ABSTRACT
Adult brachial plexus injury remains a dilemma to a reconstructive microsurgeon, especially when attempting to reconstruct cases of total root avulsion. Different degrees and different levels of injury require different strategies of reconstruction. The purpose of this article is to illustrate the author's reconstructive strategy in correlation with the injury level of classification. Nerve transfer, functioning free muscle transplantation, and other palliative surgery are reconstructive options for level 1 injuries. Neurolysis, nerve repair, nerve grafts (free nerve graft or vascularized ulnar nerve graft), nerve transfer if associated with level 1 lesion in other spinal nerves, and palliative reconstruction are chosen options for level 2, 3, and 4 lesions. A clavicle osteotomy is often required in level 3 lesions. Nerve grafts are frequently applied in level 4 lesions, which result in less aberrant reinnervation and a better prognosis.
Keywords: Brachial plexus injury, functioning muscle transfer, reconstruction
Adult brachial plexus injury (BPI) is characterized by many complex problems and remains a dilemma to the reconstructive microsurgeon, especially when attempting to reconstruct cases of total root avulsion. Neurolysis (mostly external neurolysis), nerve repair (indicated in penetrating injury), nerve grafts (free nerve grafts or vascularized ulnar nerve grafts), nerve transfer, functioning free muscle transplantation, and palliative surgery such as local muscle or tendon transfer are within the spectrum of surgical procedures for brachial plexus reconstruction.1,2,3,4,5
Different degrees and different levels of injury require different strategies of reconstruction. For example, nearly 70% of all BPIs are of the closed traction type that causes spinal nerve avulsion. This is an irreparable lesion. Nerve transfer and functioning free muscle transplantation become the only possible and reliable restorative options for the avulsed BPI.6,7
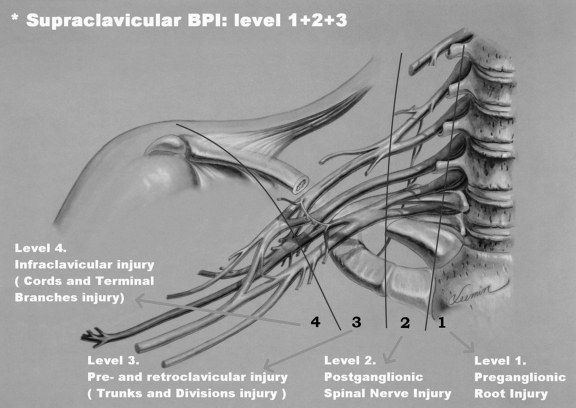
To better understand the reconstructive strategy, the author classifies brachial plexus lesions into four levels of injury (Fig. 1):
Figure 1.
Level injury of brachial plexus (Chuang's classification).
Level 1: Preganglionic root injury including spinal cord, rootlets, and root injuries.
Level 2: Postganglionic spinal nerve injury limiting the lesion to the interscalene space and proximal to the suprascapular nerve.
Level 3: Preclavicular and retroclavicular BPI including trunks and divisions.
Level 4: Infraclavicular BPI including cords and terminal branches proximal to the axillary fossa.
There are specific injury configurations and characteristics between these levels:
An “Extended Level Injury” of the same nerve is frequently observed: C7 injury, for instance, from the root down to the interscalene space (level 1 and 2 injury), or down to the trunk area (level 1, 2, and 3 injury).
A “Skip Level Injury” is rare. For instance, C5 and C7 are injured (avulsion or rupture) but C6 intact; or C5 injury involving level 1 and 3, but level 2 is intact.
A “Combined Level Injury” of different nerves is common. Example: C5 and C6 spinal nerve (level 2) rupture injury accompanied with C7-T1 root avulsion (level 1); or C5 rupture (level 2) with C6-T1 four-root avulsion (level 1).
Level 4 injuries are usually isolated, located infraclavicularly without an upward extension.
