Abstract
OpusXpress is a semi-automated system for high throughput voltage clamp recording from Xenopus oocytes. We participated in the development process for this system and were the only laboratory to field test a prototype. Subsequently, we obtained an early production model that we have used on a regular basis for the last seven years, conducting many thousands of experiments, publishing extensively, and carrying out collaborative research in drug discovery. In this chapter, we relate our experience with the OpusXpress recording system and large volume oocyte handling. We provide our standard operating procedures and outline the organization of our successful team. Some of our advice is specific to researchers fortunate enough to have access to an OpusXpress system, but most of it is applicable to any group using Xenopus oocytes for the heterologous expression of ion channels.
Introduction
It is not at all uncommon for scientists to transpose that which they wish to study from a complex setting to one which they hope to more easily control and observe. This volume has a mixture of articles dealing with Xenopus oocytes, either as experimental subjects, or as vessels used to conduct experiments which have nothing at all to do with oocytes. This paper falls most definitely in the latter category. Everything that we have learned about the oocytes themselves over the last seventeen years since the Papke lab set up shop at the University of Florida has only been applied to make the oocytes better cellular test-tubes for our experiments. We have explored the limitations of the oocytes and taken advantage of their virtues as a system for the heterologous expression of ligand-gated ion channels [1–6].
Many groups around the world have had the opportunity to take advantage of the oocyte expression system and enlarge upon those advantages in creative ways, as reported in other chapters of this book. The purpose of this chapter however, will be to present an outline for how we have tried to optimize the use of the oocyte system to take advantage of a highly developed semi-automated recording system, OpusXpress (Molecular Devices, part of MDS Analytical Technologies).
Two-electrode voltage clamp experiments with Xenopus oocytes
The Xenopus oocyte is a favored expression system for proteins which either directly or indirectly control the cell’s membrane potential. This is because, not only are the cells large and robust enough to be easily obtained and handled, they are a good size for the use of two-electrode voltage-clamp (TEVC), a method for measuring and controlling the electrical potential across the cell’s membrane. This methodology is at the core of the OpusXpress design. Each cell is penetrated by two glass microelectrodes. One electrode is the dedicated “voltage” electrode and is always used to measure the voltage across the cell membrane, relative to a reference electrode in the bath. The electronics keep track of this voltage for each of the eight cells in the separate chambers. Initially, the second electrode, which will ultimately be used as the “current” electrode, also measures transmembrane voltage when it penetrates the cell. The values from the two electrodes should be in good agreement if the “offset” on each electrode was correctly adjusted before penetrating the cell. The offset adjustment (done automatically with OpusXpress) simply involves setting the voltage difference between the electrodes in the bath and the reference (ground) electrode in the bath to zero.
After the cell is impaled by both electrodes and stable voltage readings are obtained from both electrodes, a cell can be switched to “voltage clamp” mode. There is a separate amplifier circuit for each of the eight cells. In voltage clamp an internal circuit compares the voltage measured by the dedicated voltage electrode to a “command” or “holding” potential set by the user in the OpusXpress protocol. The amplifier then injects current into the cell through the dedicated current electrode until there is zero difference between the voltage measured and the desired holding or command potential. In this way, the membrane voltage is “clamped” by the two electrodes.
Oocytes are relatively large cells, robust enough to be penetrated by large (low resistance) current electrodes. Additionally, the OpusXpress makes use of a second very low resistance electrode in the bath, referred to as the “sense electrode”. Since what is being “clamped” is the voltage difference between the inside of the cell and the bath, the sense electrode can also be used to inject current into the bath to achieve the desired difference. This is referred to as a “bath clamp” and the ground electrode for each separate chamber becomes a local measure of zero volts for that circuit and so is a “virtual” ground.
Once the OpusXpress voltage-clamp amplifiers have been set to maintain the membrane potentials of the cells, the other functions of OpusXpress, drug delivery and/or jumps in command potential are used to perturb the activity of the molecules controlling the cell’s membrane potential. The amount of current required to keep the membrane potential at the holding or the new command potential directly measures the current generated by the change in experimental conditions implemented by the OpusXpress protocol.
Better appreciation for the use of voltage clamp recordings can come from a brief consideration of the underlying biophysical principles as defined in Ohm’s law, the defining relationship between membrane voltage, ion channel conductance (conductance being the inverse of resistance), and current. The relevant measure of voltage is the driving force for current through the particular ion channel (or transporter) of interest. It is defined as the difference between the membrane potential (Em) and the zero current potential, or “reversal potential”, (Erev) for the channels of interest (Em − Erev). The total conductance (G) for the population of channels being studied can be defined as NPopenγ, where N is the total number of channels, Popen is the fraction of channels that are open in response to the stimulus, and γ is the conductance of a single open channel. Ohm’s law can then be written, I = (Em − Erev)NPopenγ, where I is the transmembrane current.
When the transmembrane voltage is being measured under non-voltage clamp conditions, the transmembrane voltage changes if a cell is stimulated and ion channels begin to open or close and the current flowing across the membrane increases or decreases. Consequently, as membrane potential changes, the driving force for current changes, resulting in a nonlinear relationship between the measured response (change in Em) and the more likely parameter of interest, Popen. The advantage of voltage clamp recording comes from the fact that, since the driving force is kept constant by the amplifier, for a given cell there is a direct linear relationship between the response measured (I), as a percent of the cell’s maximum possible response (Imax), and the fraction of the total population of ion channels that opened to create the current measured. The Imax for any given cell will be proportional to N, a value which will vary from cell to cell. That is why it is important to obtain some sort of control or reference response from every cell. It is then possible to normalize the data by calculating the experimental data relative to the control. This corrects for variations in N, and allows data from multiple cells to be pooled.
Two major classes of proteins control the transmembrane voltage, ion channels and ion transporters, such as the sodium-potassium ATP-ase. Both of these types of molecules can be studied with OpusXpress. Athough ion channel-mediated responses are usually more rapid than transporter-mediated responses and therefore more easily studied, the oocyte system has also have been used extensively for the expression cloning and characterization of various electrogenic transporters [7–12].
Most often the ion channel being studied is the one which the experimenter is using the oocyte to express, following injection of mRNA or cDNA. The OpusXpress drug delivery system allows for complete pharmacological characterization of ligand-gated ion channels. Voltage-dependent ion channels can be studied with steps in command voltage, easily combined with drug applications.
It is also possible to use ion channels that are endogenous to the oocyte as reporters of the activity of other proteins being expressed. For example, Xenopus oocytes have large numbers of calcium-dependent chloride channels. Any stimulus that causes an increase in intracellular calcium will cause activation of these channels that can be measured with the voltage-clamp amplifier. Many G-protein coupled receptors work through the IP3 pathway and cause release of calcium from intracellular stores, resulting in measurable currents in the oocytes. This allowed for the oocyte expression system to be used for the cloning [13] and continuing characterization of metabotropic glutamate receptors [14] and other G-protein coupled receptors.
Another relatively novel use for oocyte TEVC recording is for testing the membrane ion channel activity of other tissues which might be healthy or diseased [15–17]. For example, membrane vesicles can be prepared from brain tissue and used to inject oocytes. The vesicles will fuse with the oocyte’s plasma membrane, and the exogenous channels can be studied with the TEVC method. Basically, virtually anything that can be accomplished with TEVC of Xenopus oocytes can be done with OpusXpress rapidly and efficiently [4].
History of OpusXpress
Our involvement with OpusXpress began in 2001, at a time when the first author was a consultant for Axon Instruments (now part of Molecular Devices, MDS Analytical Technologies). He was asked to compose a list of features that he thought should be part of an ideal oocyte recording system.
Some of the features specified are listed below:
Acquisition of high quality data
Acquisition of large quantities of data
Logical file naming and database structuring
Automatic monitoring of cell health and consistent quality of recording throughout the course of an experiment
Sophisticated and customizable data analysis protocols
The creation of tabulated databases
Practical delivery of experimental solution from multiple sources, including both bolus deliveries of experimental solutions and flexibility to change the bulk flow of bath solutions.
