p<0.05 vs control UW.
Fibrotic changes are inhibited in grafts stored in CO-UW
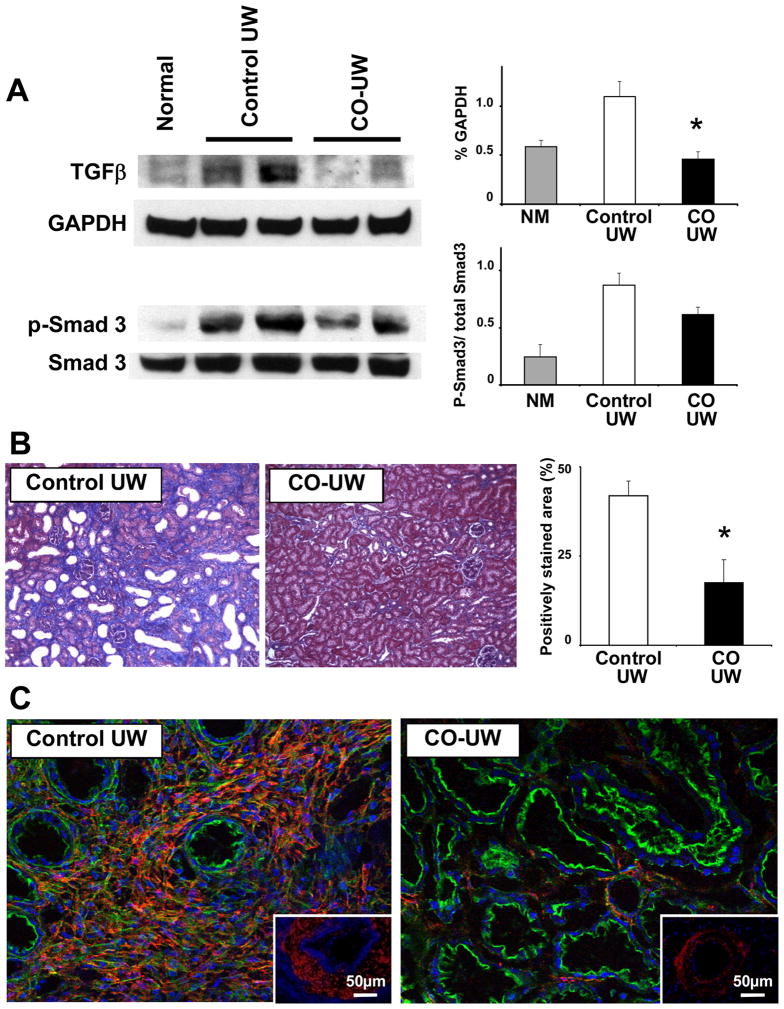
We next investigated if the amelioration of renal I/R injury with CO-UW contributed to an inhibition of profibrotic changes in injured kidney grafts. Expression of TGF-β, a key mediator in the progression of fibrosis, was significantly increased in the grafts in the control UW grafts on POD14. In contrast, CO-UW preserved grafts showed significantly less TGF-β protein expression. Likewise, Smad3, downstream signaling molecules, were less phosphorylated in the CO-UW group (Fig. 6A).
Figure 6. Fibrotic changes in kidney grafts after I/R injury.
(A) Kidney graft samples obtained POD14 were analyzed for TGF-β protein expression and Smad3 phosphorylation in Western blot. Kidney grafts preserved in CO-UW showed less TGF-β protein expression and Smad3 activation compared to those in control UW. Representative images of 3 similar experiments. * p<0.05 vs control UW.
(B) Degree of fibrosis was evaluated in sections with Masson’s Trichrome stain (blue). Grafts in CO-UW showed significantly less degree of fibrosis. Original magnification x100. n=4 in control group and n=5 in CO-UW group. * p<0.05 vs control UW.
(C) αSMA staining (red) revealed intense *αSMA expression in the area of infiltrates in control UW group Note that grafts in CO-UW group show weak *αSMA expression in peritubular area, which is normally seen in naïve kidneys. Tubular epithelial cells were outlined with falloidine (F-actin, green). The arterioles in control UW group show dense *α-SMA staining (red), while those in CO-UW group show minimum *αSMA expression (inserts). Original magnification x200.