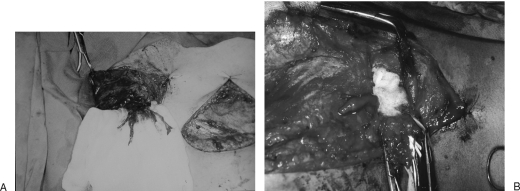
There are two types of characteristic lesions seen in the BPI: avulsion and rupture (Table 1). Avulsion refers to the nerve being torn from its attachment (proximal avulsion occurs at spinal cord level; distal avulsion at muscle level). Rupture is a nerve injury involving a traction force on an incompletely divided nerve, causing irregular proximal and distal ends. In an avulsion injury, only one disrupted end with a coiled-spring appearance can be seen in the operative field in the acute stage (Fig. 2A) or a fusiform pattern neuroma in the chronic stage (Fig. 2B). If the surgeon attempts to locate the other disrupted end, a second operative wound has to be created. However, in a rupture injury, the two nerve ends can be visualized in the same operative wound.
Table 1.
Differences between Avulsion and Rupture
| Avulsion | Rupture |
|---|---|
| Nerve tearing from its attachment or at its bone margin | Nerve division plus traction |
| In an operative wound, only one disrupted end can bee seen. Usually need second wound for seeing the other end). | Two disrupted ends can be seen in an operative wound |
| Level 1 is a proximal avulsion from spinal cord. Level 4 injury is a distal avulsion from muscle or at its bone margin. | Level 2, 3, and 4 injury |
Figure 2.
Root avulsion in level 1 lesion of the brachial plexus (A) in the acute stage and (B) in the chronic stage.
Root injury may involve the avulsion of the spinal nerve from the surface of the cord or rupture at the rootlet or root level. A novel approach to spinal cord implantation with or without nerve graft8,9 showed unsatisfactory clinical results, which implies that only the distal end is available, and the proximal end is absent or unsuitable for repair. Therefore a root injury, irrespective of the ventral or dorsal roots, is an avulsion injury from the clinical point of view. Level 1 injury in this context is an avulsion injury, whereas level 2 or 3 are rupture injuries. Root avulsion in BPI is usually accompanied with dura tearing and a CSF leak with cyst formation, called pseudomeningocele. However in some cases, the root can be avulsed at its origin, but with an intact dura cone. The nerve root may remain inside the spinal canal or at the dural orifice, giving a grossly normal appearance or loosening with curvature of the spinal nerve at the time of surgical intervention despite established paralysis. Most often, the entire avulsed root (including ventral, dorsal roots, and ganglia) retracts and migrates downward to the interscalene or preclavicular region (Fig. 2).
The following sections illustrate the author's reconstructive strategy for BPI in terms of the classification of level of BPI .
LEVEL 1 INJURY: PREGANGLIONIC ROOT INJURY (SPINAL CORD, ROOTLETS, AND ROOTS)
The incidence of level 1 lesion is 70%.5 Only one or up to all five roots may be avulsed. The reconstructive strategies include nerve transfer, functioning free muscle transplantation, and other palliative surgery.
Nerve Transfer
Nerve transfer is a surgical option that intentionally divides a physiologically active nerve (with low donor morbidity) and transfers it to a distal more important but irreparable paralytic nerve. The procedure is best done within a golden time period, within 5 months of the injury,5 to reactivate the paralyzed muscle(s) early, effectively, and successfully (aiming for M4 restored muscle strength). Nerve transfer can be broadly classified into three categories: extraplexus, intraplexus, and close-target nerve transfers.
EXTRAPLEXUS NERVE TRANSFER
Extraplexus nerve transfer involves the transfer of a neighboring nerve (from the ipsilateral or contralateral neck) to the avulsed brachial plexus for the neurotization of a paralytic nerve. The reported donor nerves in common use are mostly aimed at motor reinnervation. These include the phrenic (Ph) nerve, spinal accessory (XI) nerve (accessed by an anterior neck approach), deep motor branches of the cervical plexus (cervical motor branches; CMBs), hypoglossal nerve (XII), and the contralateral C7 (CC7) spinal nerve. Extraplexus sensory nerve transfer, such as supraclavicular sensory nerves to the median nerve transfer, is sometimes used to provide sensation to the paralytic hand.