Provide reliable handling of both the cells and experimental solutions
Once set up, it should run an entire experiment with minimal supervision
The engineers at Axon Instruments designed a system that would do all that and more (Figure 1). It was decided that the machine would be able to record from eight oocytes at a time in parallel, and drugs and bath solutions would be delivered on a cell-by-cell basis as required.
Figure 1.
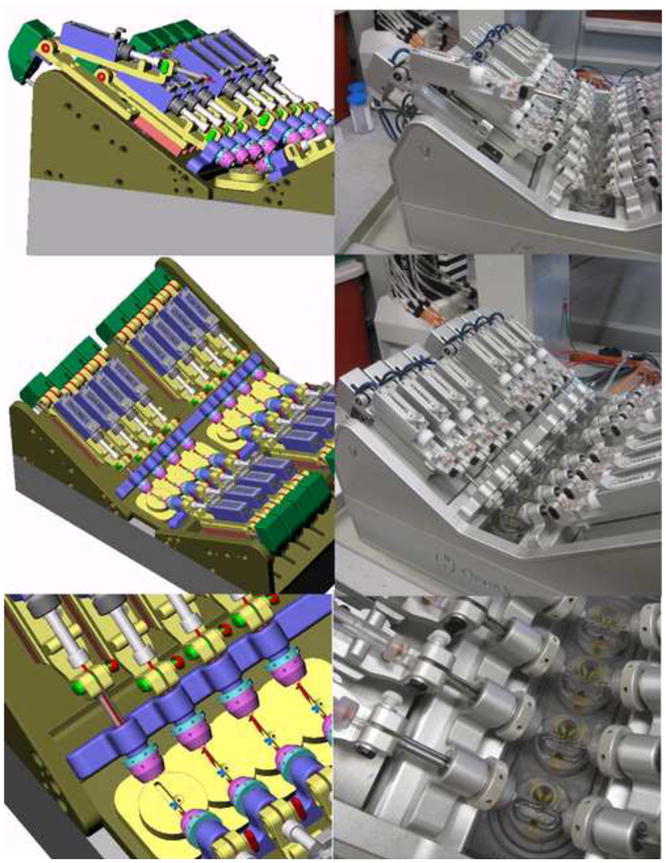
Shown on the left are preproduction images for the OpusXpress headstage and electrode arrays relative to the recording chamber. On the right are photographs of the OpusXpress that has been in service in the Papke laboratory since 2002. In the upper pictures one of the headstage-electrode units has been raised up, as it would be to change electrodes. In the middle pictures the Chamber 1 voltage headstage-electrode unit has been lowered and has begun to move toward the chambers. The bottom pictures show the final approach of the headstage-electrode unit toward one set of four recording chambers. Note that the final chamber design shows several notable modifications/refinements relative to the pre-production drawing. The electrodes point to the position for the oocyte placement. Above that point is the inflow for the bath solution and the round depression for pipette tip solution delivery. Solution delivered to the chamber flows in both directions around the cell to the outflow tube at the bottom of the chamber.
Some of the database features originally conceived of for OpusXpress ultimately became part of DataXpress, a data-handling system for Molecular Device’s automated patch clamp system. The dedicated software for OpusXpress was instead designed from the very beginning to be compatible with Axon Instrument’s pClamp, a suite of programs that have become arguably the most flexible and familiar set of applications for electrophysiology in the world. Additional custom features were added to pClamp to increase the ease with which the large OpusXpress data sets could be analyzed.
We were at first skeptical, but ultimately amazed at how well the excellent software and hardware engineers at Axon were able to put together what has effectively become the machine of our dreams. Just between 8/1/08 and 7/31/09 we conducted separate 1176 experiments on our OpusXpress, most of which yielded publication quality results. Fortunately, some of those experiments were contract research; we cannot write so many papers ourselves.
An OpusXpress is probably not suited for every lab, and was in fact originally designed for industrial research. It takes a lot of attention to details and organized team work to conduct over 1000 experiments in a year. However, considering that each experiment probably had, on average, at least sixteen drug applications made in parallel to eight cells, 128 separate data points, it is worth the trouble to get it right. Before we began to use OpusXpress, we would be happy if a summer student accomplished over the course of two months what we now typically do in a single two hour run.
Advantages
The OpusXpress is suitable for many applications, which have been reviewed elsewhere [4]. The purpose of the present review is more to provide a practical guide for potential users with advice on how they might make the most out of the machine’s potential. However, many of the general principles discussed may apply to any research laboratory.
An OpusXpress can, of course, be used for drug screening, with the capability of testing at least thirty compounds per hour in duplicates, well over two hundred a day or a thousand a week. This is very good throughput; though some would argue that it is less than industrial standards for “high throughput.”
It should be noted however, that conventional high throughput systems for ion channel screening, whether they are based on fluorescence or automated patch-clamp, require the use of cell lines, either stably transfected or naturally expressing the molecular target of interest. Such resources are not always available, and if a large number of targets need to be considered, a separate cell line has to be available for each target. If all cell lines of potential interest are constantly kept in culture, it would represent a considerable expense and there could be experimental liability if phenotypic drift occurs. Alternatively, multiple cell lines might be kept frozen down, but under those circumstances switching from one target to another could take two weeks or more, while the cells are grown up in sufficient numbers.
Any number of cDNAs or RNAs may be kept on hand for oocyte experiments, and once injected, cells can be used as soon as the next day. This allows for an incredible amount of flexibility, especially for a lab such as ours which can draw upon clones from a half dozen or more species and hundreds of site-directed mutants. This permits very effective “hypothesis screening,” with rapid and effective testing of new ideas [4].
The use of OpusXpress in an academic setting
It has been our experience that effective use of OpusXpress as a research tool very much depends on a team effort. The two anchors for the team are the principal investigator (RLP) and the lab manager/molecular biologist (CS) who both have theoretical and practical experience in all aspects of the work, although in honesty, one might say theoretical and practical, respectively. We have typically relied on a staff of two or three other technicians. Often these have been post-baccalaureates or part-time student technicians, some significantly more productive than others. Some were interested in the scientific underpinning of the work, others were not. The interested ones were encouraged to participate in data analysis and the preparation of manuscripts. Several of these young people went on to graduate or professional schools.
Of course, one of the most critical skills required for an OpusXpress technician is the careful and accurate making of experimental solutions and dilutions for concentration response studies. We provide prospective technicians with tutorials, as well as practice exercises, on these topics. No one gets hired without passing the final test with a score of 100% correct.
Somewhat ironically, the graduate students in the lab have not themselves been heavily encouraged to become OpusXpress operators. Rather, they learn how to conduct oocyte recordings with manual equipment and then typically go on to projects based on patch clamp methods and/or molecular biology. If OpusXpress experiments are needed for the student’s projects, they are often put in the weekly queue for the technical staff. On the other hand, OpusXpress has offered graduate students great opportunities to participate directly in the process of hypothesis testing and manuscript preparation. They have had the opportunity to collate and organize large data sets, extract the key concepts that emerge from the data, and then formulate hypotheses and design additional experiments, working with the principal investigator, the molecular biologist, and other collaborators.
Duties and delegation
We have found it useful if members of the technical team are both specialists at some duties but also generalists, so that when, for example, the usual frog surgeon is unavailable or an unscheduled surgery is required, the necessary work can get done. The need for redundancy not withstanding, members of the staff tend to specialize according to their talents and inclinations. One outstanding student technician worked half time throughout her entire undergraduate career, usually doing almost all the RNA injections and spending so much time at dissecting microscopes sorting and culling cells that we imagine she may see oocyte-sized spots before her eyes for the rest of her life. We truly appreciated her dedication.
Music in the lab may help lift productivity and morale. The free radio options on the internet are wonderful. We enjoy Cesaria Evora, Philip Glass, Kronos Quartet, Astor Piazolla, JS Bach, and Marin Marais. However, one lab rule is that everyone has veto power over the music. Private earbuds are discouraged. Importantly, do not use the OpusXpress dedicated computer for internet streaming.