In Masson’s Trichrome stained sections, graft fibrosis was evident in areas of infiltrates in control UW group. The graft in the CO-UW group showed significantly less fibrotic area than the control UW group (Fig. 6B). Immunofluorescent stain showed intense α-SMA expression in grafts preserved in control UW, in particular, in the area of infiltrates (Fig. 6C). However, grafts in CO-UW showed marginal α-SMA stain. In addition, the arterioles in the control UW group already showed dense α-SMA staining, changes relevant to fibrointimal hyperplasia. These results may indicate that infiltrates during the late phase of I/R injury are involved in fibrotic changes of kidney grafts, leading to CAN, and CO-UW inhibits these fibrotic changes.
Kidney grafts stored in CO-UW solution show reduced early proinflammatory responses
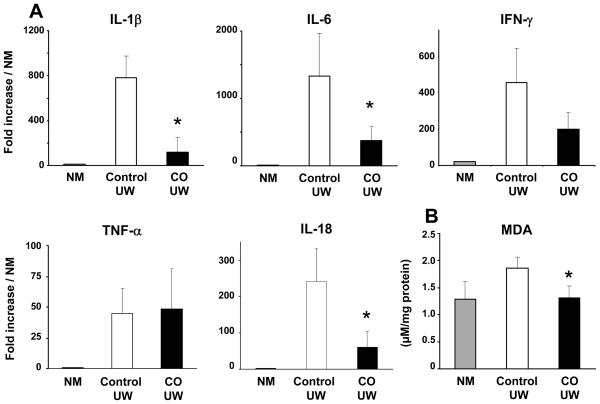
To explore the effects of CO-UW during immediate posttransplant period, we conducted the acute phase experiment and obtained kidney graft samples at 3 hrs after reperfusion to determine mRNA levels for inflammatory cytokines. Kidney grafts in CO-UW showed significantly lower mRNA levels for IL-1β, IL-6, and IL-18 than those in control UW at 3 hrs after transplantation (Fig. 7A). However, there was no difference between the two groups at this time point in mRNA levels for IFN-γ and TNF-α. MDA concentrations in kidney grafts in CO-UW were also significantly lower than those in control UW (Fig. 7B). These results may suggest that the exposure to CO during cold storage might inhibit lipid peroxidation and early proinflammatory responses.
Figure 7. Early proinflammatory responses in kidney grafts.
Six kidney grafts from 3 pig donors with 19.6 ± 5.3 kg body weight were transplanted into pig recipients in control UW (n=3, 22.3 ± 7.7 kg) and CO-UW (n=3, 30.0 ± 11.3 kg) groups after preserving in the respective solution for 48.0 ± 3.0 and 48.3 ± 3.2 hrs, respectively. Recipients were followed for 3 hrs and kidney graft samples were obtained for PCR analysis (A) and MDA assay (B). Kidney grafts stored in CO-UW showed significantly less mRNA upregulation for IL-1β, IL-6, and IL-18. MDA concentration also was significantly lower in CO-UW than in control UW group. NM: normal porcine kidney. * p<0.05 vs control UW.
DISCUSSION
Using the large animal model of KTx with 48 hrs static cold storage, this study demonstrates that ex vivo treatment of excised kidney grafts with CO during cold storage ameliorates renal I/R injury. When gaseous CO was bubbled into UW solution, soluble CO levels in UW increased corresponding to initial gaseous CO concentrations. Further, when pig kidney grafts were preserved in CO-containing UW solution, graft tissue CO levels increased by the end of 48 hrs cold storage. Grafts ex vivo exposed to CO functioned significantly better after autotransplantation with shorter initial oliguria periods, lower serum creatinine levels and less urinary protein secretion, when compared to those in control UW solution. These functional benefits with CO-UW associated with significantly less upregulations for early proinflammatory mediators and lipid peroxidation in kidney grafts. Further, histopathological analysis on POD14 revealed less severe tubular injury, reduced focal infiltration and decreased fibrotic changes in the grafts preserved in CO-UW. These beneficial effects of CO did not associate with any adverse event or COHb elevation in recipient animals.