INTRAPLEXUS NERVE TRANSFER
Intraplexus nerve transfer is applicable in cases of nonglobal root avulsion, in which at least one of the spinal nerves is a rupture injury and still available for transfer—not to its original pathway, but to other more important nerves. For example, in a case of C5 and C6 rupture injury (level 2) in which the C5 stump is healthier than the C6 stump, the C5 fibers are intentionally transferred to C6 (or the anterior division of the upper trunk) for elbow flexion. The distal C5 (or the posterior division of the upper trunk and suprascapular nerve) is then innervated by the partially injured C6. This strategy acknowledges that elbow flexion has priority over shoulder reconstruction. Intraplexus nerve transfer is individualized depending upon the intraoperative findings, the surgeon's philosophy, and the patient's condition and requirements. Extraplexus and intraplexus nerve transfers are both considered for proximal nerve neurotization.
CLOSE-TARGET NERVE TRANSFER
Close-target nerve transfer is a procedure that provides a direct coaptation at a more distal site, closer to the neuromuscular junction, thus achieving faster recovery of motor outcomes. Close-target nerve transfer is here defined as a procedure to be out of the supraclavicular and infraclavicular fossa, such as:
spinal accessory nerve transfer to the suprascapular nerve via a posterior approach
partial ulnar nerve transfer to the biceps nerve
part of median nerve transfer to the brachialis nerve
long head of triceps branch transfer to the axillary nerve
intercostal (IC) nerve transfer to the biceps nerve, or the musculocutaneous nerve, or the triceps nerve of the long head
anterior interosseus nerve transfer to the radial or posterior interosseus nerve; and
branch of the anterior interosseus nerve transfer to the deep motor branch of the ulnar nerve in the forearm.
The above are all examples of close-target nerve transfer. Selecting proximal or distal nerve transfer as a reconstructive strategy is now a subject of much debate10,11 (Table 2). Proximal nerve transfer (extraplexus and intraplexus nerve transfer) is traditionally still the main reconstructive procedure.
Table 2.
Comparison between Proximal Nerve Transfer and Distal Nerve Transfer
| Proximal Nerve Transfer | Distal Nerve Transfer | |
|---|---|---|
| Philosophy | Traditional | New strategy |
| Donor nerve | Proximal and supraclavicular nerve | Close to the target (out of supraclavicular and infraclavicular fossa) |
| Advantages | For diagnosis and treatment. | A treatment procedure. |
| Proximal nerve, move powerful. | No scars, easy dissection. | |
| Nerve cut, less functional deficits. | Shorter operation time. | |
| Nerve cut stumps: healthy, no scar. | ||
| Usually direct coaptation. | ||
| Short rehabilitation, faster recovery. | ||
| Disadvantages | Difficult dissection. | Nerve cut, risk to cause deficits. |
| Cut stumps, unpredictable. | Risk for iatrogenic injury. | |
| May need long nerve grafts. | Nerve cut, risk to cause deficits. | |
| Long operation time. | Risk for iatrogenic injury. | |
| Long rehabilitation period. | May need multiple incisions. | |
| Indication | All kinds of avulsion or rupture | Not global injury (single C5, or C5–6 two-root avulsion, or C5–7 three-root avulsion). |
| Indication | All kinds of avulsion or rupture | Median or ulnar intrinsic palsy. |
“Induction or motivation exercise” is an important muscle exercise for patients with nerve transfer.11 This is an exercise on the muscle(s) that are innervated by the transferred nerve, indicated in all cases of nerve transfer. Induction exercise is commenced when movement of the innervated muscles is palpable (M1). The action is comparable with an internal electric stimulator. Various nerve transfers have different induction exercises (Table 3).
Table 3.
