Abstract
Toll-like receptors (TLRs) are pattern-recognition receptors related to the Drosophila Toll protein. TLR activation alerts the immune system to microbial products and initiates innate and adaptive immune responses. The naturally powerful immunostimulatory property of TLR agonists can be exploited for active immunotherapy against cancer. Antitumor activity has been demonstrated in several cancers, and TLR agonists are now undergoing extensive clinical investigation. This review discusses recent advances in the field and highlights potential opportunities for the clinical development of TLR agonists as single agent immunomodulators, vaccine adjuvants and in combination with conventional cancer therapies.
Keywords: bacillus Calmette–Guerin, CpG, imiquimod, immunotherapy, monophosphoryl lipid A, polyriboinosinic-polyribocytidylic acid, resiquimod, Toll-like receptor, Toll-like receptor agonist, vaccine adjuvant
Background
The innate immune system utilizes a variety of transmembrane or secreted pattern-recognition receptors (PRRs), which are vital for activation of complement and coagulation cascades, opsonization, phagocytosis, apoptosis and induction of proinflammatory mediators. Toll-like receptors (TLRs) are important members of PRRs, initially discovered as Toll involved in Drosophila dorsoventral embryonic development and antifungal immunity [1]. In 1997, Medzhitov et al. cloned a human homolog of the Drosophila Toll protein, now known as TLR4, and showed that the Toll/NF-κB signaling pathway is conserved in humans, and that Toll signaling stimulated adaptive immune responses [2]. Shortly after, TLR4 was shown to be involved in the recognition of lipopolysaccharide (LPS), a major cell wall component of Gram-negative bacteria, which established the link between mammalian TLRs and recognition of pathogen-associated molecular patterns (PAMPs) [3–5]. PAMPs are conserved motifs shared by many bacteria, viruses, protozoa and fungi; examples are LPS and lipoteichoic acid (all recognized by TLR4); peptidoglycans (cell walls), lipoproteins (bacterial capsules) and zymosan (all recognized by TLR2 following heterodimerization with TLR1 or TLR6); flagellin (in bacterial flagella, recognized by TLR5), unmethylated bacterial or viral CpG DNA (recognized by TLR9) and viral RNA (single-stranded recognized by TLR7 and TLR8, double-stranded by TLR3).
Toll-like receptors belong to the TLR-IL-1 receptor (TIR) superfamily; they share an ectodomain composed of leucine-rich repeats for recognition of PAMPs and an intracytoplasmic TIR domain that mediates the recruitment of adapter molecules such as myeloid differentiation factor-88 (MyD88), TIR-associated protein (TIRAP), Toll receptor-associated-activator of interferon (TRIF) and/or Toll-receptor-associated molecule (TRAM). While most TLRs are expressed on the cell surface, TLR3, 7, 8 and 9 are found within endosomes, where they are activated following capture and internalization of pathogens or their products [6,7]. Some cell-surface TLRs are internalized after ligand binding; TLR2, for example, is recruited to macrophage phagosomes [8].
Toll-like receptor signaling is detailed in Figure 1; pathway activation results in transcription of type I IFN genes and proinflammatory cytokine genes such as TNF-α, IL-1 and IL-6 [6]. The cytokine induction pattern is determined by the type of TLR-activated cell. Stimulation of human TLR7, for instance, induces IFN-α from plasmacytoid dendritic cells (pDCs) important for innate antiviral immunity and the development of adaptive immunity, whereas it induces IL-12 from myeloid dendritic cells (mDCs), associated with the induction of a Th1 response [9]. It has also been suggested that, at least for murine TLR4, relative activation of its two distinct downstream signaling pathways affects the therapeutic index of the agonist [10]. Despite differences in the induced cytokine pattern defined by dendritic cell (DC) subset, TLR agonist and signaling adaptors, TLR activation generally results in activation and phenotypic maturation of all DCs.
Figure 1. Toll-like receptor signaling.
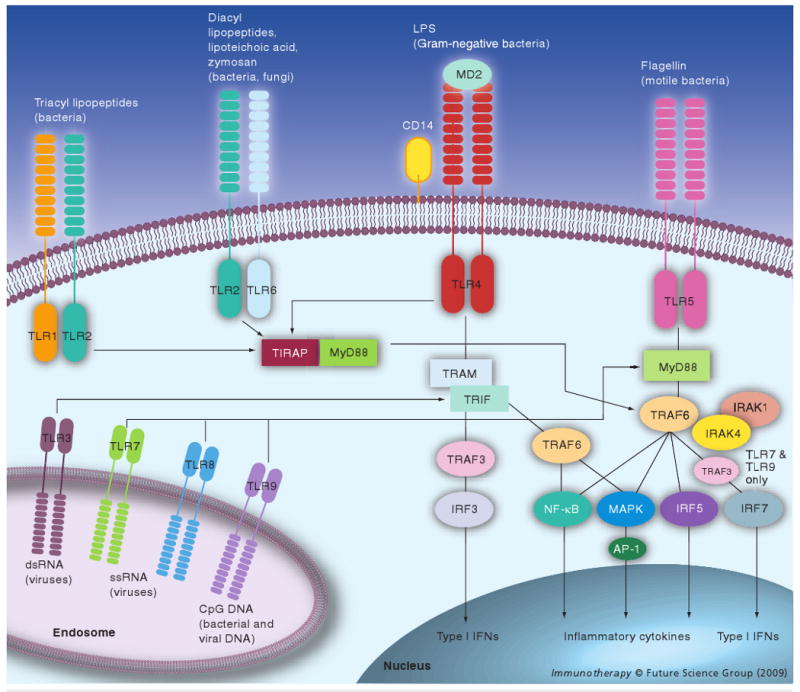
Toll-like receptors (TLRs) recognize microbial products, endogenous ligands (such as HGMB1, not shown) and synthetic agonists (not shown) either directly or aided by accessory molecules, such as CD14 and MD2. Dimerization of the receptor (heterodimerization for TLR2/6 and TLR1/6) is followed by downstream signaling. All TLRs engage the MyD88 adaptor molecule with the exception of TLR3, which signals through TRIF. TLR4 signaling can follow MyD88/TIRAP-dependent and/or TRIF/TRAM-dependent pathways. Activation of NF-κB, activator protein (AP)-1 and interferon regulatory factors (IRFs) induces expression of genes encoding inflammatory cytokines, type 1 IFN and IFN-inducible genes.
MyD88: Myeloid differentiation factor-88; TIRAP: TIR-associated protein; TRAM: Toll-receptor-associated molecule; TRIF: Toll receptor-associated-activator of interferon.
Toll-like receptors are invariant receptors, preferentially expressed in cells of the innate immune system. They provide an organism-wide sensing system; therefore, the expression pattern is aligned with the cell's frontline effector role in host defense. The expression and regulation of TLR genes in humans differ from those of other species, for instance mice [11], placing important limitations on the interpretation of animal models. In humans, ten distinct TLRs have been described to date. mDCs express TLR1–6 and 8 whereas pDCs express TLR7 and 9 [9,12–15]. TLR7 expression remains controversial in mDCs and monocyte-derived DCs (moDCs) [12,14]. Neutrophils express TLR1, 2 and 4–10, natural killer (NK) cells express TLR1, monocytes express all TLRs except TLR3 and B lymphocytes express TLR9 and 10 [16–19]. Activated T-cell subsets; including memory cells, express TLR2 as a costimulatory receptor [20], whereas regulatory T cells (Tregs) can express TLR8 and 10 [21,22]. While TLRs are primarily expressed in hematopoetic cells, they have also been described in keratinocytes [23] and epithelial cells of the intestinal, urogenital and respiratory tracts [24–26], and are likely to provide antimicrobial defense in addition to the mechanical barrier of the epithelial layer.
The importance of TLRs in the host response to tumors is evident from the fact that a link between polymorphisms in TLR genes has been established not only for susceptibility to infections but also for cancers [27]. For instance, carriers of the TLR2 rs3804100 variant are at greater risk for developing marginal zone lymphoma (MZL) [28]. The association between TLR2 and MZL is conceivable given the strong evidence linking Helicobacter pylori to the pathogenesis of mucosa-associated lymphoid tissue lymphoma, a common subtype of MZL, and the role of TLR2-modulated immune responses to this pathogen [29,30].
Rationale for TLR agonists as cancer therapeutics
Systemic antitumor effects of streptococcal infections were reported over a century ago [31]. These observations were followed by attempts to treat cancer with Coley's toxin (a mixture of heat-killed Streptococcus pyogenes and Serratia marcescens), which cured a subset of patients with inoperable sarcoma [32]. In retrospect, antitumor effects were probably due to TLR-activation by endotoxins and unmethylated sequences in bacterial DNA.