Although the pig kidney autotransplantation model does not directly mimic human kidney allotransplantation, the model is useful in studying direct consequences of renal I/R injury after transplantation without additional factors caused by alloantigen-dependent reactions. Several previous studies have used the porcine kidney autotransplantation model as an ultimate preclinical evaluation of strategies in ameliorating renal I/R injury. The majority of these studies used 24 hrs cold preservation and showed variable transplant outcomes, ranging from 60 to 100% survival with peak creatinine levels of 6–13 mg/dl (23–27). Thus, 24 hrs cold storage of kidneys appears to induce relatively mild I/R injury with large deviations. On the other hand, very limited numbers of porcine kidney transplant studies used cold storage for 48 hrs or more and showed severe injury. Hauet evaluated the efficacy of trimetazidine in the 48 hrs storage model and showed 57% animal survival with a peak creatinine level of 17 mg/dl in control UW (23). Our study using 48 hrs cold storage showed relevant results in the control group and significant effects of CO in improving severe I/R injury. Although findings in the experimental porcine autotransplantation model do not allow direct adjustment to human kidney transplantation, function and histopathology of porcine renal grafts with 48 hrs cold storage are substantially improved using a simple strategy by supplementing CO to preservation solution. Our preliminary study in the porcine kidney allotransplantation model with 48 hrs cold storage showed the similar efficacy of CO in UW solution in protecting porcine renal allografts from initial I/R injury.
Untreated porcine kidney grafts functionally recovered from severe I/R injury induced by 48 hrs cold storage, and serum creatinine levels returned to nearly normal levels by POD14 in this study; however, histopathological analysis of grafts revealed that I/R injury was not resolved by this time point, and it rather showed continual active reactions to injury. Kidney grafts showed tubular dilatation, glomerular injury, and numerous infiltrates including neutrophils and CD3+ T cells. Indeed, longer follow-up for 16 weeks of porcine kidney autografts with 48 hrs cold preservation resulted in progressive interstitial fibrosis and deterioration of renal function (14, 28). Initial I/R injury and loss of functioning renal mass have been shown to cause functional and morphologic changes relevant to CAN in the alloantigen-independent way in experimental kidney isografts (29–30). CO in this study shows less severe fibrotic changes on POD14, indicating that initial inhibition of I/R injury with CO-UW will provide long-term benefits.
Apart from the kidney transplantation model, recent studies in the pig using an isolated organ-perfusion system with autologous pig blood show that CO releasing molecules (CORM) protect the kidneys against I/R injury. Renal blood flow, creatinine clearance, and urine output are improved during 3-hr reperfusion period in an experimental model of 60 min warm ischemia with 16 hrs cold storage (15). Although it is difficult to directly compare the results in our transplant study and those with CORM, they certainly support the efficacy of CO in inhibiting renal I/R injury in preclinical large animal experiments.
The effects of CO might be dose-dependent. Although fine correlation between the dose and efficacy needs to be studied, the use of 5~10% CO gas (CO solubility of 40~100 μM) appeared to be beneficial, as shown in our previous studies (11, 13, 31) and in those using CORM (32–33). We were not able to determine the difference between 5% and 10% CO-UW in this study; however, one pig kidney preserved in 20% CO-UW (CO solubility of 188μM) for 48 hrs in the current study did not associate with improved creatinine levels (peak value of 10.7 mg/dl) with normal recipient COHb levels, suggesting that 20% CO-UW might not be effective. In studies using CORM, the dose 50–100 μM showed benefits, while higher doses of 200–400 μM CORM resulted in renal dysfunction (15–16). These findings may suggest that high doses of CO are toxic to porcine kidney grafts. Toxic effects might be induced by chemical interactions of CO with the different components of UW solution. However, as graft tissue CO levels increase by the end of cold storage depending on the concentration of soluble CO in UW solution, CO binding to kidney grafts may introduce both beneficial and toxic effects. The major molecular targets of CO are believed to be transition metals. Since excised bloodless kidney grafts contain minimum amounts of erythrocytes, the binding of CO to hemoglobin is negligible in this study, and CO might efficiently bind to graft heme proteins that play crucial roles in regulating cellular function (e.g. cyclooxygenase, cytochrome c, cytochrome P450, cytochrome c oxidase, guanylyl cyclase). In fact, previous in vitro studies show that CO is able to activate guanylyl cyclase and inhibit cytochrome c oxidase (34–35). CO thus might alter graft heme protein function to elicit protective effects. However, at the same time, higher doses of CO could result in impaired function of graft heme proteins (31). In addition, CO has been shown to induce strong erythropoietin release. However, as CO is exposed to kidney grafts during cold preservation period in this study, additional protein synthesis/release in the hypothermic condition does not appear to take place. Further studies certainly are required to determine the mechanisms of beneficial and adverse effects induced by CO.
In this study, application of CO-UW to kidney grafts during donor surgery was slightly different between the acute phase and survival experiments. We initially attempted to use CO-UW for the entire donor procedures including in situ kidney graft perfusion in the donor and subsequent back table procedure. However, CO could be quickly liberated from CO-UW solution when CO-UW is exposed to the air. Additionally, there are concerns regarding potential hazardous effects caused by CO released from CO-UW solution. Therefore, in the survival experiments CO-UW was applied in a fume hood to excised kidney grafts after they were retrieved from the donor using control UW solution. In fact, the use of control UW for the initial kidney procurement and subsequent application of CO-UW to excised kidney grafts are technically simpler and safer, and could be more suitable to clinical kidney transplantation.