Different “Induction Exercises” for Different Nerve Transfers
| Donor Nerve | Induction Exercise |
|---|---|
| IC nerve | Aerobic exercise (e.g., run, walk, or climb hill) to frequently induce deep breaths |
| Ph nerve | Aerobic exercise |
| XI nerve | Shoulder moves up or back exercise with resistance |
| XII nerve | Tongue to palate push-up exercise |
| Contralateral C7 | Shoulder adduction or grasp exercise on the healthy limb |
| Partial ulnar or median nerve | Hand grasp exercise |
For Shoulder
Reconstruction for shoulder abduction in a level 1 lesion should take priority over shoulder adduction. If the supraspinatus, infraspinatus, and deltoid muscles are innervated simultaneously, results are predictably better. The Ph and XI are the main donor nerves for shoulder abduction. The XII nerve, CMBs, part of C5 or C6, long thoracic nerve, branch to the long head of triceps, medial pectoral nerve, IC nerve, and CC7 have also been reported as the donor nerve for shoulder abduction. The recipient nerves for shoulder abduction in order of priority are the distal C5, suprascapular nerve, dorsal division of the upper trunk, then axillary nerve. A study of a series of patients operated between 2000 and 2003 demonstrated that triple nerve transfers produced the best and consistently reliable results for shoulder elevation: An average of 160 degrees shoulder abduction was achieved after triple nerve transfers but 85 degrees after double nerve transfers and 65 degrees after single nerve transfer.12
For Elbow
In level 1 injury, reconstruction for elbow flexion is always the first priority. Reported donor nerves for elbow flexion include IC nerve, XI nerve with a nerve graft, Ph nerve with or without nerve graft, partial ulnar nerve, partial median nerve, pectoral nerve, thoracodorsal nerve, and CC7. The recipient nerve is the (mixed) musculocutaneous nerve, the nerve branch to biceps, or the nerve branch to brachialis. Elbow extension is usually the lesser priority for reconstruction. Ph nerve transfer to the distal C5 or to the posterior division of the upper trunk or the radial nerve with a nerve graft can often yield elbow extension, usually in the third year of rehabilitation. Some authors describe transferring two or three IC nerves to the branch of the long head of triceps to achieve elbow extension.13
For Finger
In global (C5-T1) level 1 injury, reconstructive priority for finger function depends on the procedure used: nerve transfer or functioning free muscle transplantation. Traditionally, reconstruction for finger flexion is preferable to finger extension, which can sometimes be helped by a dynamic extension splint. In C5 rupture with C6-T1 four-root avulsion, transfer of C5 to the median nerve, and in a total root (C5-T1) avulsion, transfer of the CC7 to the median nerve to achieve finger and wrist flexion as well as finger sensation is often performed. Either procedure requires a vascularized ulnar nerve graft to achieve a one-stage full reconstruction when accompanied with other nerve transfers for shoulder and elbow function.11 In total root avulsion, multiple nerve transfers including CC7 in the acute stage can provide a one-stage full reconstruction, which is the author's preferred method (Fig. 3). Functioning free muscle transplantation (FFMT) is used predominately as an adjuvant palliative reconstruction to enhance results at a later stage.
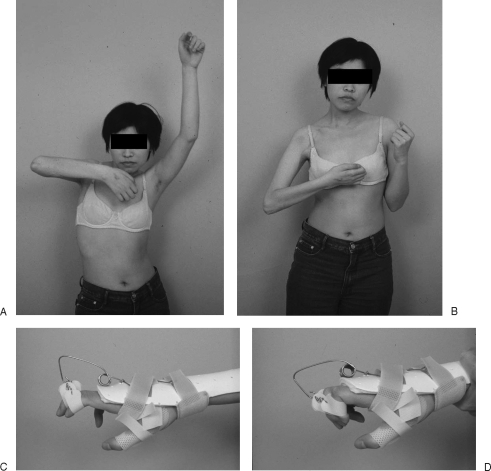
Figure 3.
A case of total root avulsion (C5-T1) of the right upper limb. Results 5 years after the multiple nerve transfers: (1) Ph nerve transfer to the suprascapular nerve for shoulder function, (2) IC nerve (T3–5) transfer to the musculocutaneous nerve for elbow function, and (3) contralateral C7 transfer to the median nerve with a vascularized ulnar nerve graft for the hand function. The patient achieves improving (A) shoulder elevation, (B) elbow flexion, and (C, D) hand function with help of interphalangeal extension dynamic splint.