TLR3, 4, 7/8 and 9 agonists represent promising cancer immunotherapeutics and have been included in the ranked National Cancer Institute's list of immunotherapeutic agents with the highest potential to treat cancer [201]. They provide essential requirements for initiating T-cell immunity: antigen uptake, processing and presentation by DCs and other APCs, DC maturation and T-cell activation. TLR-mediated DC activation leads to enhanced phagocytosis, maturation with upregulation of MHC and costimulatory molecules (CD80, CD86 and CD40), upregulation of CCR7 and migration to draining lymph nodes, secretion of directive cytokines and antigen presentation to lymphocytes [15]. Type I IFNs are the most prevalent cytokines induced upon TLR3, 7, 8 and 9 activation. While often associated with antiviral defense, type I IFNs are also essential for several aspects of the adaptive immune response including facilitation of antigen cross-presentation, proliferation of memory T cells, prevention of T-cell apoptosis, induction of DC maturation and activation of NK cells [15]. Of particular interest to the development of TLR agonists for anticancer therapy are the effects of the TLR-induced cytokines IL-6 and IL-12 [33,34]. IL-6 can enhance antigen-specific T-cell activation through suppression of Tregs; IL-12 skews the effector response toward a Th1 profile [35], although IL-12-independent pathways for Th1 differentiation have been recently described after TLR4 stimulation [36]. TLR7/8 activation by imidazoquinolines can also enhance antitumor effects through inhibition of angiogenesis, NK-mediated cytotoxicity and direct apoptosis of tumor cells [37–39]. Furthermore, local TLR7 activation by imiquimod has been shown to alter the tumor microenvironment and create an inflammatory environment suitable for antigen cross-presentation and infiltration by effector T cells and DCs with cytotoxic potential [40].
Toll-like receptor agonists undergoing clinical investigation for cancer are reviewed in the following section and include live or killed bacteria, viral agents and synthetic compounds (Table 1).
Table 1.
Toll-like receptor agonists under clinical investigation for cancer therapy
| TLR | Agent | As monotherapy | As vaccine adjuvant | As ex vivo dendritic cell stimulator | In combination with chemotherapy | In combination with radiotherapy | In combination with targeted therapies, monoclonal antibodies or cytokines |
|---|---|---|---|---|---|---|---|
| 2/4/9 | BCG | Bladder cancer*, malignant melanoma [46] | Malignant melanoma [87], colon cancer [88], urothelial cancer [89], breast cancer [152] | Breast cancer [153], acute myelogenous leukemia [154] | |||
| 3 | Poly I:C, Poly ICLC | Advanced cancers [80], malignant melanoma [82] | Malignant glioma (I/II), malignant melanoma (I/II), HCGβ-expressing tumors (I) | Malignant melanoma [112] | |||
| 4 | MPL | NSCLC [96,98] and (III), malignant melanoma [90,91,99] and (III), breast and ovarian cancer [93,94], breast cancer (III), mucin-expressing tumors [92], ras-mutated tumors [95] | |||||
| 4 | LPS | Ductal carcinoma in situ [113], NHL (I/II), malignant melanoma (I/II) | |||||
| 7 | Imiquimod | Basal cell carcinoma*, actinic keratosis*, breast cancer (II), malignant melanoma (I/II) | Malignant melanoma [104] | Breast cancer (I/II) | Malignant melanoma [155] | ||
| 7 | 852A | Advanced cancers [64,65] | |||||
| 7/8 | Resiquimod | Melanoma (I), HCGβ-expressing tumors (I) | |||||
| 8 | VTX-2337 | Advanced cancers (I) | |||||
| 9 | CpG ODN | Glioblastoma [156], NHL [157], malignant melanoma [74,75], renal cell cancer (I), CTCL (I), CLL (I), malignant melanoma [75,77] | NY-ESO-1-expressing tumors [101], MAGE-3-expressing tumors (I/II), NSCLC (II/III), malignant melanoma [100], renal cell carcinoma (I/II) | NSCLC [133], colorectal cancer (I) | NHL (I/II)[120] | NSCLC (II), NHL [132,138,158], NSCLC (I), colorectal cancer (I) |
I/II/III indicates ongoing Phase I, II or III studies, as listed in [202].
US FDA-approved for this indication.
852A: Synthetic imidazoquinoline mimicking viral ssRNA; VTX-2337: Small-molecule selective TLR8 agonist mimicking viral ssRNA; BCG: Bacillus of Calmette–Guerin, Mycobacterium bovis; CpG ODN: CpG oligodeoxynucleotide; CLL: Chronic lymphoid leukemia; CTCL: Cutaneous T-cell lymphoma; Imiquimod: Synthetic imidazoquinoline mimicking viral ssRNA; LPS: Lipopolysaccharide; MPL: Monophosphoryl lipid A; NHL: Non-Hodgkin's lymphoma; NSCLC: Non-small-cell lung cancer; Poly I:C: Polyriboinosinic-polyribocytidylic acid; PolyICLC: Poly I:C-poly-l-lysine; Resiquimod: Synthetic imidazoquinoline mimicking viral ssRNA; TLR: Toll-like receptor
TLR agonists as monotherapy for cancer
Toll-like receptor agonists used as single agents especially when applied locally can effectively eradicate tumors due to their potent stimulation of innate and adaptive immunity as well as their effects on the tumor microenvironment (Figure 2). Two TLR agonists, bacillus Calmette–Guerin (BCG) and imiquimod are US FDA approved for clinical use as monotherapy for cancer.
Figure 2. Toll-like receptor agonists and their immune effects on the tumor microenvironment.
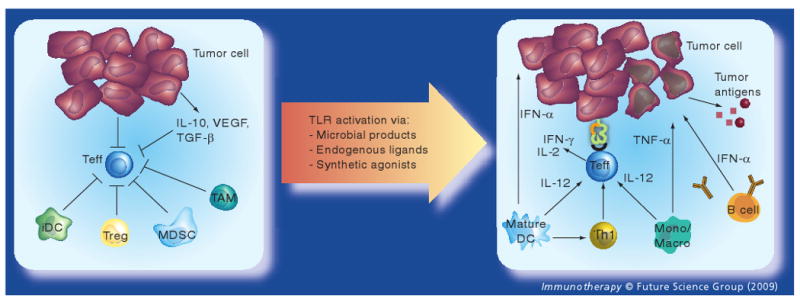
Left panel: several immunosuppressive mechanisms inhibit Teff function in the tumor microenvironment. Tumor-derived soluble factors such as IL-10, TGF-β and VEGF recruit MDSCs, prevent maturation of dendritic cells and inhibit Teff function. Furthermore, tumors deficient in B7–1 and B7–2 expression lead to inefficient T-cell stimulation; and expression of programmed death (PD)-ligand 1 on tumors inhibits T-cell activation (not shown). Tregs, often infiltrating tumors, can also directly suppress Teff function and trigger IL-10 production, which leads to upregulation of B7-H4 on antigen-presenting cells (APCs), and inhibition of DC maturation. B7-H4+ APCs such as TAM can induce cell cycle arrest in T cells. iDCs promote the accumulation of Tregs and result in T-cell anergy. Right panel: Toll-like receptor (TLR) activation enhances antitumor immunity via several mechanisms. TLR-mediated DC activation leads to enhanced phagocytosis, maturation with upregulation of MHC and costimulatory molecules (CD80, CD86 and CD40), secretion of Th1 cytokines (especially IL-12) and antigen presentation to lymphocytes resulting in the generation of Teff and antigen-specific B cells. Furthermore, local TLR activation can enhance natural killer cell-mediated cytotoxicity (not shown), upregulate MHC class I on tumor cells, directly induce tumor cell apoptosis and shift the tumor microenvironment toward an inflammatory milieu conducive to antigen cross-presentation and infiltration by functional Teff.
DC: Dendritic cell; iDC: Immature dendritic cell; MDSC: Myeloid-derived suppressor cell; Mono/Macro: Monocyte/macrophage; TAM: Tumor-associated macrophage; Teff: Effector T cell; Treg: Regulatory T cell.
Bacillus Calmette–Guerin
A live, attenuated Mycobacterium bovis prepartaion of bacillus of the Calmette–Guerin strain (TICE™ and PACIS™) stimulates TLR2 and TLR4 via its mycobacterial components, including cell wall skeleton and peptidoglycan, as well as TLR9 via its bacterial DNA [41–45]. BCG is approved for intravesical therapy of bladder carcinoma in situ and superficial bladder cancers. Initially tested by local injections into melanoma metastases, it was observed that inflammation was accompanied by tumor regression in skin or urinary bladder [46,47]. Extensive clinical investigation of BCG in patients with bladder cancer showed antitumor activity in the majority of patients. Compared to intravesical doxorubicin chemotherapy, BCG led to greater complete response rates for Tis lesions (70 vs 34%; p < 0.001) and improved 5-year disease-free survival in patients with Ta/T1 tumors (37 vs 17%; p = 0.015) as well as Tis tumors (45 vs 18%; p = 0.01) [48]. BCG's antitumor activity has been linked to cytokines locally secreted by the host, especially IFN-γ and IL-2 [49–51] and the development of antigen-specific T-cell immunity [52]. While BCG is typically well tolerated, with only mild to moderate side effects (mainly cystitis and influenza-like symptoms), serious infections can occur that may require antituberculous treatment [53]. BCG's regulatory approval marked the first compound (pathogen) approved for the treatment of cancer, which primarily acts via TLR stimulation.