There is one primary molecular biologist in the lab, but students and post-docs make their own mutants and other constructs, prepare their own RNA, and usually do their own injections. While out of preference or practical necessity, some researchers may have taken upon themselves the job of animal (i.e. Xenopus) husbandry; we do not, so we will leave that subject to be covered elsewhere [18–22].
Logistics
Each week, the list of desired experiments is updated and prioritized by the PI, with input as appropriate from students and post-docs. The team leader then has to plan the injection list and the time(s) for frog surgery. The logistics involved can be rather complicated. Considerations have to be given to factors such as how long the different constructs take to express, how long is the time window for when they will be good, and when OpusXpress operators will be available to run experiments. It makes no sense to plan experiments so that everything has to be done on a single day, or for days when no one will be in the lab.
The knowledge necessary to make these decisions comes only with experience. For example, we have learned that cells injected with cDNA for the human α7 nAChR are ready in 24 hours for experiments that will utilize a powerful positive allosteric modulator, which greatly amplifies responses. After 48 hours, potentiated responses will be too large to maintain the voltage-clamp. However, if we are conducting experiments without the modulators, the same cells will be useful for an entire week. In contrast, it is our experience that for some reason cells injected with cDNA for the mouse α7 nAChR only begin to be ready for experiments about seven days after injection. It helps us to keep little charts of expected useful expression times for the various genes we study, along with lists of the reference concentrations to be used with them.
Oocyte care
Successful experimentation utilizing OpusXpress depends on quality oocytes. They must be treated with care at every step of the way, beginning with the frog surgery to obtain them. We provide our standard operating procedure for frog surgery as Appendix 1. We have found it best to have two people work together during the process of harvesting oocytes. One is the designated surgeon who handles the frog and performs the actual operation. The surgeon’s assistant should not even touch the frog, but rather is responsible for the oocytes and protecting them from exposure to any non-sterile contacts. We employ a fully aseptic surgical stage and procedures. We try to disinfect the entire surface of the frog. However, our experience has taught us that these procedures may not be sufficient to protect the cells from bacterial infections following surgery. While the surgeon finishes the procedure, and minds the frog through the recovery, it is the assistant’s job to continue the processing of the oocytes.
Our post-surgical processing of the oocytes is fairly standard. We, of course, try to maintain aseptic conditions with all sterile solutions and pipettes for oocytes handling. The collagenase is sterile-filtered. However, we do not have the luxury of sterile hoods for the collagenase procedure and cell checking, so we wipe surfaces frequently with 70% ethanol.
Cell selection, injections, and post-injection cell checking are all important jobs on the team’s roster. In an ordinary week we may work with a dozen or more injection sets, requiring a thousand or more cells to be selected immediately after the collagenase procedure. Injections are normally done on the day after surgery. The Drummond Nanoject microinjector is used to expediently inject the many desired oocytes with in vitro transcribed RNA coding for the receptor subunits of choice.
Anyone at all familiar with Xenopus oocytes knows that they are like apples in a barrel, it only takes one or two sick oocytes in a dish to seal the fate of remaining healthy cells. Therefore, we store cells in multiple dishes and check them frequently, culling any questionable cells and changing the media. It is most critical to check the cells within a few hours of injection, and again the next morning. Thereafter we check the cells and change the media daily. We have learned that, oddly, tissue culture-treated dishes, made to help adhesion in cultured cell lines, are better for the oocytes than untreated dishes because the oocytes do not stick to them, as long as the vitilline membrane is intact. Cells are maintained at approximately 14°C, and may become noticeably less robust if left out at warmer temperatures for extended periods.
Using OpusXpress
Features of OpusXpress fluid handling and data acquisition
OpusXpress is a very flexible machine and may accommodate the breadth of imagination of the investigator. Fluid handling and mechanical operations are integrated with data acquisition protocols that are based on standard pClamp (ClampEx) formats. Fluid can be directed to the cells in one of two ways, either through tubing that provides bath perfusion with running buffer or by using the automated pipetting system to pick up and deliver specific drugs or solutions that have been aliquotted into multiwell plates. Figure 2 provides illustrations of the OpusXpress components and operations. Flow rates for these two delivery systems can be programmed sequentially and variously, as desired. The chambers are designed with a conical delivery station for the pipetted drugs above the buffer entry hole, which is located just above and behind the oocyte recording chamber so that there is excellent mixing with minimal turbulence when switching from one solution source to the other. Air bubbles do not reach the oocytes.
Figure 2.
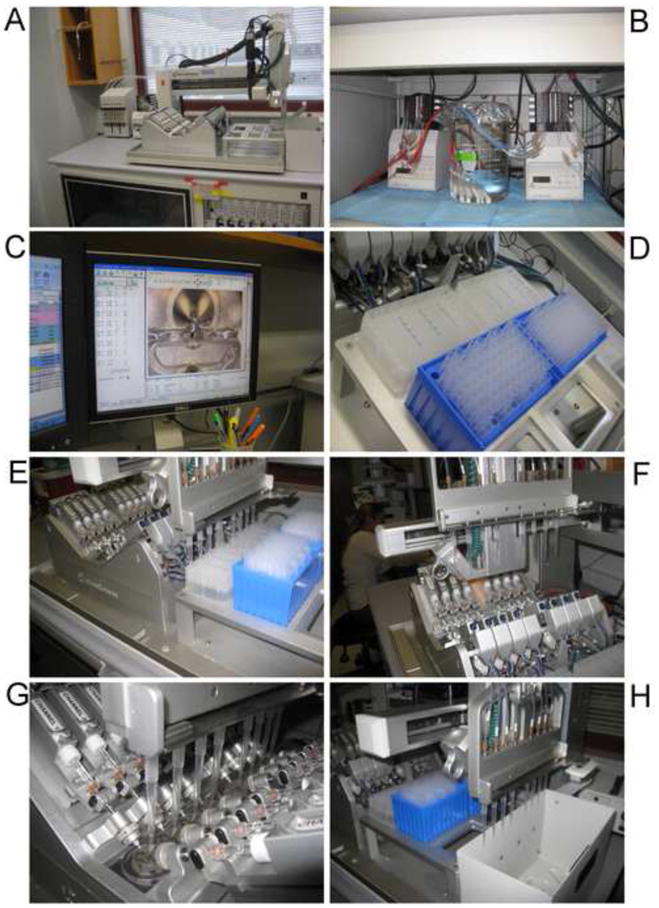
OpusXpress in action. A) The flight deck: the “V8” array of chambers is in the center. Behind the chambers to the left is the peristaltic pump for bath outflow and the syringe pumps for the pipette delivery system. To the right of the V8 are the racks for drug well plates and pipette tips, and furthest right is the bin for used tips. The video camera arm is in the rear, in its “homed” position. B) The two peristaltic pumps for the bath buffers. Tubes for the eight “buffer A” lines are in a single reservoir beaker. Each line has a replaceable filter at the end. C) One of the computer monitors with a chamber in view on the camera window. D) Drug wells and pipette tips in place. E) Aspiration of drug solutions. F) Movement of the tips toward the chambers. G) Drug delivery. H) Disposal of used tips.
The bath perfusion system can be supplied from either of two sources (buffer A or buffer B) via two sets of eight tubes and two peristaltic pumps. The lines to each individual chamber can draw from a single reservoir or alternatively, the lines to each chamber can draw from their own smaller reservoirs. The user can program changes between the two buffer sources at any time during an experiment. The junction for the two buffer inputs is close to the chambers, so after a change in the buffer source, there is minimal latency before the delivery of the new solution. To prevent cross contamination between the two buffers at the junction, whenever the active buffer is switched, a small amount of the new active buffer is drawn up into the inactive line.
It is possible to apply pulses of a reference drug from buffer B and achieve consistent reproducible control responses. Compared to our usual method of using the pipette system to make repeated application of the reference compound, using buffer B to make applications of the reference solution would have the advantage of freeing up extra wells in the solution plate for holding experimental solutions. However, the effect of this approach on overall throughput would be offset by the fact that, although more drugs could be delivered in a single experiment, the experiments themselves would be proportionately longer.