The alternative approach is FFMT13: A long FFMT from the clavicle down to the extensor digitorum communis (EDC), innervated by the XI nerve, is performed in the first stage, followed by a second long FFMT from the second rib to the flexor digitorum profundus (FDP), innervated by IC nerves in the second stage. Arthrodesis of the wrist and thumb are usually required for stability and grip. Doi's FFMT13 across the elbow with a below-elbow pulley for hand reconstruction in the acute proven root avulsion illustrates a “distal to proximal” reconstruction approach, which is different from the traditional “proximal to distal” reconstruction order. The proximal-to-distal reconstructive strategy (nerve reconstruction first, FFMT later) compared with the distal-to-proximal approach (FFMT first, then nerve reconstruction later) in level 1 injury is illustrated in Table 4.
Table 4.
Comparison between Proximal-to-Distal and Distal-to-Proximal Reconstructive Priority
| Proximal to Distal | Distal to Proximal | |
|---|---|---|
| Philosophy | Traditional nerve first,* FFMT next | New strategy; FFMT first, nerve next |
| Reconstruction priority | Shoulder, elbow first, then finger | Fingers and elbow first, then shoulder |
| Brachial plexus exploration | Yes | Possibly no |
| Nerve reconstruction | ||
| For shoulder | Yes | May or may not be required |
| For elbow | Yes | Need FFMT |
| For finger | Yes | Need FFMT |
| Stage requirement | May be one stage | Multiple stages |
| Rehabilitation period | Longer (at least 4 years) | Usually 2 years |
| Patient selection | Highly motivated, intelligent, and compliant | Low compliance, impatient patient |
| Outcome prediction | ||
| Shoulder elevation | Better (≥60 degrees) | Shoulder fusion (10 to 30 degrees) |
| Elbow flexion | Usually better (M4) | M3–4 |
| Finger flexion | M2–4 | M2–3 |
| Finger extension (EDC) | M0 | M2–3 |
Nerve first: nerve reconstruction first.
Functioning Free Muscle Transplantation
FFMT is the transfer of a muscle using microvascular anastomoses for revascularization and subsequent microneural coaptation to the recipient motor nerve for reinnervation. The use of FMT in brachial plexus reconstruction is one example of the application of nerve transfer (including extraplexus, intraplexus, and close-target nerve transfer). It has been shown to be effective and has become increasingly popular. The gracilis myocutaneous FMT is the most common and best choice of donor muscle in brachial plexus reconstruction.6,13 The commonly used extraplexus donor nerves include the XI, the IC, the Ph nerve, and the CC7 nerve. The intraplexus donor nerves include part of the ulnar nerve, part of the median nerve, or more proximally from the infraclavicular or suprascapular nerve, which requires nerve elongation (with a nerve graft) and FMT in a two-stage procedure. The results from FFMT are more satisfactory than those achieved with a local muscle transfer. It is especially useful for elbow and hand function restoration in global plexopathy. Indications for FFMT in BPI include acute or chronic root avulsion, root injury with failed nerve transfer (muscle strength less than M3), or BPI with associated Volkmann's contracture of the forearm.
Palliative Reconstruction
Palliative reconstruction procedures include muscle transfer, tendon transfer, functioning muscle transplantation, tenodesis, and arthrodesis. Alternatively, orthotics and prosthetics can be used. Local pedicled muscle transfer, although an alternative restorative option, is often not reliable due to the presence of a partial nerve injury. For example, using a local latissimus dorsi muscle transfer for elbow flexion in C5 and C6 ± C7 avulsion injury usually results in M3, but not M4 muscle strength, compared with latissimus dorsi transfer for the traumatic loss of biceps and brachialis, which always results in M4 muscle strength. The reason for this difference in outcome is the state of the thoracodorsal nerve, which originates from C6–8. In the former case it is an injured nerve, but in the latter it is an uninjured one. Palliative reconstruction can be considered when the injury involves C8 and T1 level, called Klumpke's palsy in adults, or when deformities persist after maximum recovery, either with or without nerve reconstruction.