Imiquimod
Imiquimod is a synthetic imidazoquinoline that targets TLR7. Formulated as imiquimod 5% cream (Aldara™), it is indicated for the treatment of a variety of diseases ranging from human papillomavirus (HPV)-related external genital and perianal warts (condylomata acuminata) to primary skin malignancies (superficial basal cell carcinoma [sBCC]) or premalignant conditions (actinic keratosis). Treatment of sBCC with imiquimod 5% cream resulted in histologic clearance rates of 79–82% in Phase III studies [54]. Objective responses have also been observed in selected melanoma patients when imiquimod was directly applied onto cutaneous metastases [55,56]. A Phase II study of imiquimod in breast cancer patients with chest wall recurrences or skin metastases is ongoing [202]. Imiquimod's antineoplastic effects result from the induction of innate and adaptive immunity, reversal of local tumor-induced immunosuppression and induction of tumor-cell specific apoptosis [39,57–60]. Topical use of imiquimod cream has shown an excellent safety profile with reversible local reactions and minimal systemic exposure [61,62]. Orally administered imiquimod induced systemic IFN-α in patients with advanced cancers, but tumor responses were not observed as immune-related side effects limited dosing [63]. A newer imidazoquinoline TLR7 agonist, 852A, administered parenterally as monotherapy has shown only modest clinical efficacy with disease stabilization observed in some patients in Phase I and II studies of advanced cancer [64,65], raising again the possibility that systemic side effects preclude the evaluation of greater doses.
While not yet investigated for the topical treatment of invasive cancers, resiquimod, an imidazoquinoline TLR7/8 agonist in humans, holds great promise as a single agent or vaccine adjuvant [66,67]. In addition to TLR7-induced IFN-α production by pDCs, TLR8 activation of human monocytes and mDCs leads to the secretion of proinflammatory cytokines and chemokines such as TNF, IL-12 and macrophage inflammatory protein (MIP)-1 [68]. The induction of IL-12, enhancement of NK-cell cytotoxicity and TLR8-mediated suppression of Tregs are particularly desirable features for cancer immunotherapeutics [21,68,69]. When studied in patients with actinic keratosis, topical treatment with resiquimod resulted in 77–90% complete clearance of lesions [70].
CpG oligodeoxynucleotides
CpG are single-strand oligodeoxynucleotides (ODNs), characterized by motifs containing cytosines and guanines. Based on their immunologic effects, CpG ODNs are divided into three different classes: CpG-A, a potent stimulator of NK cells owing to its IFN-α-producing effect on pDCs; CpG-B, a moderate IFN-α inducer, and enhancer of antigen-specific immune responses (upregulates costimulatory molecules on pDCs and B cells, induces Th1 cytokine production and stimulates antigen presentation by pDCs); and CpG-C, which combines the stimulatory capacity of both CpG-A and CpG-B [71,72].
CpG 7909 (PF-3512676, a CpG type B and TLR9 agonist) has been evaluated in several tumor types including renal cell carcinoma, glioblastoma, melanoma, cutaneous T-cell lymphoma and non-Hodgkin's lymphoma [73]. A 10% response rate in patients with metastatic melanoma was observed, along with evidence of immune activation in a Phase II trial of CpG given as subcutaneous injection [74]. Intralesional CpG showed antitumor activity against basal cell carcinoma and melanoma in a Phase I study [75] in the setting of local and systemic immune activation. When injected intradermally around primary melanoma excision sites CpG resulted in pDC and mDC activation, a Th1 cytokine profile, and reduced Treg frequencies in the draining lymph node [76], augmenting melanoma-antigen specific T lymphocytes and NK-cell responses [77].
Polyriboinosinic-polyribocytidylic acid
Polyriboinosinic-polyribocytidylic acid (Poly I:C) is a synthetic analog of viral dsRNA that stimulates endosomal (TLR3) and/or cytosolic melanoma differentiation-associated gene 5 (MDA5), leading to increased production of type I IFNs [78,79]. Poly I:C and its more stable cousin Poly I:C-poly-l-lysine (Poly ICLC) were evaluated as single agents in cancer patients several decades ago. Intravenous or intramuscular administration of Poly I:C showed no clinical activity in several cancers [80], probably due to its rapid degradation in human plasma [81]. The more stable compound Poly ICLC did induce systemic IFNs; however, dosing was limited by constitutional symptoms and no antitumor efficacy was seen in patients with metastatic melanoma [82]. Single-agent treatment of recurrent anaplastic glioma with Poly ICLC at lower doses to reduce toxicity did not translate into clinical benefit when compared with historical controls [83]; a lack of benefit with low-dose Poly ICLC was also observed in renal cell carcinoma [84].
In summary, TLR ligands are successfully and safely used as monotherapy for some cancers when applied locally (intravesical and percutaneous), but their systemic use remains limited because immunostimulatory effects required for antitumor efficacy come at the expense of systemic toxicity.
TLR agonists as adjuvants for cancer vaccines
Toll-like receptor agonists represent promising vaccine adjuvants as they are potent DC activators, augment T-cell responses and downregulate suppressive effects of Tregs. The following section focuses on the clinical development of TLR agonists as vaccine adjuvants for cancer therapy. Many infectious disease vaccines, both licensed and experimental, engage TLRs through intrinsic or added adjuvants, a topic that has been reviewed elsewhere [85,86].
Bacillus Calmette–Guerin
Bacillus of the Calmette–Guerin adjuvant has been extensively evaluated with cellular vaccines in colorectal cancer (CRC) and malignant melanoma [87,88]. Despite promising Phase II data, the randomized Phase III trial of BCG administered with an allogeneic melanoma vaccine (Canvaxin™) failed to improve disease-free and overall survival compared to BCG alone in patients with resected stage III and IV melanoma. Better outcomes were instead reported for the BCG alone arm [87]. The reason for this unexpected observation remains unclear, though it is possible that antigen-specific immune responses may have been suppressed by the mixture of irradiated melanoma lines.
Clinical benefit, along with an excellent safety profile, was observed in a placebo-controlled, randomized Phase III trial after vaccinating CRC patients with autologous tumor cells and BCG in the adjuvant setting [88]. The vaccine arm showed significantly reduced recurrence rates (44%; p = 0.023) and improved recurrence-free survival (42%; p = 0.032) for patients with stage II disease, leading to regulatory approval in The Netherlands. However, no benefit was seen in stage III patients.
Bacillus Calmette–Guerin followed by granulocyte–macrophage colony-stimulating factor (GM-CSF) as vaccine adjuvant for NY-ESO-1 protein induced antigen-specific humoral and cellular immune responses in a single-arm pilot study of patients with urothelial carcinoma [89].
Monophosphoryl lipid A
Lipid A molecules that target the TLR4 complex are among the most commonly used vaccine adjuvants. One promising candidate is monophosphoryl lipid A (MPL), a derivative of lipid A from Salmonella minnesota. It was identified in the 1980s through systematic modification of LPS (the prototype TLR4 agonist) of S. minnesota, lacking LPS toxicity in humans. MPL adjuvant has shown an excellent safety and efficacy profile in several thousands of patients, received regulatory approval in Europe as adjuvant for a hepatitis B vaccine (Fendrix™) and is used as adjuvant in a HPV vaccine (Cervarix™) for which a Biologics License Application has been filed with the US FDA.
DETOX adjuvant, which contains MPL and cell wall skeletons of Mycobacterium phlei, has been evaluated in Phase I/II vaccine studies in several cancers. In melanoma patients, cellular immune responses to vaccination with a combination of melanoma cell lysates (Melacine™), or irradiated autologous tumor cells with DETOX, correlated with clinical responses [90,91]. Synthetic sialyl-Tn haptens conjugated to keyhole limpet hemocyanin (STn-KLH, Theratope™), together with DETOX adjuvant, have been used in pilot vaccination studies for mucin-expressing epithelial tumors, including breast cancers, and have shown safety and occasional tumor regressions [92]. Vaccination after high dose chemotherapy and autologous stem cell rescue resulted in humoral and cellular immune responses; however, no clinical benefit was demonstrated with this approach [93,94]. Immune responses were also demonstrated in a Phase I study of mutated ras peptides combined with DETOX [95].