Another consideration is that the design of the pipette delivery system is optimized to present a sharper leading edge to a solution exchange than the bath perfusion system typically provides. However, the pipette delivery system is flexible enough that, with some amount of trial and error, the pipette delivery could be tuned to emulate the bath application, for example by specifying a slow initial rate of application.
Most commonly, the buffer B system is used for providing prolonged preapplications of drugs that have slow kinetics, or for simply changing the baseline ionic or pharmacologic condition. Sometimes pre-incubations are necessary to study drug-drug interactions, such as the effects of potentiators or antagonists. However, using buffer B will not be sufficient if preincubations with multiple drugs, or multiple concentrations of a single drug, need to be tested. For these types of experiments, the preincubation solutions also have to be delivered from the pipette system. Normally the bath perfusion stops while the pipette delivery takes place and begins again as soon as the pipette delivery is over. We have found that it works well to simply delay the restart of the bath perfusion system after a pipette-delivery of a preincubation solution, so that the cell sits in a “static bath” until the next programmed pipette drug delivery is made. The “static bath” incubation can be as long as desired, but has to be at least as long as it takes for the delivery system to change pipette tips, draw up and deliver the next solution (approximately 34 s).
Another level of flexibility in experimental design is provided by the data acquisition protocols. Potentially, several acquisition protocols could be called up during a single fluid handling step or likewise a single episode of data acquisition could run over several fluid handling steps, for example including both the data from a pre-incubation period, a drug delivery, and a prolonged washout step. The routine protocols for the study of nAChR are relatively simple, but the pClamp protocol generator is intrinsically able to design complex sequences of voltage commands, multiple sweeps, and event driven acquisition. The reader can refer to the various manuals provided by Molecular Devices for details on the OpusXpress and pClamp (ClampEx) protocol generators.
Preparation for experiments with OpusXpress
Do we impale cells first or prepare drug well plates first? That depends on several factors. Which is more precious, the cells or the test compounds? Is the clone being used a reliable expressor? Has the injection set already been tested and confirmed to be working well? Are the cells healthy and plentiful? Are the drugs stable in solution? Are they light or temperature-sensitive? Are they expensive or rare?
For optimal efficiency, one team member might prepare solutions while another readies the cells. Nonetheless, before we conduct the experiment of interest, we run what we call the “first control”. That is, the freshly impaled and clamped cells are given one application of the reference solution. This preliminary test serves several purposes: Firstly, it indicates whether the cells are expressing well enough for an experiment, with responses in an acceptable range, whether gain adjustments should be made, or whether the experiment should be postponed. It also allows us to confirm whether the individual cells are healthy and will be stable in voltage clamp. Bad cells can be replaced before the actual experiments.
Each experiment is associated with a hardcopy datasheet outlining the OpusXpress protocol and the drug solutions (see Figure 3 for an example). Data includes specifics about the oocytes’ injections and the experimental stock solutions used. These datasheets also indicate which wells were drawn upon for each step in the experiment. As each drug delivery to each single cell draws from 24 (3×8) wells, our datasheets include a map of these wells for a single cell, representing one of our eight replicates (Figure 4A). We fill in the map with the concentrations of each drug. This makes a good aid when loading the well plates. We also plan the arrangement of drugs in the wells for ease of pipetting, which also reduces error. We use a multi-channel pipettor for our controls, all in one column. We mark the 96-well plates by the columns of three wells (Figure 4B). On the datasheets there are lines for each cell (channel) with room for notes. Injection dates help us cross-reference with our injection logs and RNA lots, which we maintain in separate, searchable Excel files. Notes are added to these sheets while the experiment runs: experiment filenames and any anomalies that might occur during the experiment. Further notes are added during the analysis: which cells were analyzed, any anomalies seen, and the analyzed data filename. These log sheets are the keys to unlock the experiments as they allow the translation of the raw data to the pClamp data output which will be later stored in other electronic spreadsheets.
Figure 3.

Sample experiment sheet for an ACh concentration-response study of muscle-type (α1β1γδ) nAChR conducted on October 28, 2009 using cells injected on October 27, 2009 and an ACh stock solution made up the same day as the experiment. The ACh controls were 30 μM and the test solutions ranged for 100 nM to 300 μM. The initials (RLP) indicate that the data were analyzed by the first author.
Figure 4.
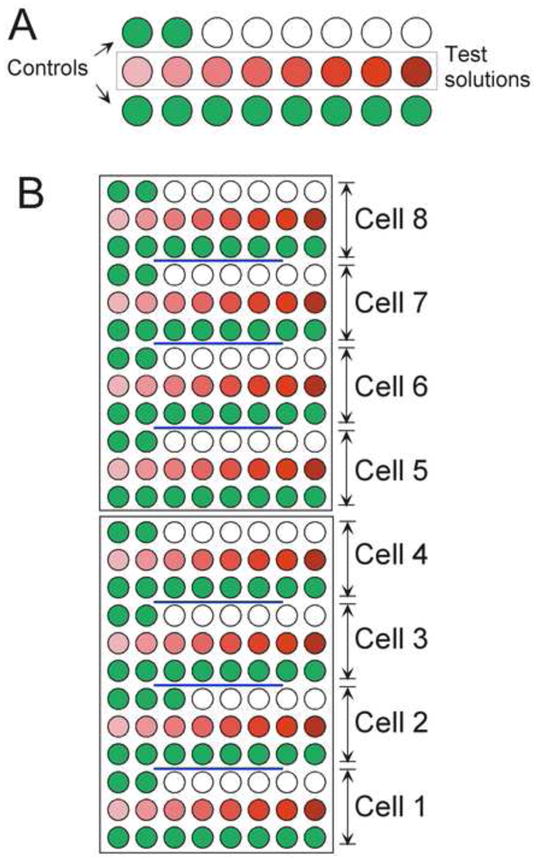
The loaded drug wells for the experiment described by the data sheet in Figure 3. A) The loading of the wells for a single cell. Control solutions are green, experimental solutions are in graduated shades of red. Note that this experiment utilized only 18 out of the possible 24 steps. B) The loading of the two-plate set to provide drug delivery for all eight cells.
Drug and buffer solutions
Experimental solutions need to be prepared for bath delivery and the bolus drug delivery from the eight channel Gilson fluid handler. The bath solution is usually a standard Ringer’s (115 mM NaCl, 10 mM HEPES, 1.8 mM CaCl, 2.5 mM KCl, 1μM atropine, pH 7.2.), which is stable at room temperature, and made up in bulk well in advance. In contrast, there may be more than a dozen different experimental solutions, which may or may not be stable if made up too far in advance. In order to minimize errors, we stick to a standard stock solution concentration, 10mM, and we have charts on the wall with dilution volumes.
Consideration must be given to the solvent of the drug stock. Concentrations of DMSO, for example, greater than 3% show responses in control oocytes. Solutions with acidic pH (≤6.0) will also cause responses in uninjected oocytes. The buffer commonly used as the diluent may affect the chemistry and activity of a drug.
Electrodes
Electrodes can be purchased from Molecular Devices, but we have a suitable puller (Sutter P-97, Novato CA), and making electrodes is another job taken care of by the team. We order borosilicate glass, 6 inches long, with a filament, 1.5mm OD, 1.2mm ID (King Precision Glass, Claremont CA). The electrodes are filled with 3M KCl: First, they are allowed to rest for a few minutes inverted in a small container with a little 3M KCl, and the capillary action of the filament will draw the salt solution up into the tip of the electrode. Then, the glass is backfilled, using a 28 gauge MicroFil syringe needle (World Precision Instruments, Sarasota FL), leaving approximately 1 inch headspace in order to avoid excessive salt buildup in the electrode holders. It is likely that a small air bubble near the tip will need to be dislodged by a little judicious tapping of the glass before the glass is slid over the silver-silver chloride pellet in the electrode holder. The pellet must be well immersed in the salt solution.