LEVEL 2: POSTGANGLIONIC SPINAL NERVE INJURY LIMITING THE LESION IN THE INTERSCALENE SPACE AND PROXIMAL TO THE SUPRASCAPULAR NERVE
The distance from the distal part of the dorsal root ganglion to where the ventral and dorsal rootlets join is only 1 to 2 mm. This part, the postganglionic root zone, should be disregarded as it is too short to be of surgical significance. After the ganglion it becomes the spinal nerve located between the anterior and middle scalene muscles. This terminology has been defined by Sunderland.14 The clinical differential diagnosis between preganglionic root (level 1) and postganglionic spinal nerve (level 2) injury is of great importance with regard to the surgical approach and prognosis.
Level 2 injury is defined as an injury distal to the dorsal root ganglion (or outside the intervertebral foramen) between the scalene muscles and proximal to the suprascapular nerve. If the suprascapular nerve is intact, the lesion will be in level 3 to 4 and not in level 2. The incidence of a pure level 2 injury is ∼8%.5 Disruption at the spinal nerve with neuroma formation and dense scar tissue involving the scalene muscles (especially the middle scalene muscle) are the main findings in this type of injury. Nerve rupture may occur at one or multiple spinal nerves. Reconstruction for this level of injury includes neurolysis, nerve repair, nerve grafts (free nerve graft or vascularized ulnar nerve graft), nerve transfer if associated with a level 1 lesion in other spinal nerves, and palliative reconstruction.
Neurolysis
When the nerve lesion is in continuity, it commonly shows neuroma-in-continuity, implying that some function might remain. Neurolysis is sometimes helpful. The surgical technique should be either an epifascicular epineurotomy/epineurectomy (external neurolysis) or an interfascicular epineurectomy (internal neurolysis). In brachial plexus lesions, external neurolysis is usually performed.
Nerve Repair
Direct nerve repair is only possible after penetrating injuries.
Nerve Graft
Nerve grafting is the predominant technique employed in level 2, 3, or 4 brachial plexus repair. There are two popular types of nerve grafts in brachial plexus reconstruction: free nerve graft and vascularized ulnar nerve graft. Sural nerves are the source of free nerve grafts in the majority of cases. The medial cutaneous nerves of the arm and forearm and the saphenous nerve from the thigh are sometimes also used. The outcome is influenced by the length of the nerve graft, presence of scar tissue at the wound site, number of grafts used, and the presence of a healthy proximal stump available for grafting. In cases of total root avulsion or lower plexus root avulsion (C8-T1 ± C7), the entire ulnar nerve from the axilla to the wrist can be used as a vascularized nerve graft, either on a pedicle or as a free tissue transfer. Vascularized ulnar nerve grafts are used frequently in contralateral C7 elongation and transfer. Nerve grafting is always necessary in level 2 injuries. These are frequently associated with level 3 injury of the same spinal nerve or with a level 1 injury on other spinal nerves. If combined level 1 and level 2 injury on different spinal nerves is encountered, nerve grafts and nerve transfers are the main procedures for reconstruction of this injury. For example, ruptures of C5 and C6 with root avulsions of C7 to T1 are frequently seen. C5 nerve grafting to the suprascapular nerve and posterior division of the upper trunk for shoulder elevation, C6 nerve grafts to the distal C8 spinal nerve or to the median nerve with a segment of vascularized ulnar nerve graft for hand function, and IC nerve transfer to the musculocutaneous nerve for elbow function are a good option for a full one-stage reconstruction. If combined level 2 and level 3 injury on the same spinal nerve is noted, long nerve grafts (greater than 10 cm in length) are usually required to cover the distance from the spinal nerve to the terminal branches in the infraclavicular fossa. The clavicle can be elevated through Chuang's triangle approach5 without requiring an osteotomy.