Monophosphoryl lipid A adjuvant is also a component of the liposomal Stimuvax® vaccine targeting MUC-1 in advanced non-small-cell lung cancer (NSCLC), and is currently undergoing Phase III evaluation worldwide. The Phase II study suggested clinical benefit in NSCLC stage IIIb patients in the absence of a malignant pleural effusion [96].
Monophosphoryl lipid A is also used as part of a proprietary immunological adjuvant system (AS15, AS02b) in a MAGE A3 vaccine against MAGE A3-expressing NSCLC and malignant melanoma [97]. AS15 is a liposomal formulation of CpG, MPL and QS21 (a natural saponin molecule extracted from the bark of the South American tree Quillaja saponaria molina). AS02b consists of MPL and QS21 in an oil-in-water emulsion. A placebo-controlled, randomized Phase II trial of MAGE A3/AS02b confirmed an excellent safety profile and suggested clinical benefit with prolonged disease-free survival for patients with resected stage I and II NSCLC (HR = 0.73; 95% CI: 0.45– 41.16) [98], leading to the initiation of a global Phase III trial of MAGE A3/AS15 in patients with resected stage Ib–IIIa NSCLC. The Phase II trial in patients with metastatic melanoma confirmed a safe vaccination approach and showed superiority of adjuvant AS15 over AS02b suggesting that the addition of TLR9 agonist CpG to MPL increased vaccine efficacy [99]. A follow-up Phase III study of MAGE A3/AS15 is ongoing for patients with resected, high-risk melanoma.
CpG oligodeoxynucleotides
CpG ODNs, short DNA sequences containing unmethylated CpG motifs stimulating TLR9, have been used successfully as adjuvants in cancer vaccines when added to cancer-testis antigens emulsifed in Montanide ISA 51 [100,101]. While studies lacked a concurrent non-CpG control arm, comparisons with vaccines containing antigen/Montanide ISA 51 are suggestive of substantially increased immunogenicity of the CpG-containing formulation [100]. The vaccine combination with antigen and/or emulsification in Montanide ISA 51 may be necessary for CpG's adjuvant effect, as CpG did not induce antigen-specific immunity in patients with advanced cancers when given sequentially after GM-CSF before antigen injection [102]. Interestingly, CpG induced profound transient peripheral lymphopenia that coincided with lymphocyte infiltration at the vaccination site, probably due to pDC-derived chemoattractants. Intratumoral injection of CpG may therefore be a promising strategy to direct the effector response after vaccination. CpG is also part of the adjuvant system AS15 discussed above, which is being evaluated in two Phase III MAGE A3 vaccination trials.
Imiquimod
Imiquimod, a synthetic imidazoquinoline that targets TLR7 has demonstrated immunomodulating effects as a topical vaccine adjuvant [103,104]. Injection of immature DCs into imiquimod-pretreated skin led to DC activation and migration to draining lymph nodes in cancer patients, as well as to enhanced DC immunostimulatory capacity [105]. Topical imiquimod treatment augmented the immunogenicity of a melanoma peptide vaccine when administered with systemic Flt-3 ligand [103]. Our group recently demonstrated imiquimod's adjuvant activity when administered topically with NY-ESO-1 protein vaccination in patients with resected melanoma. Imiquimod induced recruitment and activation of mDCs and pDCs in the treated skin, as well as inflammatory infiltrates (Figure 3). The vaccine combination showed an excellent safety profile, elicited humoral and cellular NY-ESO-1-specific immune responses in a significant fraction of patients and was easy to administer [104]. Indoleamine 2′3′-dioxygenase (IDO), an enzyme involved in catabolism of tryptophan and generation of kynurenine metabolites that results in suppression of activated T cells and induction of functional Tregs after TLR7 activation of human pDCs [106], was not detected in our study. A follow-up study of topical resiquimod, a TLR7/8 agonist, in combination with a NY-ESO-1 protein in Montanide ISA 51 emulsion, is ongoing.
Figure 3. Dendritic cells recruited into skin treated with a Toll-like receptor-7 agonist.
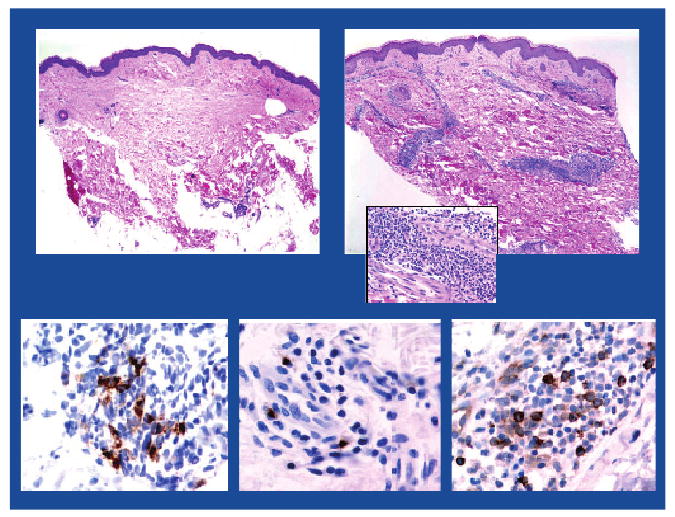
Imiquimod 5%, applied topically to patients' healthy skin for five days (in order to condition the site for vaccination) results in marked cellular infiltration in the dermis (H&E-stained sections, ×40 magnification). Upper left panel: untreated skin (control), Upper right panel: imiquimod-treated skin with perivascular and periadnexal mononuclear cell infiltrates (insert ×200 magnification). Infiltrates were mainly composed of lymphocytes as well as myeloid and plasmacytoid dendritic cells (as shown in representative immunohistochemistry sections). Lower left panel: mature DC (CD83), Lower center panel: activated myeloid dendritic cells (DC-LAMP) and Lower right panel: plasmacytoid dendritic cells (CD123).
Polyriboinosinic-polyribocytidylic acid
Polyriboinosinic-polyribocytidylic acid and derivatives are being studied as vaccine adjuvants in cancer trials, as potent DC maturation and induction of a Th1 cytokine profile has been demonstrated in vitro [107], and preclinical studies have shown promising results [108]. Depending upon formulation and delivery, endosomal (TLR3) and/or cytoplasmic (MDA5) pathways are differentially accessed, as recently shown in preclinical models [109]. Several studies of Poly ICLC administered with peptide or DC vaccines are ongoing in patients with various advanced malignancies, including malignant glioma [202]. Efficacy data have not yet been reported.
Manipulation of TLR to improve the immunogenicity of ex vivo prepared DC vaccines
We have recently shown that mDCs matured ex vivo with a cytokine cocktail (IL-1β, IL-6, TNF-α and PGE2) are suboptimal in inducing cellular immune responses in cancer patients when compared with Montanide, a commonly used vaccine adjuvant [110]. Ex vivo TLR-activated DCs or genetically modified DCs expressing TLR-stimulating receptors may be superior for immunization protocols. Preliminary clinical data are promising; human DCs electroporated with constitutively active TLR4-encoding mRNA are potent T-cell stimulators [111] and Poly I:C-activated DCs produced high amounts of the Th1 cytokine IL-12p70 [112]. LPS-activated DCs pulsed with Her2 peptides injected intranodally prior to surgical resection of Her2+ ductal carcinoma in situ resulted in the regression of tumors, accompanied by the induction of Her2-specific cellular immune responses, loss of Her2 expression and lymphocytic infiltration in the tumor [113].
TLR modulation by chemotherapy & radiation
Recently demonstrated in mice, TLR4 signaling in DCs triggered by chemo- and radiotherapy-induced cell death contributes to the generation of an antitumor immune response [114]. Furthermore, TLR4 and its activation were required for the efficacy of radiation and chemotherapy. These findings are consistent with clinical observations that TLR4 polymorphism (TLR4 Asp299Gly) is associated with worse outcomes in patients with operable breast cancers receiving adjuvant anthracycline-based chemotherapy [114].
Lymphodepleting preparative regimens employed prior to adoptive T-cell transfer in tumor-bearing mice can also activate the innate immune system via TLR4, as total-body irradiation or chemotherapy-induced gut injury permitted translocation of microbes [115,116]. TLR4 activation by bacterial LPS was one of several mandatory factors contributing to the effectiveness of adoptively transferred cells; therefore, manipulation of TLRs could be employed to further enhance adoptive immunotherapy for cancer. In addition, the understanding of genetic variations of TLR polymorphisms and TLR activation in response to conventional cancer therapies will greatly enhance our ability to tailor treatments to the individual patient.