For each experiment, cells are placed in the eight separate chambers. With the bath fluidics on standby (0.5 ml/min), electrodes are lowered into the bath using the “Find All Solutions” button. Offsets will be automatically adjusted to 0. However, we have experienced enough accidental problems with our electronic connections allowing the electrodes to crash on the bottom of the bath, that we have learned to watch carefully with the cursor poised over the “Stop” button. Or, a simpler (and faster) method is to lower all the electrodes using the “Safe Position All” button, followed by the “Remove All Pipette Offsets” button. The Safe Position is selected for a depth where the glass tips will be submerged but not so deep as to touch the cells. Next, electrode resistances are checked. For our purposes, voltage electrode resistances must be between 0.5 and 10 MΩ and current electrode resistances must be between 0.5 and 3 MΩ. If the current electrode resistance is too high, large responses will not be clamped and recorded well.
Impaling cells
OpusXpress is reasonably good at automatic impalement, but in our experience, manual fine-tuning of the final steps is worth the time and attention. OpusXpress has a digital camera to serve as long working distance dissecting-type microscope. It can be automatically positioned over any of the eight chambers. The camera window is opened and positioned close to the “impaling” window on the screen so both windows may be seen readily. One channel at a time is selected (both V and I). Offsets are checked again and rezeroed if necessary before impaling. Typically, the electrodes can be advanced a couple times in 1 mm increments (by holding down the “Control” key while clicking the nudge button), depending on the safe position setting and bath depth, then the electrodes can be moved in 0.3 mm increments (by holding down the “shift” key while clicking the nudge button), watching both the offsets (mV column) and the camera. Small changes in the offset voltage occur just as the electrodes begin to touch the cell membrane. An ideal impaling would have both electrodes barely dimpling the cell and then simultaneously popping through the membrane. Likely one electrode will reach the cell first. Deselect it and proceed with the other. If the voltage jumps to a negative number, the cell is impaled. The membrane potential should be between −30 and −80 mV. If a cell needs to be replaced, “home” the electrodes by pressing the software button that draws them out of the bath. The camera may remain where it is if the headstages are not lifted up, but it needs to be sent to its “home” location if the electrodes need work (requiring lifting the headstages). There are sometimes difficulties with the alignment of the glass impaling the cell. Solutions are: rotate the electrode holder in the headstage to take advantage of any little eccentricity of the glass taper, use the alignment tool, or, as a last resort, replace the white plastic nose cone.
After impalement, the cells may then be clamped using the desired voltage, gain, and filter settings. It is useful to recheck the impaling window, looking at the clamped voltages and the holding currents. If the currents are large (< −0.1μA), the cell will probably not hold up very long, but often this baseline current decreases within a few minutes after impaling.
Running the experiment
Saved experiment “procedures” are named and accessed through the “Experiment” tab. Double clicking the procedure window opens it up for editing. Acquisition protocols may be edited through here as well. Procedures detail the experimental steps and fluidics. Protocols detail the data acquisition. Once the “go” button is pressed, the actual operation of the machine is usually quite troublefree, requiring no more than cursory attention in case a rare fault with the fluid handler should occur. The “Progress” window indicates the time remaining in the procedure, so preparations for the next experiment may begin as appropriate.
At the end of the day, experiments may be left to run on their own after the operator leaves. There are selectable settings to unclamp the cells, withdraw the electrodes to the safe position, and set the fluidics to an overnight rate when the experiment is completed. The electrodes may remain useable the next day if the tips are submerged in the baths overnight. A slow overnight flow rate will keep the baths from drying out, the source buffer from running out, and the waste container from overfilling. However, at the beginning of the next day, remember to deselect these checkboxes, or your first cells will be unclamped and unimpaled after the first control test, when you may prefer to keep them clamped and ready for an experiment.
Additional cautions and suggestions
Be careful to position tip boxes exactly.
Watch the fluid levels in the buffer reservoirs and the waste container.
Watch the waste box for used pipette tips. Tips always drop at one edge and might pile up there, sometimes enough to interfere with the drop.
If a tip fails to drop, the liquid handler (Gilson arm) will fault when it tries to pick up the next set of tips. The experiment will abort. After the liquid handler homes and reorients itself, you may be able to resume the experiment with the same cells if you edit your procedure, preferably starting with a fresh control; reset the nozzle; and replace tips and drugs as needed. Sometimes this jamming will push the nozzle head out of alignment so that it does not pick up tips properly. This can be adjusted with the four allen screws.
When the liquid handler delivers the drug to the baths, the tip ideally touches the back side of the cone above the bath level and deflects slightly. The positioning can be adjusted, by 1 mm increments in the x, y, and z directions using “Configure Fluidics”, “Syringe Pump”. Having this correct is important for response consistency.
Maintenance
Crucial for good data is quality control and routine maintenance. At a nontrivial cost, Molecular Devices will extend a service contract. For us it has always been money well spent because their support is tremendous and our machine gets very heavy use.
The OpusXpress team needs to pay attention to details. They need to be mindful of whether impalements are becoming more difficult or if bath solution is not running out as rapidly as expected. Any unusual noises signal a need to look for potential trouble. In our fortunate experience to date, everything about OpusXpress, like an old British motorcycle, is adjustable or replaceable. While the replacement parts usually have to come from Molecular Devices, there are a lot of adjustments that the team can learn to make on their own. To check the performance of the machine, we conduct regular flow tests (Figure 5). An overview of our routine maintenance procedures is provided in Appendix 2.
Figure 5.
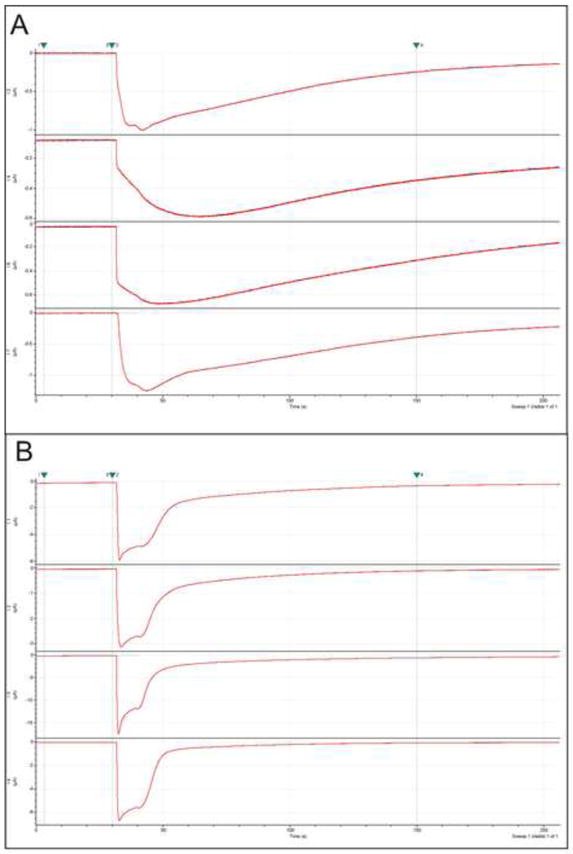
Sample traces from α4β2 nAChR expressing cells, viewed in Clampfit. A) The slow decay of these responses indicated to the users that the flow dynamics were suboptimal. B) Sample traces from α4β2 nAChR expressing cells obtained after the tubing was replaced in the perfusion system.
Data
OpusXpress automatically names each experiment with the procedure name, date, and a sequential number each day. Each experiment is saved in its own folder. Also in this folder is a log file detailing the procedure in text. They are stored in the heierarchy: Projects (drugs in our case), then Screens (genes being expressed in our case).