LEVEL 3: PRECLAVICULAR AND RETROCLAVICULAR (TRUNKS AND DIVISIONS) INJURY
Level 3 injury involves the trunks and divisions. In our series of more than 1600 cases, only 5% of patients had an isolated injury in this zone.5 Nerve rupture with neuroma formation and dense scars were the most common findings. Bypass nerve grafting is required to reestablish the connection between the supraclavicular and infraclavicular brachial plexus. A clavicle osteotomy is often required, especially for injuries involving the lower trunk, to allow grafting or direct neurolysis. Multiple nerve grafts are required and need to be harvested from different locations. A C-loop vascularized ulnar nerve graft is sometimes helpful to reduce the amount of nerve grafts especially in cases of extensive injury.
LEVEL 4: INFRACLAVICULAR BRACHIAL PLEXUS INJURY
Level 4 BPI involves the cords and their terminal branches. The incidence is high (17%), second after level 1 injury.5 Level 4 injury is most commonly limited to this zone only and rarely has upward level involvement. It is an injury associated with nerve ruptures. However, nerve avulsion is occasionally seen. In such a case it is usually a distal avulsion occurring at the bone margin (such as a suprascapular nerve avulsion at the scapular notch, or an axillary nerve at the humeral neck), or at the muscle attachment (such as the musculocutaneous nerve avulsed from the surface of biceps muscle).
In closed level 4 lesions, the nerve damage is variable, ranging from simple isolated nerve injury to lesions of all cords or all terminal branches. Level 4 injuries show particular characteristics that present specific difficulties in surgical dissection and repair due to dense scar tissue. Nerve grafts are frequently applied, which result in less aberrant reinnervation and a better prognosis. There is a high incidence (30%) of vascular injury, rupture, or segmental occlusion of the subclavian or axillary artery. For ease of dissection, detachment of the insertion of the pectoralis major muscle by Z-lengthening incision is often required. In penetrating injuries, vascular and nerve repairs are usually performed simultaneously. The golden time for primary direct repair of the divided nerves in level 4 penetrating injuries without nerve grafts is within 2 weeks, in contrast with 1 week only in level 2 or 3 injuries. Traction injury in level 4 is usually associated with a fracture of the proximal humerus or the glenoid scapula. The nerve segments in such a case are extensively damaged. Long nerve grafts are usually required to bridge the gap, commonly more than 8 cm in length. Sometimes a C-loop vascularized ulnar nerve graft is harvested from the paralytic forearm and used for median and radial nerve reconstruction. The results are usually good. In nerve avulsion from the muscle, nerve grafting of the proximal nerve stump and direct implantation into the muscle (nerve to muscle neurotization) can yield fair results (around M3). Functioning muscle transplantation is another option of reconstruction in such cases.
RECONSTRUCTIVE STRATEGIES FOR DIFFERENT TYPES OF LESIONS
Single-Root Avulsion
In an isolated C5 root injury, mass nerve transfer including the spinal accessory, phrenic, and cervical motor branches directly to the C5 spinal nerve will achieve good supraspinatus, infraspinatus, and deltoid muscle strength and obtain powerful shoulder abduction of more than 90 degrees.
Single C6 root avulsion is usually associated with a C5 rupture. Nerve grafts from the proximal C5 stump to the anterior division of the upper trunk can achieve better results of elbow flexion than IC nerve transfer to the musculocutaneous nerve. Shoulder abduction can be achieved by spinal nerve accessory nerve transfer to the suprascapular nerve and Ph nerve transfer to the posterior division of the upper trunk.
Single C7 root avulsion is usually associated with a rupture of the upper trunk. Only repair of the upper trunk is required. C7 spinal nerve reinnervation is not required.
Two-Root Avulsion
In combined C5 and C6 two-root avulsion, multiple nerve transfers are recommended: For shoulder elevation, the author recommends transfer of the XI nerve to the suprascapular nerve, combined with Ph nerve transfer to the posterior division of the upper trunk. For elbow flexion restoration, the author routinely performs three IC nerve transfers to the musculocutaneous nerve. In combined C6 and C7 two-root avulsions, C5 is usually ruptured. The proximal C5 stump, if healthy, is transferred to the anterior division of the upper trunk for elbow flexion. Shoulder function can be obtained by XI nerve and Ph nerve transfer as described earlier. If the proximal C5 stump is of doubtful viability, it is recommended to transfer it to the posterior division of the upper trunk in addition to XI nerve transfer to the suprascapular nerve to achieve a more reliable shoulder function. Elbow flexion can then be obtained by IC nerve transfer to the musculocutaneous nerve.