Combination of TLR agonists & conventional cancer therapies
Promising areas for TLR agonists are their use in combination with conventional cancer treatments to enhance cytotoxic chemotherapies, ionizing radiation or monoclonal antibodies. TLR activation enhances the immune response to antigens released, potentially resulting in therapeutic synergy with traditional cancer treatments. Additional benefits of chemotherapy, ionizing radiation and monoclonal antibodies are the reduction of tumor bulk (leading to a smaller target for the immune effectors as well as decreased tumor-associated immunosuppression), upregulation of MHC expression necessary for effective immunity, sensitization of tumor cells to immune destruction via upregulation of FasL and removal of immunosuppressive Tregs [117–120]. In addition, cytotoxic cancer therapies can release molecules such as high-mobility group box 1 protein (HMGB1) and heat-shock proteins, which serve as endogenous ligands for TLR2 and/or TLR4. Clinical relevance of TLR4 modulation by endogenous ligands has been demonstrated in breast cancer, as discussed above [114]. Furthermore, HMGB1 released from necrotic cells can also modulate pDC and B-cell function in response to DNA in a TLR9-dependent manner [121–123].
TLR2/4 agonists also enhance the effect of chemotherapy via TNF-α and inducible nitric oxide synthase (iNOS)-dependent tumor cell apoptosis in animal models [124].
In addition, TLR2/4 and 7 agonists inhibit tumor-associated angiogenesis [38,124]. The restoration of ‘normal’ vasculature decreases interstitial pressure that allows efficient drug delivery into the tumor and possibly decreases metastases [125].
Synergy of TLR agonists with chemotherapeutic approaches, radiotherapy and monoclonal antibodies has been successfully demonstrated in murine models of sarcoma, mammary carcinoma and lymphoma [126–129]. Pilot studies in patients with malignant glioma and lymphoma support the notion of synergistic effects of TLR9 agonist CpG with chemotherapy, radiation or monoclonal antibodies (rituximab) and showed clinical benefit and an excellent safety profile [130–132].
In a randomized Phase II study, the addition of TLR9 agonist PF-3512676 (CpG 7909, which belongs to the CpG-B class) to first-line treatment with platinum-based chemotherapy in stage IIIb–IV NSCLC improved the overall response, and suggested a trend toward improved survival (12.3 vs 6.8 months; p = 0.18) [133]. The confirmatory Phase III trial was discontinued at the scheduled interim analyses, as the chemotherapy/PF-3512676 combination did not result in clinical benefit for this population [134]. Failure of this approach may be owing to a lack of ‘context’ as CpG 7909 was delivered distant from the tumor as subcutaneous injection. Furthermore, NSCLC is a relatively chemotherapy resistant malignancy, possibly compromising the availability of tumor antigens; therefore, this combinatorial approach may be more effective in malignancies such as ovarian cancer which are highly sensitive to front-line cytotoxics. It is also possible that the chemotherapeutic regimen (taxane and platinum) interfered with the development of an antigen-specific immune response or antagonized the effects of the CpG, possibly at the MyD88 level as paclitaxel can recruit MyD88 as a TLR4 agonist [135] as well as in a TLR-independent manner [136].
Intratumoral injections of CpG after local radiation is currently under clinical investigation in patients with B-cell lymphoma [202]. This approach is designed to activate intratumoral DCs to facilitate antigen presentation after radiation-induced apoptosis of the tumor [120]. CpG is also being studied in combination with monoclonal antibodies such as anti-CD20 (rituximab), as CpG ODN have been shown to upregulate CD20 on human malignant B cells [137] and increase the expression of several IFN-inducible genes [132]. A Phase II study confirmed the safety of this approach, and while the contribution of CpG to clinical and immune responses can only be determined in a randomized, controlled trial, significant CD8+ T-cell infiltration observed in the tumor after treatment is suggestive of an enhanced immune response [138].
Conclusion & future perspective
Toll-like receptor agonists possess important properties that can be exploited for the immunotherapy against tumors. Their ability to promote tumor-specific Th1 and cytotoxic T lymphocyte responses has translated into antitumor activity in many preclinical and clinical studies, leading to the approval of two TLR agonists for cancer therapy when applied locally to tumors. However, systemic use of TLR agonists may be restricted by dose-limiting toxicities owing to systemic cytokine induction.
Combinatorial approaches of TLR agonists with conventional therapies such as chemotherapy or radiation are promising, as dosing requirements may be lower, and one can selectively target several pathways. In addition, the recent discovery that the therapeutic effects of conventional cancer treatments such as anthracyclines and radiation depend upon intact TLR signaling will greatly enhance our ability to test novel combinations, as well as to use genetic screening for TLR polymorphisms to guide the selection of individual treatments.
The use of TLR agonists as cancer vaccine adjuvants remains a promising area of investigation. New approaches conjugate the TLR agonist to the antigen of interest or utilize TLR agonist combinations as synergism have been demonstrated for several agonists [139,140]. As discussed above, two international Phase III studies are currently evaluating the clinical efficacy of adjuvant AS15, which contains a combination of TLR4 and 9 agonists, in MAGE A3 vaccination. Finally, combinations of TLR agonists with anti-CTLA4 antibody or targeted therapies may prove advantageous. Inhibition of p38 in DCs for instance, has been shown to attenuate TLR-induced IL-10 thus selectively suppressing the induction of Tregs as well as to augment TLR-induced IL-12 production critical for Th1 polarization [141]. Consistent with these observations, p38 inhibition enhanced the antitumor efficacy of TLR9-activated DCs in B16 murine melanoma [141].
It is important to carefully choose the agonist as TLR ligation could potentially trigger autoimmune diseases or severe inflammation, which have recently emerged as side effects of immunotherapies such as CTLA-4 blockade including dermatitis, colitis, hepatitis and hypophysitis [142]. Experimental asthma for instance is exacerbated by TLR2 agonists through the induction of a Th2 profile in the setting of vaccination, whereas TLR9 agonists confer protection [143].
Executive summary.
Toll-like receptors & their activation
Toll-like receptors (TLR) recognize pathogen-associated molecular patterns, which are conserved motifs shared by bacteria, viruses, protozoa and fungi.
TLR signaling activates innate and adaptive immune responses. TLR activation results in transcription of type I IFN and proinflammatory cytokine genes. The cytokine induction pattern is determined by dendritic cell (DC) subset, TLR agonist and signaling adaptors. TLR-mediated DC activation leads to enhanced phagocytosis, maturation and migration to draining lymph nodes, secretion of T helper cell-1 cytokines and antigen presentation to lymphocytes. Type I IFNs support an antitumor immune response by facilitating antigen cross-presentation, T-cell proliferation, DC maturation and natural killer cell activation.
TLR agonists as cancer therapeutics
Synthetic agonists, microbial products and endogenous ligands can modulate TLRs.
TLR agonists represent promising cancer immunotherapeutics, agents undergoing clinical investigation are reviewed in Table 1.
Locally applied TLR agonists can effectively eradicate tumors owing to the induction of innate and adaptive immunity as well as their multiple effects on the tumor microenvironment.
TLR agonists are also potent vaccine adjuvants as they activate DCs, augment T-cell responses and downregulate suppressive effects of regulatory T cells. While cancer vaccines remain investigational, many infectious disease vaccines, both licensed and experimental, engage TLRs through intrinsic or added adjuvants.
Combinations of TLR agonists with chemotherapy, ionizing radiation, monoclonal antibodies and targeted therapies are being explored.
TLRs are modulated by chemotherapy & radiation
TLR signaling can be triggered by chemotherapy- and radiotherapy-induced cell death and contributes to an antitumor immune response.
TLR polymorphisms can affect outcomes in patients treated with conventional cancer therapies.
Conclusion
Several TLR agonists possess important properties that can be exploited for the immunotherapy against tumors.
The understanding of genetic variations of TLR polymorphisms and TLR activation in response to cancer therapies will greatly enhance the ability to tailor treatments to the individual patient.
The successful clinical development of TLR agonists requires careful selection and study of the agent as activation of some TLRs, albeit through tumor-derived soluble mediators, has been shown to enhance tumor growth and metastasis [144,145]. Furthermore, functional TLRs can be expressed by human tumors that may promote tumor progression, confer resistance to apoptosis as well as suppress antitumor immune responses, leading to immune evasion [146,147]. In contrast, TLR-induced apoptosis of several human cancers has been demonstrated, for instance treatment of cutaneous melanoma metastases with 5% imiquimod cream induced tumor-cell death in the majority of biopsied lesions [148]. TLRs may not be required for this process, although Poly I:C-triggered apoptosis of breast cancer in vitro is mediated through TLR3 expressed by tumor cells [149].