A central key to managing and finding our data is a large Excel document which is renamed with the date every day and archived. The first worksheet lists the experiments to be done, and the second worksheet contains the completed experiments, one experiment per row. It is our practice to fill colors by groups of experiments. When an experiment is run, it is copied from the first worksheet to the second, and the data filename is added. The fill color is removed from the first worksheet row, but the listing is not deleted until the experiment is analyzed and confirmed to be okay. The columns of the completed side are set to autofilter; in practice, this is searched quite frequently for various reasons. Our column headings are: cell, drug, experiment (a shorthand synopsis of the procedure, e.g. CRC), control (reference concentration), details (a complete listing of the procedure, usually only visible when the cell is selected), objective, date initiated, date finished, and raw data filename. This file serves as a communication coordinator amongst the lab personnel regarding what has been done and what needs to be done. At this writing, there are 30 rows filled in on the first sheet (experiments to be conducted or redone) and 6094 on the second sheet (completed experiments).
Data analysis
When things are working well, the amount of data generated by the team using OpusXpress is enormous. While the temptation is there to keep running experiments, it is important that data not pile up without being analyzed. No experiment can be taken off the list of experiments to be run until the data have been analyzed and confirmed to be of satisfactory quality. Problems can come up. For example, as mentioned above, we typically alternate applications of reference agonist with our experimental applications. We do this to ensure that our repeated measures from single cells always reference equivalent starting conditions. If we run a concentration-response study with ten drug concentrations but discover the control responses were suppressed after the first application of the experimental drug, then the only data we can use are from the first drug application. All of the other concentrations will likely need to be tested on individual sets of cells.
The analysis of large OpusXpress data sets can actually go very rapidly through the pClamp analysis module, Clampfit, especially if the data are being analyzed for a simple feature like peak current amplitude over baseline. Clampfit will store all the parameters used on your last analysis, so when you open the first file in a set of twenty-four files that have all the control and experimental responses of a concentration response study, you may be able to begin analysis at once. The cursors will be preset to define your baseline region and the appropriate segment to search for the peak response. If the Clampfit analysis tool bar is set up with the “quick statistics” button next to the “next file button” the entire experiment can be analyzed in the thirty seconds that it takes to press those two buttons in sequence twenty five times. We find it most convenient to copy the Clampfit “results” (i. e. the Clampfit-generated spreadsheet) into an Excel spreadsheet. The Excel spreadsheet may of course include an analysis template which, once the data has been pasted in, instantly conducts any normalization procedure required (e.g. experimental responses relative to controls) and then calculates means, standard errors, etc. With a nice set of data, the process can literally be accomplished in no more than a minute.
However, for as good a system as OpusXpress may be, no biological data are perfect. Many OpusXpress data sets are close enough to perfect that analysis can go very rapidly. By no means, though, can the analysis process be reduced to fifty button clicks in Clampfit without circumspection of the data as it passes through the analysis engine. For this reason, it is best if the analysis is being conducted by someone really involved with the science, or at least someone who can distinguish real data from artifacts that sometimes occur. Since we begin each experiment with eight replicates (i.e. normally we give the same treatments to each of the eight cells), we find it a good practice to quickly look through the entire sequence of files. If one or two cells are lost midway through the experiments, we can exclude those from the beginning and generate a complete high quality data set.
If net charge (area) is being measured relative to the baseline [23], the quality of the baseline is critical. In these cases, we manually adjust each baseline of each trace, carefully and consistently, taking care to not alter the response itself.
Often our team leaders have had enough experience to conduct the experimental analyses, so that what are passed on to the PI (or the students) are the reduced data in Excel. If there were problems with the analysis, they can usually be flagged by unduly large standard errors or other anomalous features.
Of course, any but the most banal data sets have to be analyzed by the individuals who will ultimately be writing the manuscripts or research reports. Someone prepared to try to interpret unexpected results has to look at the actual raw data. We recently published one such example of unexpected results [24]. We were studying an experimental activator of human α7 receptors, 3-2,4, dimethoxy-benzylidene anabaseine (diMeOBA or GTS-21), in the presence of the strong positive allosteric modulator PNU-120596. In the absence of PNU-120596, applications of diMeOBA produced only small transient inward currents and inhibition of subsequent responses to control applications of ACh. When we applied diMeOBA repeatedly in the presence of PNU-120596 (in the bath buffer B solution) it seemed almost as if we were losing the cells, since there were large increases in the holding currents. Curiously, when we made additional applications of diMeOBA to the apparently unstable cells, we recorded what appeared to be outward currents. It was not until we repeated the experiments and obtained essentially the same results that we understood what was going on. The increase in holding current that we recorded was not due to cells dying. It was an accurate reflection of the fact that over time diMeOBA and PNU-120596 together were creating so much channel activation that it appeared as a steady-state current. When we made bolus applications of diMeOBA, the drug showed that it had a secondary effect of blocking the already open channels, so that what looked like outward currents were temporary blockades of the steady-state inward currents. You have to be prepared to learn whatever it is that the data has to teach you.
Data storage
We archive our analyzed data, mainly in the form of excel sheets, with the same file heierarchy as the raw data files: drug, then receptor type. Needless to say, frequent and multiple backups are mandatory.
The handwritten datasheets are kept in chronological order in a single set of notebooks. We have a portable copy machine at hand to make duplicates of the log sheets that will travel with the raw data files to the person who conducts the actual analysis of the data. After the analysis, the annotated copies of the datasheets are also archived.
Planning for success and for serendipity
In pre-OpusXpress days, we had a colleague who hoped he had cloned a new calcium channel gene. He wanted data from five oocytes expressing his new gene so he injected seven cells. A single cell survived long enough to give uninterpretable results. We cite this as an example of planning for failure, and our advice is to plan for success. In this article we have tried to share some of our experiences which we hope will lead to successful cell survival and experimental results. If data is needed from ten cells, best to inject 100 cells, better yet to inject 200, using two separate lots of RNA!
As discussed above, another part of planning for success is to never let the data go cold. If there has been a problem with an experiment, it is best to run it again right away, while cells and drug stock solutions are still available. If the data is unanalyzed for even a single day, the resulting delay before the experiment can be repeated may be a week or more.
Try to be ready for follow-up experiments by having plenty of cells expressing your primary targets ready all the time. New experiments may suggest themselves constantly, especially when are preparing a manuscript. There are times when a hypothesis may suggest itself in the process of composing a sentence for a manuscript. The sentence could be written as a speculative hypothesis, but if there are injected oocytes available, with OpusXpress, in the matter of just a of couple hours it may be possible to state a conclusion rather than merely make a speculation. This is a really beautiful aspect of a system like OpusXpress in an academic environment. On numerous occasions the first author has had an experiment come to mind during the morning motorcycle ride to work and, thanks to the machine and the excellence of the laboratory team, has had publication-quality results in the afternoon. It is a wonderful thing to be discussing science while visiting colleagues out-of-town and when a question comes up, to be able to phone into the lab, request an experiment, and get an answer just a few hours later.
OpusXpress has tremendous potential, enough to inspire many investigators if they can make the most out of it. While this article has focused on our experiences with OpusXpress, most of the philosophies about lab management can be applied to many settings. You can never go wrong with teamwork, quality control, planning for success, and keeping your mind open to serendipitous discovery.
Acknowledgments
We would like to acknowledge all of the following people from Axon Instruments who helped make OpusXpress a reality; Rich Lobdill, Greg Hamersly, Philip Churchward, Chris Johnson, Peter Kay, Steve Ames, Scott Ferguson, Nick Fitton, Jason Williams, Alan Christiansen, Eric Harris, Andy Blatz, JoAnna Delgado, Chris Mathes, Eric Fung, Lynette Sarandi, and most especially Cathy Smith-Maxwell, the product line manager and Alan Finkel, the former CEO of Axon Instruments.
We also want to acknowledge and thank the following members of the Papke lab who were appreciated members of the Opus team past and present; Adriane Argenio, Bernadette Schoneburg, Brian Jack, Chad Brodbeck, Chris Coverdill, Dolan Abu-Aouf, Hillary Schiff, Irena Garić, Joshua Buhr, Julia Papke, Lisa Jacobs, Lynda Cortes, Matt Beatty, Sara Braley, Shehd Abdullah Abbas Al Rubaiy, and Theresa Kappel.