This study demonstrates that ex vivo application of CO in UW preservation solution improves the outcome of KTx in the large animal kidney autotransplantation model. Porcine kidney grafts preserved in CO containing UW solution for 48 hrs show improved graft function and survival compared to those in control UW. Ex vivo CO application does not associate with any adverse effect and may therefore be a safe strategy to reduce I/R injury in human renal transplantation.
Acknowledgments
We thank Mike Tabacek, Lisa Chedwick, Nethravathi Guthalu Puttaraju, and Tam Pham for their excellent technical support; Drs. Fadi Lakkis and Ron Shapiro for their helpful suggestions and input into the study; Drs. Atsushi Sugitani and Masao Tanaka for their guidance and support; the Department of Bioengineering at the University of Pittsburgh for their assistance in measuring blood gas; the DLAR at the University of Pittsburgh for their assistance; and Carla Forsythe for the preparation and organization of the manuscript.
This work was supported by the National Institutes of Health Grant DK071753
ABBREVIATIONS
- CO
carbon monoxide
- COHb
carboxyhemoglobin
- KTx
kidney transplantation
- I/R
ischemia/reperfusion
- HO
heme oxygenases
- MDA
malondialdehyde
References
- 1.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004 Nov 13–19;364(9447):1814–27. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 2.Shoskes DA, Halloran PF. Delayed graft function in renal transplantation: etiology, management and long-term significance. J Urol. 1996 Jun;155(6):1831–40. doi: 10.1016/s0022-5347(01)66023-3. [DOI] [PubMed] [Google Scholar]
- 3.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–55. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–4. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 5.Suematsu M, Goda N, Sano T, Kashiwagi S, Egawa T, Shinoda Y, et al. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver. J Clin Invest. 1995 Nov;96(5):2431–7. doi: 10.1172/JCI118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins CC, Boehning D, Kaplin AI, Rao M, Ferris CD, Snyder SH. Carbon monoxide mediates vasoactive intestinal polypeptide-associated nonadrenergic/noncholinergic neurotransmission. Proc Natl Acad Sci U S A. 2004 Feb 24;101(8):2631–5. doi: 10.1073/pnas.0308695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000 Apr;6(4):422–8. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 8.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000 Oct 2;192(7):1015–26. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, et al. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001 May;7(5):598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 10.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, et al. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004 Nov;287(5):F979–89. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- 11.Nakao A, Faleo G, Shimizu H, Nakahira K, Kohmoto J, Sugimoto R, et al. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008 Oct;74(8):1009–16. doi: 10.1038/ki.2008.342. [DOI] [PubMed] [Google Scholar]
- 12.Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, et al. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008 Jun 27;85(12):1833–40. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- 13.Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, et al. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant. 2006 Oct;6(10):2243–55. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 14.Hauet T, Goujon JM, Vandewalle A, Baumert H, Lacoste L, Tillement JP, et al. Trimetazidine reduces renal dysfunction by limiting the cold ischemia/reperfusion injury in autotransplanted pig kidneys. J Am Soc Nephrol. 2000 Jan;11(1):138–48. doi: 10.1681/ASN.V111138. [DOI] [PubMed] [Google Scholar]
- 15.Bagul A, Hosgood SA, Kaushik M, Nicholson ML. Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation. 2008 Feb 27;85(4):576–81. doi: 10.1097/TP.0b013e318160516a. [DOI] [PubMed] [Google Scholar]
- 16.Hosgood SA, Bagul A, Kaushik M, Rimoldi J, Gadepalli RS, Nicholson ML. Application of nitric oxide and carbon monoxide in a model of renal preservation. Br J Surg. 2008 Aug;95(8):1060–7. doi: 10.1002/bjs.6174. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, et al. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int. 2009 Jan;75(2):156–66. doi: 10.1038/ki.2008.509. [DOI] [PubMed] [Google Scholar]