Coinduction or promotion of Tregs that may attenuate antitumor effects is also of concern for use of TLR agonists in cancer immunotherapy (reviewed in [150]). Strategies to limit Treg-mediated suppression may include p38 inhibition as described above [141] and the selective use of TLR4, 8 or 9 agonists. In murine models for instance, stimulation of TLR4 and TLR9 on DCs has been demonstrated to overcome Treg-mediated suppression of effector cells [33,151]. TLR8 activation of human Tregs has been shown to reverse their function in vitro, and adoptive transfer of TLR8-stimulated Tregs into tumor-bearing mice enhanced antitumor immunity [21]. TLR8 manipulation is a particularly appealing approach for clinical studies as human Tregs express relatively high levels of TLR8.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure: Sylvia Adams was supported by grants from the American Society of Clinical Oncology (Career Development Award), the Cancer Research Institute and the National Cancer Institute (NCI K23CA125205, NIH 5PCA016087–29). The author wishes to thank Nina Bhardwaj, Tze-Chiang Meng, Patrick Ott and Dusan Bogunovic for helpful discussions and critical review of the manuscript. Sylvia Adams receives consulting fees as a member of the global steering committee for the Phase III NSCLC vaccination trial for Glaxo-Smith-Kline Biologicals. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]; •• Characterization of the role of Toll in fungal resistance in Drosophila.
- 2.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]; •• Characterization of the first human Toll (now referred to as Toll-like receptor [TLR]4) and Toll-like signaling molecules.
- 3.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3h/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi ST, Lariviere L, Leveque G, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (TLR4) J Exp Med. 1999;189(4):615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162(7):3749–3752. [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]; • Comprehensive review of TLR signaling.
- 7.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5(2):190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 8.Underhill DM, Ozinsky A, Hajjar AM, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401(6755):811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 9.Ito T, Amakawa R, Kaisho T, et al. Interferon-α and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195(11):1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid a as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 11.Rehli M. Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 2002;23(8):375–378. doi: 10.1016/s1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194(6):863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Description of differential expresssion of TLRs by human antigen-presenting cells.
- 13.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168(9):4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 14.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31(11):3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 16.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: upregulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101(11):4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102(7):2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 18.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of Toll-like receptors (TLRs) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164(11):5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 19.Hasan U, Chaffois C, Gaillard C, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174(5):2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 20.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101(9):3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T-cell function. Science. 2005;309(5739):1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]; • Demonstration that human regulatory T cells express high levels of TLR8 and that TLR8 triggering prevents their inhibitory phenotype.
- 22.Bell MP, Svingen PA, Rahman MK, Xiong Y, Faubion WA., Jr FoxP3 regulates TLR10 expression in human T regulatory cells. J Immunol. 2007;179(3):1893–1900. doi: 10.4049/jimmunol.179.3.1893. [DOI] [PubMed] [Google Scholar]
- 23.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15(4):396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 24.Guillot L, Le Goffic R, Bloch S, et al. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2004;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174(2):992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 26.Vora P, Youdim A, Thomas LS, et al. β-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173(9):5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 27.El-Omar EM, Ng MT, Hold GL. Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene. 2008;27(2):244–252. doi: 10.1038/sj.onc.1210912. [DOI] [PubMed] [Google Scholar]
- 28.Purdue MP, Lan Q, Wang SS, et al. A pooled investigation of Toll-like receptor gene variants and risk of non-Hodgkin lymphoma. Carcinogenesis. 2009;30(2):275–281. doi: 10.1093/carcin/bgn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MF, Jr, Mitchell A, Li G, et al. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-κB activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278(35):32552–32560. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]
- 30.Torok AM, Bouton AH, Goldberg JB. Helicobacter pylori induces interleukin-8 secretion by Toll-like receptor 2- and Toll-like receptor 5-dependent and -independent pathways. Infect Immun. 2005;73(3):1523–1531. doi: 10.1128/IAI.73.3.1523-1531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991;(262):3–11. [PubMed] [Google Scholar]
- 32.Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529–564. doi: 10.1016/0163-7258(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 33.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 34.Reis e Sousa C, Hieny S, Scharton-Kersten T, et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin-12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186(11):1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 36.Skokos D, Nussenzweig MC. CD8-DCs induce IL-12-independent Th1 differentiation through δ4 notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204(7):1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumitru CD, Antonysamy MA, Gorski KS, et al. NK1.1+ cells mediate the antitumor effects of a dual Toll-like receptor 7/8 agonist in the disseminated B16-F10 melanoma model. Cancer Immunol Immunother. 2009;58(4):575–587. doi: 10.1007/s00262-008-0581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majewski S, Marczak M, Mlynarczyk B, Benninghoff B, Jablonska S. Imiquimod is a strong inhibitor of tumor cell-induced angiogenesis. Int J Dermatol. 2005;44(1):14–19. doi: 10.1111/j.1365-4632.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 39.Schon M, Bong AB, Drewniok C, et al. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst. 2003;95(15):1138–1149. doi: 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- 40.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204(6):1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuji S, Matsumoto M, Takeuchi O, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette–Guerin: Involvement of Toll-like receptors. Infect Immun. 2000;68(12):6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uehori J, Matsumoto M, Tsuji S, et al. Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus calmette-guerin peptidoglycan. Infect Immun. 2003;71(8):4238–4249. doi: 10.1128/IAI.71.8.4238-4249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokunaga T, Yamamoto H, Shimada S, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984;72(4):955–962. [PubMed] [Google Scholar]
- 44.Tokunaga T, Yamamoto T, Yamamoto S. How BCG led to the discovery of immunostimulatory DNA. Jpn J Infect Dis. 1999;52(1):1–11. [PubMed] [Google Scholar]
- 45.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163(7):3920–3927. [PubMed] [Google Scholar]
- 46.Morton DL, Eilber FR, Holmes EC, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg. 1974;180(4):635–643. doi: 10.1097/00000658-197410000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silverstein MJ, Dekernion J, Morton DL. Malignant melanoma metastatic to the bladder. Regression following intratumor injection of BCG vaccine. JAMA. 1974;229(6):688. [PubMed] [Google Scholar]
- 48.Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette–Guerin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325(17):1205–1209. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 49.Jackson AM, Alexandroff AB, Kelly RW, et al. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette–Guerin (BCG) immunotherapy. Clin Exp Immunol. 1995;99(3):369–375. doi: 10.1111/j.1365-2249.1995.tb05560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson AM, Prescott S, Hawkyard SJ, James K, Chisholm G. The immunomodulatory effects of urine from patients with superficial bladder cancer receiving intravesical evans BCG therapy Cancer Immunol. Immunother. 1993;36(1):25–30. doi: 10.1007/BF01789127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Jong WH, De Boer EC, van Der Meijden AP, et al. Presence of interleukin-2 in urine of superficial bladder cancer patients after intravesical treatment with bacillus Calmette–Guerin. Cancer Immunol Immunother. 1990;31(3):182–186. doi: 10.1007/BF01744734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruno S, Machi AM, Semino C, et al. Phenotypic, functional and molecular analysis of lymphocytes associated with bladder cancer. Cancer Immunol Immunother. 1996;42(1):47–54. doi: 10.1007/s002620050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mangiarotti B, Trinchieri A, Del Nero A, Montanari E. A randomized prospective study of intravesical prophylaxis in non-muscle invasive bladder cancer at intermediate risk of recurrence: mitomycin chemotherapy versuss BCG immunotherapy. Arch Ital Urol Androl. 2008;80(4):167–171. [PubMed] [Google Scholar]
- 54.Geisse J, Caro I, Lindholm J, Golitz L, Stampone P, Owens M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two Phase III, randomized, vehicle-controlled studies. J Am Acad Dermatol. 2004;50(5):722–733. doi: 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 55.Wolf IH, Smolle J, Binder B, Cerroni L, Richtig E, Kerl H. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol. 2003;139(3):273–276. doi: 10.1001/archderm.139.3.273. [DOI] [PubMed] [Google Scholar]
- 56.Bong A, Bonnekoh B, Franke I, Schon MP, Ulrich J, Gollnick H. Imiquimod, a topical immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology. 2002;2:135–138. doi: 10.1159/000063904. [DOI] [PubMed] [Google Scholar]; • Clinical observation of antitumor activity of TLR7 agonist imiquimod.