Work in the Papke lab has been supported by numerous sources including: N.I.H grants: PO1 AG10485, GM57481, DA017548, the McKnight Foundation, Targacept, Memory Pharmaceuticals, Servier, Critical Therapeutics and Lundbeck. Our primary OpusXpress was donated by Axon Instruments, a second OpusXpress is on loan from Dr. Lynn Wecker (University of South Florida).
Appendix 1
Frog surgery and harvesting oocytes
Justification
Xenopus oocytes are internationally accepted as a valuable system of choice for the study of artificially expressed receptors. The use of Xenopus oocytes to study human or mammalian brain receptors is a method of using the lowest phylogenetic species available. By using these animals, our results can be directly compared with hundreds of other studies. That is, our work is more meaningful because our results can be considered in this large context. Moreover these animal respond well to handling and can be kept healthy in captivity.
It is of scientific value to be able to do repeated survival surgeries on the same animals because, due to the biological variability of the oocytes, it may be important to return to the same animal for additional samples of oocytes to evaluate whether experimental results are associated with that variability. Since batches of oocytes from different frogs may vary greatly in the potential for expression or the constitutive balance of metabolic mediators (kinases, cyclic nucleotides etc.), repeated use of a single frog may provide a reproducible assay for sensitive or unique effects. Additionally, since there is a large amount of variability amongst animals in regard to the expression of the injected RNAs, it is important to identify those animals which provide oocytes most suitable for the experiments and to maximize the experimental potential of those animals. While some oocytes may be adequate for experiments when robust responses to established experimental protocols are expected, occasionally experiments require the use of oocytes that produce large responses to small stimuli. This can most effectively be accomplished by using oocytes from animals previously evaluated for their experimental suitability.
In brief
Frogs are purchased from approved vendors and maintained by Animal Care Services. The oocytes are obtained by surgical removal from the frog’s belly. First, the frog is anesthetized by being placed in a basin of water containing the anesthetic, 3-aminobenzoic acid ethyl ester (MS222), which is absorbed through the frog skin. After it is fully anesthetized, the surgery is performed aseptically. The muscle and skin layers are sutured separately, using absorbable suture so there is no need to re-anesthetize the frog to remove sutures. The frog is watched and kept moist until she fully recovers and is returned to the animal care facility, where she is monitored daily. We may do up to five survival surgeries and the sixth is terminal. During the first surgery on an animal, colored beads are sewn onto a toe to identify the frog. We wait at least two months between surgeries on the same animal, and then cut into the opposite side of the belly from the most recent surgery. Frogs are housed separately for one to two days after surgery for monitoring, and they are normally housed in group tanks. We never observe any signs of pain or distress in the frogs.. The frog behavior after the surgery is indistinguishable from the behavior before the surgery.
Presurgical preparation
Clean surgical instruments (blunt forceps, tissue forceps, hemostats, fine scissors, and blunt scissors) are autoclaved together with a section of surgical drape and two packets of sterile gauze all in a surgical autoclave pack. The surgical area, surrounding counter tops, and the surgical tray are washed first with soap and water, rinsed, then wiped with 70% ethanol. A large autoclaved drape is draped over equipment in the back of the hood area where surgeries are performed. A germicidal light is turned on for 20 minutes. (We use an enclosed area dedicated to surgeries and oocyte injections.) Ice is placed on the surgical tray and covered with a new piece of waterproof drape.
We have found it to be a good idea to have both a surgeon and an assistant. It is important to be conscious that the frog tank water normally contains pseudomonas and aeromonas bacteria, which we have found to be resistant to penicillin, streptomycin, and gentamicin. And these bacteria, it seems, are the main reason that laboratories frequently report seasonal problems with their oocyte health [25]. Therefore, we separate duties as much as possible, wear gloves, and keep the frog basins on a different counter than where we work with oocytes.
We maintain an Excel file listing bead colors (3 small colored beads), date frog received, natural markings (if any; beads are sometimes lost), dates and sides of surgeries, surgeon’s initials, egg quality, and medical notes.
Frogs are anesthetized for surgery by being placed in a basin containing 1.5 liters of frog tank water and 1.0 g MS222 buffered with 0.65 g sodium bicarbonate. The anesthetic is readily absorbed through the frog’s skin. The basin is set in ice to further immobilize the frog. The frog is removed from the anesthesia water after 15 min immersion and visually and tactually inspected for lack of responsiveness.
After the frog has been in the anesthesia water 15 min, it is disinfected before the surgery. As noted above, there are pseudomonas/aeromonas bacteria normally present in frog water. These bacteria are highly mobile, very small and swift, and they could easily traverse the moist skin of the frog from the back to the belly during the surgery if we only disinfected the belly. We put petrolatum opthalmic ointment (Paralube) on the frog’s eyes to protect them and then place the frog in an autoclaved beaker containing 200 ml of 1% chlorhexidine diacetate (Nolvasan) solution, keeping the eyes and nose out of the solution. After no more than 3 minutes in the disinfectant, the frog is rinsed with e-pure water, the skin of the belly wiped gently with a sterile gauze pad to remove any loose debris, and rinsed again. The frog is then laid on the drape over the ice in the surgical area.
Surgical procedure
The surgeon puts on sterile gloves and observes the sterile field. A window is cut in the autoclaved surgical drape and laid over the frog belly. The skin is cut with a #15 scalpel blade, holding the belly taut, making an incision approximately 1.5 – 2 cm long. Tissue forceps are used to grasp the muscle layer, which is then cut in an upward direction with a #11 scalpel blade. Fine pointed scissors are used to lengthen the incision to approximately 1 – 1.5 cm long. The incision in the muscle layer will be centered under the incision in the skin layer and will be a little shorter. Oocytes are usually presenting, in ovarian lobes, and can be easily, gently pulled out with forceps. The desired quantity is cut off with scissors and placed in a petri dish containing filter-sterilized calcium-free Barth’s solution (88 mM NaCl, 1 mM KCl, 0.33 mM MgS04, 2.4 mM NaHCO3, 10 mM HEPES (pH 7.6), 12 mg/l tetracycline chloride). There is no need to tie off the remaining ovarian tissue, which is allowed to slip back into the belly cavity. Care is taken that no oocytes are left between the muscle layer and the skin.
The muscle layer and the skin are sutured separately with 4-0 synthetic absorbable monofilament suture, Three knots are made for each stitch. Care is taken to include the membrane over the muscle layer. The first two knots are loose, just butting the sides of the incision together. The third knot is pulled tight. There are usually 2–3 stitches made in the muscle layer and 3–4 stitches in the skin. The incision is sealed with VetBond tissue adhesive (3M Animal Care Products, St. Paul MN). Gentamicin solution (2–4mg/kg) is injected into muscle of a hind leg. The surgery takes approximately 30 minutes.
At the first surgery, colored beads are attached to a hind toe using 4-0 nylon monofilament suture in order to mark the frog for identification.
Recovery
The frog is then placed on her back on wetted paper towels in a rectangular basin and kept moist with E-pure water while she recovers from the anesthesia. The frog is judged to be recovered when she flips herself over onto her belly. This usually takes between 30 minutes and two hours. She is then placed in a basin containing some frog tank water and is carried back down to the Animal Care Facility. The frog is placed in a recovery tank for daily observation for a maximum of two days unless otherwise directed by the clinical veterinarian and then returned to the communal frog tank.
No signs of any pain, distress, or discomfort are seen in the frogs before, during, or after surgeries. The behavior of the frogs after recovery from the surgery is indistinguishable from their behavior before a surgery. The frogs are typically motionless at the bottom of the tank and will swim when startled, and occasionally they will swim to the surface for air.
“Xenopus lacks a cerebral cortex, and although some nociception possibly occurs in the thalamus, it is reasonable to assume that “pain” perception in Xenopus is much more primitive than in mammals. The absence of a limbic cortex or cerebral cortex diminishes the potential for pain in amphibians. Amphibians do not have the ballooning neocortex of humans and other mammals, nor do they possess any complex cortical tissue arising from telencephalic specialization. By default, processing of sensory information ends at the diencephalon or midbrain areas of the frog brain. Even these midbrain areas are much less complex than homologous areas in mammals, lacking distinct clusters of neurons or laminar structure.” -- Craig W. Stevens, MS, PhD, associate professor of pharmacology at Oklahoma State University, College of Osteopathic Medicine, Tulsa, OK.