- 18.Borca MV, Gudmundsdottir I, Fernandez-Sainz IJ, Holinka LG, Risatti GR. Patterns of cellular gene expression in swine macrophages infected with highly virulent classical swine fever virus strain Brescia. Virus Res. 2008 Dec;138(1–2):89–96. doi: 10.1016/j.virusres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Sipos W, Duvigneau C, Pietschmann P, Holler K, Hartl R, Wahl K, et al. Parameters of humoral and cellular immunity following vaccination of pigs with a European modified-live strain of porcine reproductive and respiratory syndrome virus (PRRSV) Viral Immunol. 2003;16(3):335–46. doi: 10.1089/088282403322396136. [DOI] [PubMed] [Google Scholar]
- 20.Cikos S, Bukovska A, Koppel J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol Biol. 2007;8:113. doi: 10.1186/1471-2199-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983 Dec 22;309(25):1543–6. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 22.Price CP, Newall RG, Boyd JC. Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: a systematic review. Clin Chem. 2005 Sep;51(9):1577–86. doi: 10.1373/clinchem.2005.049742. [DOI] [PubMed] [Google Scholar]
- 23.Hauet T, Han Z, Doucet C, Ramella-Virieux S, Hadj Aissa A, Carretier M, et al. A modified University of Wisconsin preservation solution with high-NA+ low-K+ content reduces reperfusion injury of the pig kidney graft. Transplantation. 2003 Jul 15;76(1):18–27. doi: 10.1097/01.TP.0000062663.85992.FB. [DOI] [PubMed] [Google Scholar]
- 24.Baldan N, Toffano M, Cadrobbi R, Codello L, Calabrese F, Bacelle L, et al. Kidney preservation in pigs using celsior, a new organ preservation solution. Transplant Proc. 1997 Dec;29(8):3539–40. doi: 10.1016/s0041-1345(97)01013-0. [DOI] [PubMed] [Google Scholar]
- 25.Maathuis MH, Manekeller S, van der Plaats A, Leuvenink HG, t Hart NA, Lier AB, et al. Improved kidney graft function after preservation using a novel hypothermic machine perfusion device. Ann Surg. 2007 Dec;246(6):982–8. doi: 10.1097/SLA.0b013e31815c4019. discussion 9–91. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson ML, Hosgood SA, Metcalfe MS, Waller JR, Brook NR. A comparison of renal preservation by cold storage and machine perfusion using a porcine autotransplant model. Transplantation. 2004 Aug 15;78(3):333–7. doi: 10.1097/01.tp.0000128634.03233.15. [DOI] [PubMed] [Google Scholar]
- 27.Lodge JP, Perry SL, Skinner C, Potts DJ, Giles GR. Improved porcine renal preservation with a simple extracellular solution--PBS140. Transplantation. 1991 Mar;51(3):574–9. doi: 10.1097/00007890-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Faure JP, Petit I, Zhang K, Dutheil D, Doucet C, Favreau F, et al. Protective roles of polyethylene glycol and trimetazidine against cold ischemia and reperfusion injuries of pig kidney graft. Am J Transplant. 2004 Apr;4(4):495–504. doi: 10.1111/j.1600-6143.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 29.Tullius SG, Heemann U, Hancock WW, Azuma H, Tilney NL. Long-term kidney isografts develop functional and morphologic changes that mimic those of chronic allograft rejection. Ann Surg. 1994 Oct;220(4):425–32. doi: 10.1097/00000658-199410000-00002. discussion 32–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azuma H, Nadeau K, Takada M, Mackenzie HS, Tilney NL. Cellular and molecular predictors of chronic renal dysfunction after initial ischemia/reperfusion injury of a single kidney. Transplantation. 1997 Jul 27;64(2):190–7. doi: 10.1097/00007890-199707270-00002. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda A, Ueki S, Nakao A, Tomiyama K, Ross MA, Stolz DB, et al. Liver graft exposure to carbon monoxide during cold storage protects sinusoidal endothelial cells and ameliorates reperfusion injury in rats. Liver Transpl. 2009 Nov;15(11):1458–68. doi: 10.1002/lt.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandouka A, Fuller BJ, Mann BE, Green CJ, Foresti R, Motterlini R. Treatment with CO-RMs during cold storage improves renal function at reperfusion. Kidney Int. 2006 Jan;69(2):239–47. doi: 10.1038/sj.ki.5000016. [DOI] [PubMed] [Google Scholar]
- 33.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3) J Heart Lung Transplant. 2007 Nov;26(11):1192–8. doi: 10.1016/j.healun.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994 May 10;33(18):5636–40. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 35.Zuckerbraun BS, Chin BY, Bilban M, d’Avila JC, Rao J, Billiar TR, et al. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. Faseb J. 2007 Apr;21(4):1099–106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]