- 57.Wolf IH, Kodama K, Cerroni L, Kerl H. Nature of inflammatory infiltrate in superficial cutaneous malignancies during topical imiquimod treatment. Am J Dermatopathol. 2007;29(3):237–241. doi: 10.1097/01.dad.0000211531.33670.94. [DOI] [PubMed] [Google Scholar]
- 58.Broomfield SA, van der Most RG, Prosser AC, et al. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009;182(9):5217–5224. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- 59.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 60.Clark RA, Huang SJ, Murphy GF, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205(10):2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison LI, Skinner SL, Marbury TC, et al. Pharmacokinetics and safety of imiquimod 5% cream in the treatment of actinic keratoses of the face, scalp, or hands and arms. Arch Dermatol Res. 2004;296(1):6–11. doi: 10.1007/s00403-004-0465-4. [DOI] [PubMed] [Google Scholar]
- 62.Lebwohl M, Dinehart S, Whiting D, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two Phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol. 2004;50(5):714–721. doi: 10.1016/j.jaad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Witt PL, Ritch PS, Reding D, et al. Phase I trial of an oral immunomodulator and interferon inducer in cancer patients. Cancer Res. 1993;53(21):5176–5180. [PubMed] [Google Scholar]
- 64.Dudek AZ, Yunis C, Harrison LI, et al. First in human Phase I trial of 852A, a novel systemic Toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer. Clin Cancer Res. 2007;13(23):7119–7125. doi: 10.1158/1078-0432.CCR-07-1443. [DOI] [PubMed] [Google Scholar]
- 65.Dummer R, Hauschild A, Becker JC, et al. An exploratory study of systemic administration of the Toll-like receptor-7 agonist 852A in patients with refractory metastatic melanoma. Clin Cancer Res. 2008;14(3):856–864. doi: 10.1158/1078-0432.CCR-07-1938. [DOI] [PubMed] [Google Scholar]
- 66.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27(2):190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 67.Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev Vaccines. 2007;6(5):835–847. doi: 10.1586/14760584.6.5.835. [DOI] [PubMed] [Google Scholar]
- 68.Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174(3):1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 69.Gorski KS, Waller EL, Bjornton-Severson J, et al. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol. 2006;18(7):1115–1126. doi: 10.1093/intimm/dxl046. [DOI] [PubMed] [Google Scholar]
- 70.Szeimies RM, Bichel J, Ortonne JP, Stockfleth E, Lee J, Meng TC. A Phase II dose-ranging study of topical resiquimod to treat actinic keratosis. Br J Dermatol. 2008;159(1):205–210. doi: 10.1111/j.1365-2133.2008.08615.x. [DOI] [PubMed] [Google Scholar]
- 71.Vollmer J, Weeratna R, Payette P, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34(1):251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 72.Wooldridge JE, Weiner GJ. CpG DNA and cancer immunotherapy: orchestrating the antitumor immune response. Curr Opin Oncol. 2003;15(6):440–445. doi: 10.1097/00001622-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27(2):161–167. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- 74.Pashenkov M, Goess G, Wagner C, et al. Phase II trial of a Toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24(36):5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 75.Hofmann MA, Kors C, Audring H, Walden P, Sterry W, Trefzer U. Phase I evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31(5):520–527. doi: 10.1097/CJI.0b013e318174a4df. [DOI] [PubMed] [Google Scholar]
- 76.Molenkamp BG, Van Leeuwen PA, Meijer S, et al. Intradermal CpG-B activates both plasmacytoid and myeloid dendritic cells in the sentinel lymph node of melanoma patients. Clin Cancer Res. 2007;13(10):2961–2969. doi: 10.1158/1078-0432.CCR-07-0050. [DOI] [PubMed] [Google Scholar]
- 77.Molenkamp BG, Sluijter BJ, Van Leeuwen PA, et al. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14(14):4532–4542. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- 78.Schulz O, Diebold SS, Chen M, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433(7028):887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 79.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 80.Robinson RA, Devita VT, Levy HB, Baron S, Hubbard SP, Levine AS. A Phase I–II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patients with leukemia or solid tumors. J Natl Cancer Inst. 1976;57(3):599–602. doi: 10.1093/jnci/57.3.599. [DOI] [PubMed] [Google Scholar]
- 81.Nordlund JJ, Wolff SM, Levy HB. Inhibition of biologic activity of polyI:C by human plasma. Proc Soc Exp Biol Med. 1970;133(2):439–444. doi: 10.3181/00379727-133-34492. [DOI] [PubMed] [Google Scholar]
- 82.Hawkins MJ, Levin M, Borden EC. An Eastern Cooperative Oncology Group Phase I–II pilot study of polyriboinosinic-polyribocytidylic acid poly-l-lysine complex in patients with metastatic malignant melanoma. J Bio Response Mod. 1985;4(6):664–668. [PubMed] [Google Scholar]
- 83.Butowski N, Lamborn KR, Lee BL, et al. A North American brain tumor consortium Phase II study of poly-ICLC for adult patients with recurrent anaplastic gliomas. J Neurooncol. 2009;91(2):183–189. doi: 10.1007/s11060-008-9705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Giantonio BJ, Hochster H, Blum R, et al. Toxicity and response evaluation of the interferon inducer poly-ICLC administered at low dose in advanced renal carcinoma and relapsed or refractory lymphoma: a report of two clinical trials of the Eastern Cooperative Oncology Group. Invest New Drugs. 2001;19(1):89–92. doi: 10.1023/a:1006458232384. [DOI] [PubMed] [Google Scholar]
- 85.Van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27(1):49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 86.Mckee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Morton DL, Mozzillo N, Thompson MC, et al. An international, randomized, Phase III trial of bacillus Calmette–Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J Clin Oncol ASCO Annual Meeting Proceedings Part I. 2007;25(18 Suppl) [Google Scholar]
- 88.Vermorken JB, Claessen AM, Van Tinteren H, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353(9150):345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 89.Sharma P, Bajorin DF, Jungbluth AA, Herr H, Old LJ, Gnjatic S. Immune responses detected in urothelial carcinoma patients after vaccination with NY-ESO-1 protein plus BCG and GM-CSF. J Immunother. 2008;31(9):849–857. doi: 10.1097/CJI.0b013e3181891574. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell MS, Kan-Mitchell J, Kempf RA, Harel W, Shau HY, Lind S. Active specific immunotherapy for melanoma: Phase I trial of allogeneic lysates and a novel adjuvant. Cancer Res. 1988;48(20):5883–5893. [PubMed] [Google Scholar]
- 91.Eton O, Kharkevitch DD, Gianan MA, et al. Active immunotherapy with ultraviolet B-irradiated autologous whole melanoma cells plus detox in patients with metastatic melanoma. Clin Cancer Res. 1998;4(3):619–627. [PubMed] [Google Scholar]
- 92.Maclean GD, Reddish M, Koganty RR, et al. Immunization of breast cancer patients using a synthetic sialyl-Tn glycoconjugate plus Detox adjuvant. Cancer Immunol Immunother. 1993;36(4):215–222. doi: 10.1007/BF01740902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holmberg LA, Oparin DV, Gooley T, et al. Clinical outcome of breast and ovarian cancer patients treated with high-dose chemotherapy, autologous stem cell rescue and theratope STn-KLH cancer vaccine. Bone Marrow Transplant. 2000;25(12):1233–1241. doi: 10.1038/sj.bmt.1702430. [DOI] [PubMed] [Google Scholar]
- 94.Holmberg LA, Oparin DV, Gooley T, Sandmaier BM. The role of cancer vaccines following autologous stem cell rescue in breast and ovarian cancer patients: experience with the STn-KLH vaccine (theratope) Clin Breast Cancer. 2003;3(Suppl. 4):S144–S151. doi: 10.3816/cbc.2003.s.004. [DOI] [PubMed] [Google Scholar]
- 95.Khleif S, Abrams S, Hamilton J, et al. A Phase I vaccine trial with peptides refecting ras oncogene mutations of solid tumors. J Immunother. 1999;22(2):155–165. doi: 10.1097/00002371-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Butts C, Murray N, Maksymiuk A, et al. Randomized Phase IIb trial of BLP25 liposome vaccine in stage IIIb and IV non-small-cell lung cancer. J Clin Oncol. 2005;23(27):6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 97.Brichard VG, Lejeune D. GSK'S antigen-specific cancer immunotherapy programme: pilot results leading to Phase III clinical development. Vaccine. 2007;25(Suppl. 2):B61–B71. doi: 10.1016/j.vaccine.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 98.Vansteenkiste J. Final results of a multicenter, double-blind, randomized, placebo-controlled Phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in Stage Ib/II non-small cell lung cancer (NSCLC) J Clin Oncol ASCO Annual Meeting Proceedings Part I. 2007;25(18 Suppl) [Google Scholar]
- 99.Kruit WH. Immunization with recombinant MAGE-A3 protein combined with adjuvant systems AS15 or AS02B in patients with unresectable and progressive metastatic cutaneous melanoma: a randomized open-label Phase II study of the EORTC Melanoma Group. J Clin Oncol. 2008;26(Suppl) Abstract 9065. [Google Scholar]
- 100.Speiser DE, Lienard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115(3):739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clinical trial showing strong cellular immune responses after cancer vaccination with TLR9 agonist CpG as adjuvant.