The MS222 is reported to provide a residual analgesic effect, so additional local analgesics are not necessary. Green [26] reports that use of additional analgesia may be dangerous to Xenopus laevis after surgical harvest of oocytes because the therapeutic window is narrow, there is danger of oversedation and drowning, modes of action and side-effects of the analgesic agents are not known, and amphibian pain sensation is not known. Furthermore, local anesthetics such as bupivacaine affect the ion channels which we study expressed in the oocytes [27], and bupivacaine is highly lipophilic and has a long half-life.
We may do five survival surgeries on each animal and a sixth terminal surgery. There will be at least two months between surgeries on the same animal, and the incisions will be made on opposing sides of the belly for sequential surgeries.
Euthanasia
After the sixth surgery, or if the frog has no more eggs in its belly, or if the eggs are of consistently poor quality, frogs are euthanized after anesthesia by decapitation. For the terminal surgery, the suturing and prophylactic gentamicin injection are omitted. Frogs are re-anesthetized after the surgery (even though they have not yet recovered from their initial anesthesia) by being placed in 0.75 g/liter MS222 buffered with sodium bicarbonate for 30–60 minutes. The anesthetic is readily absorbed through the frog skin. The “decapitation” is accomplished by inserting one blade of a gross anatomy cutter in the frog’s mouth so that the outer blade is at the back of the top of the head. The skull and brain are cut in this manner as a physical assurance of death.
Appendix 2
OpusXpress routine maintenance
General
Minimize algae growth. We autoclave our Ringer’s vessels and keep them covered. We filter-sterilize our HEPES stock.
Clean up salt and solution spills promptly.
Try to prevent oocytes from slipping into the aspiration tube. It happens sometimes, and the tube might get blocked and need clearing.
Daily
Whenever electrodes are changed, the electrode holder is inspected for cracks, corrosion, and salt buildup. Usually the port hole has some salt crust around it, which is easily rinsed off with a squeeze bottle of distilled water. Water is directed into the port hole, followed by a blast of a canned air duster. This is also a good way to remove broken glass which might be stuck in there.
Mind the level of fluid in the bath. It should be generally as low as possible for efficient solution exchange. The level is adjusted by screwing the aspiration tube up or down. We use a 1 ml pipette tip to turn this little pipe.
Weekly
Perform a flow test and adjust peristaltic pump or change tubing as needed (Figure 4). The red-red tygon tubing in pumps for buffers A and B are most vulnerable to wear. Flow tests are most easily performed by unscrewing the tubes at the beige fittings on the outflow side of the pump. Each one is placed in a numbered 15 ml graduated tube. Using the manual pump controls and a timer, set the pump to run for a set time and rate, for example 2 ml/min for 5 min or 4 ml/min for 2 min. Then you can see just what your true flow rate is. Theoretically, tightening the screws in the back of the peristaltic pump will increase the flow rate. In our experience, it helps to add some tape-covered cardboard padding for the screws to press against. Even so, we generally replace this tygon tubing (Cole Parmer, Vernon Hills IL) every two weeks. The outflow rate is less important, and this tubing (purple-black) is changed less often.
Replace the buffer A and B vessels. It helps keep the filters and tubing clean.
Firmly wipe the metal nozzles on the Gilson arm with a Kimwipe and 70% ethanol. This seems to help the tip drop. It seems that some sort of plastic residue builds up over time with use and makes them stick.
Monthly
Run 50% isopropanol through the lines. If you run this through the baths, have the electrodes homed (i.e. out of the bath). The isopropanol seems to ruin the resistances.
Likely the white filters will need changing (UpChurch/IDEX, Oak Harbor WA). Change them if they are discolored or feel slimy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bossi E, Fabbrini MS, Ceriotti A. Methods Mol Biol. 2007;375:107–31. doi: 10.1007/978-1-59745-388-2_6. [DOI] [PubMed] [Google Scholar]
- 2.Morera FJ, Vargas G, Gonzalez C, et al. Methods Mol Biol. 2007;400:571–85. doi: 10.1007/978-1-59745-519-0_38. [DOI] [PubMed] [Google Scholar]
- 3.Sigel E, Minier F. Mol Nutr Food Res. 2005;49:228–34. doi: 10.1002/mnfr.200400104. [DOI] [PubMed] [Google Scholar]
- 4.Papke RL, Smith-Maxwell C. Comb Chem High Throughput Screen. 2009;12:38–50. doi: 10.2174/138620709787047975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Methfessel C, Witzemann V, Takahashi T, et al. Pflüegers Arch Eur J Physiol. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- 6.Miledi R, Parker I, Sumikawa K. Embo J. 1982;1:1307–12. doi: 10.1002/j.1460-2075.1982.tb01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwamoto H, Blakely RD, De Felice LJ. J Neurosci. 2006;26:9851–9. doi: 10.1523/JNEUROSCI.1862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng JB, Chen XZ, Berger UV, et al. J Biol Chem. 1999;274:22739–46. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- 9.Kanai Y, Nussberger S, Romero MF, et al. J Biol Chem. 1995;270:16561–8. doi: 10.1074/jbc.270.28.16561. [DOI] [PubMed] [Google Scholar]
- 10.Klockner U, Storck T, Conradt M, et al. J Biol Chem. 1993;268:14594–6. [PubMed] [Google Scholar]
- 11.Mager S, Naeve J, Quick M, et al. Neuron. 1993;10:177–88. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- 12.Kavanaugh MP, Arriza JL, North RA, et al. J Biol Chem. 1992;267:22007–9. [PubMed] [Google Scholar]
- 13.Masu M, Tanabe Y, Tsuchida K, et al. Nature. 1991;349:760–5. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- 14.Pin J, Waeber C, Prezeau L, et al. Proc Natl Acad Sci USA. 1992;89:10331–10335. doi: 10.1073/pnas.89.21.10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miledi R, Eusebi F, Martinez-Torres A, et al. Proc Natl Acad Sci U S A. 2002;99:13238–42. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miledi R, Palma E, Eusebi F. Methods Mol Biol. 2006;322:347–55. doi: 10.1007/978-1-59745-000-3_24. [DOI] [PubMed] [Google Scholar]
- 17.Palma E, Amici M, Sobrero F, et al. Proc Natl Acad Sci U S A. 2006;103:8465–8. doi: 10.1073/pnas.0602979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfrey EW, Sanders GE. Comp Med. 2004;54:170–5. [PubMed] [Google Scholar]
- 19.Gouchie GM, Roberts LF, Wassersug RJ. Lab Anim (NY) 2008;37:165–9. doi: 10.1038/laban0408-165. [DOI] [PubMed] [Google Scholar]
- 20.Hilken G, Dimigen J, Iglauer F. Lab Anim. 1995;29:152–62. doi: 10.1258/002367795780740276. [DOI] [PubMed] [Google Scholar]
- 21.Schultz TW, Dawson DA. Lab Anim (NY) 2003;32:34–9. doi: 10.1038/laban0203-34. [DOI] [PubMed] [Google Scholar]
- 22.White-James J, McAndrew D, Badman J, et al. Lab Anim (NY) 2008;37:161–3. doi: 10.1038/laban0408-161. [DOI] [PubMed] [Google Scholar]
- 23.Papke RL, Papke JKP. Br J of Pharm. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papke RL, Kem WR, Soti F, et al. J P E T. 2009;329:791–807. doi: 10.1124/jpet.108.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsner HA, Honck HH, Willmann F, et al. Comp Med. 2000;50:206–11. [PubMed] [Google Scholar]
- 26.Green SL. Comp Med. 2003;53:244–7. [PubMed] [Google Scholar]
- 27.Aracava Y, Ikeda SR, Daly JW, et al. Mol Pharmacol. 1984;26:304–13. [PubMed] [Google Scholar]