- 101.Valmori D, Souleimanian NE, Tosello V, et al. Vaccination with NY-ESO-1 protein and CpG in montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104(21):8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haining WN, Davies J, Kanzler H, et al. CpG oligodeoxynucleotides alter lymphocyte and dendritic cell trafficking in humans. Clin Cancer Res. 2008;14(17):5626–5634. doi: 10.1158/1078-0432.CCR-08-0526. [DOI] [PubMed] [Google Scholar]
- 103.Shackleton M, Davis ID, Hopkins W, et al. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 104.Adams S, O' Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181(1):776–784. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nair S, Mclaughlin C, Weizer A, et al. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol. 2003;171(11):6275–6282. doi: 10.4049/jimmunol.171.11.6275. [DOI] [PubMed] [Google Scholar]
- 106.Manches O, Munn D, Fallahi A, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118(10):3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Navabi H, Jasani B, Reece A, et al. A clinical grade Poly I:C-analogue (ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine. 2009;27(1):107–115. doi: 10.1016/j.vaccine.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 108.Zhu X, Nishimura F, Sasaki K, et al. Toll-like receptor-3 ligand Poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Trumpfheller C, Caskey M, Nchinda G, et al. The microbial mimic Poly I:C induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105(7):2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O'Neill DW, Adams S, Goldberg JD, et al. Comparison of the immunogenicity of montanide ISA 51 adjuvant and cytokine-matured dendritic cells in a randomized controlled clinical trial of melanoma vaccines. J Clin Oncol. 2009;27(15 Suppl) Abstract 3002. [Google Scholar]
- 111.Wilgenhof S, Van Nuffel AM, Benteyn D, et al. Therapeutic vaccination with an autologous trimix-dendritic cell vaccine combined with sequential interferon α-2b in patients with advanced melanoma. J Clin Oncol. 2009;27(15 Suppl) Abstract 9024. [Google Scholar]
- 112.Trakatelli M, Toungouz M, Blocklet D, et al. A new dendritic cell vaccine generated with interleukin-3 and interferon-β induces CD8+ T cell responses against NA17-A2 tumor peptide in melanoma patients. Cancer Immunol Immunother. 2006;55(4):469–474. doi: 10.1007/s00262-005-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/ν in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]; • Clinical investigation of lipopolysaccharide-activated dendritic cells (DCs), which showed strong immunogenicity and DCs that secrete high levels of IL-12p70.
- 114.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]; • Demonstration of the contribution of TLR4 and HMGB1 to antitumor effects mediated by chemotherapy and radiotherapy.
- 115.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117(8):2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13(18 Pt. 1):5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Demaria S, Bhardwaj N, Mcbride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Demaria S, Santori FR, Ng B, Liebes L, Formenti SC, Vukmanovic S. Select forms of tumor cell apoptosis induce dendritic cell maturation. J Leukoc Biol. 2005;77(3):361–368. doi: 10.1189/jlb.0804478. [DOI] [PubMed] [Google Scholar]
- 119.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: Indispensable for therapeutic success? J Clin Invest. 2008;118(6):1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Summary of immunological effects of conventional cancer treatments.
- 120.Houot R, Levy R. Vaccines for lymphomas: idiotype vaccines and beyond. Blood Rev. 2009;23(3):137–142. doi: 10.1016/j.blre.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 121.Ivanov S, Dragoi AM, Wang X, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110(6):1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Popovic PJ, Demarco R, Lotze MT, et al. High mobility group B1 protein suppresses the human plasmacytoid dendritic cell response to TLR9 agonists. J Immunol. 2006;177(12):8701–8707. doi: 10.4049/jimmunol.177.12.8701. [DOI] [PubMed] [Google Scholar]
- 123.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 124.Garay RP, Viens P, Bauer J, et al. Cancer relapse under chemotherapy: why TLR2/4 receptor agonists can help. Eur J Pharmacol. 2007;563(1–3):1–17. doi: 10.1016/j.ejphar.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 125.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8(4):309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 126.Weigel BJ, Rodeberg DA, Krieg AM, Blazar BR. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res. 2003;9(8):3105–3114. [PubMed] [Google Scholar]
- 127.Milas L, Mason KA, Ariga H, et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64(15):5074–5077. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 128.Mason KA, Ariga H, Neal R, et al. Targeting Toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin Cancer Res. 2005;11(1):361–369. [PubMed] [Google Scholar]
- 129.Mason KA, Neal R, Hunter N, Ariga H, Ang K, Milas L. CpG oligodeoxynucleotides are potent enhancers of radio- and chemoresponses of murine tumors. Radiother Oncol. 2006;80(2):192–198. doi: 10.1016/j.radonc.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 130.Butowski N, Chang SM, Junck L, et al. A Phase II clinical trial of Poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01–05) J Neurooncol. 2009;91(2):175–182. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salazar AM, Levy HB, Ondra S, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38(6):1096–1103. discussion 1103–1094. [PubMed] [Google Scholar]
- 132.Friedberg JW, Kim H, Mccauley M, et al. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-α/β-inducible gene expression, without significant toxicity. Blood. 2005;105(2):489–495. doi: 10.1182/blood-2004-06-2156. [DOI] [PubMed] [Google Scholar]
- 133.Manegold C, Gravenor D, Woytowitz D, et al. Randomized Phase II trial of a Toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(24):3979–3986. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 134.Schmidt C. Clinical setbacks for Toll-like receptor 9 agonists in cancer. Nat Biotechnol. 2007;25(8):825–826. doi: 10.1038/nbt0807-825. [DOI] [PubMed] [Google Scholar]
- 135.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of taxol. Eur J Immunol. 2001;31(8):2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 136.Wang J, Kobayashi M, Han M, et al. MyD88 is involved in the signalling pathway for taxol-induced apoptosis and TNF-α expression in human myelomonocytic cells. Br J Haematol. 2002;118(2):638–645. doi: 10.1046/j.1365-2141.2002.03645.x. [DOI] [PubMed] [Google Scholar]
- 137.Warren TL, Weiner GJ. Synergism between cytosine-guanine oligodeoxynucleotides and monoclonal antibody in the treatment of lymphoma. Semin Oncol. 2002;29(1 Suppl. 2):93–97. [PubMed] [Google Scholar]
- 138.Friedberg JW, Kelly JL, Neuberg D, et al. Phase II study of a TLR-9 agonist (1018 ISS) with rituximab in patients with relapsed or refractory follicular lymphoma. Br J Haematol. 2009;146(3):282–291. doi: 10.1111/j.1365-2141.2009.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Makela SM, Strengell M, Pietila TE, Osterlund P, Julkunen I. Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J Leukoc Biol. 2009;85(4):664–672. doi: 10.1189/jlb.0808503. [DOI] [PubMed] [Google Scholar]
- 141.Jarnicki AG, Conroy H, Brereton C, et al. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180(6):3797–3806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]
- 142.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Redecke V, Hacker H, Datta SK, et al. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172(5):2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 144.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang B, Zhao J, Li H, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65(12):5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 147.Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27(2):218–224. doi: 10.1038/sj.onc.1210904. [DOI] [PubMed] [Google Scholar]
- 148.Schon MP, Wienrich BG, Drewniok C, et al. Death receptor-independent apoptosis in malignant melanoma induced by the small-molecule immune response modifier imiquimod. J Invest Dermatol. 2004;122(5):1266–1276. doi: 10.1111/j.0022-202X.2004.22528.x. [DOI] [PubMed] [Google Scholar]
- 149.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176(8):4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 150.Conroy H, Marshall NA, Mills KH. TLR ligand suppression or enhancement of treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27(2):168–180. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 151.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5(5):508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 152.Dols A, Smith JW, 2nd, Meijer SL, et al. Vaccination of women with metastatic breast cancer, using a costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic, breast cancer cell line: clinical and immunological results. Hum Gene Ther. 2003;14(11):1117–1123. doi: 10.1089/104303403322124828. [DOI] [PubMed] [Google Scholar]
- 153.Gutterman JU, Cardenas JO, Blumenschein GR, et al. Chemoimmunotherapy of advanced breast cancer: prolongation of remission and survival with BCG. Br Med J. 1976;2(6046):1222–1225. doi: 10.1136/bmj.2.6046.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Powles RL, Lister TA, Crowther D, Mcelwain T, Alexander P, Fairley GH. Immunotherapy for acute myelogenous leukemia. Bibl Haematol. 1975;(40):737–749. doi: 10.1159/000397596. [DOI] [PubMed] [Google Scholar]
- 155.Green DS, Bodman-Smith MD, Dalgleish AG, Fischer MD. Phase I/II study of topical imiquimod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol. 2007;156(2):337–345. doi: 10.1111/j.1365-2133.2006.07664.x. [DOI] [PubMed] [Google Scholar]
- 156.Carpentier A, Laigle-Donadey F, Zohar S, et al. Phase I trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro-oncol. 2006;8(1):60–66. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Link BK, Ballas ZK, Weisdorf D, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29(5):558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 158.Leonard JP, Link BK, Emmanouilides C, et al. Phase I trial of Toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin's lymphoma. Clin Cancer Res. 2007;13(20):6168–6174. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
Websites
- 201.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunological Reviews. 2008;222(1):357–368. doi: 10.1111/j.1600-065X.2008.00604.x. www3.interscience.wiley.com/journal/119398015/issue. [DOI] [PubMed]
- 202.List of ongoing Phase I/II/III studies. www.clinicaltrials.gov.