Combined C8 and T1 root avulsions usually occur with C5 to C7 ruptures. Shoulder elevation is regained with nerve grafts from C5 to the suprascapular nerve and to the posterior division of the upper trunk. C6 nerve fibers are intentionally transferred to the median nerve to achieve motor and sensory hand function. C7 is usually connected to itself by short nerve grafts due to its high incidence of concurrent root injury. Elbow flexion is achieved by IC nerve transfer. Pure C8 and T1 root injury with intact C5 to C7 is a very rare finding. Early exploration is not indicated. In the late stage, tendon transfers are performed in two separate procedures.
Three-Root Avulsion
Combined C5 to C7 root avulsions with intact C8 to T1 are reasonably common injuries. Ph and XI nerve transfers for shoulder abduction and IC nerve transfer for elbow flexion are recommended. The Ph nerve is transferred to the posterior division of the upper trunk giving the possibility of deltoid, triceps, and wrist extensor (extensor carpi radialis longus, [ECRL]) muscle neurotization. If the Ph nerve is also avulsed, XII nerve transfer to the axillary nerve with a nerve graft can replace the role of Ph nerve function.
Combined C7 to T1 three-root avulsion is usually associated with a rupture of the upper trunk. Using nerve grafts, C5 fibers are transferred to the suprascapular nerve and posterior division of the upper trunk for shoulder elevation. The proximal C6 stump is intentionally transferred to the distal C8 spinal nerve or median nerve for hand function. For elbow flexion, IC nerve transfer to the musculocutaneous nerve is performed.
Four-Root Avulsion
C6 to T1 four-root avulsion usually associated with a rupture of C5 is not an uncommon injury. If the proximal C5 fibers are grossly healthy, it is preferred to transfer these to the anterior division of the upper trunk for elbow flexion. If the proximal C5 stump is of questionable viability, it is then transferred to posterior division of the upper trunk plus a XI nerve transfer to the suprascapular nerve for a more reliable outcome of shoulder function. Contralateral C7 transfer to the median nerve with a pedicle or free vascularized ulnar nerve graft for hand function (finger flexion and sensation) can be done simultaneously, thus obtaining a total reconstruction in a single-stage procedure.
Total (Five) Root Avulsion
Total root avulsion is unfortunately the most common traction injury of the adult brachial plexus.5 In the context of the severely disabling nature of this injury, it has been proposed that “for a patient who has nothing, a little is a lot.” In a single-stage procedure for total reconstruction, the author's preferred method is a contralateral C7 transfer to the median nerve, using a vascularized ulnar nerve graft to regain some hand function. In addition, IC nerve transfer to the musculocutaneous nerve for elbow flexion and Ph nerve/XI nerve transfer for shoulder elevation are performed simultaneously. Once the patient obtains hand function (finger flexion and sensation), wrist and thumb fusion are performed to improve hand stability. A dynamic extension splint is used to aid finger extension. This procedure can lead to hand prehension (grasp and sensation).
DISCUSSION
Using a “Number” Instead of an “Anatomic Description” for the Different Levels of BPI
Classification of brachial plexus lesions facilitates interpretation of clinical findings, provides guidelines for surgical reconstruction, and gives information with regard to the prognosis of the injury. Several classifications are used including Narakas,15 Milesi,16 Alnot,17 Terzis et al18 (Table 5), Boome (eight levels),19 Leffert (two levels),20 and Chuang.5 To avoid confusion, the use of a number instead of an anatomic description for the different levels of BPI provides a simple and easy solution. Level 1 injury is a brachial plexus lesion inside the vertebral bone, or a preganglionic root injury. Level 2 is defined as an injury at the interscalene space or a postganglionic spinal nerve injury. Level 3 is a BPI around the clavicle or a preclavicular and retroclavicular injury including the trunks and divisions. Level 4 injury is an infraclavicular BPI including the cords and terminal branches. The simplicity of using numbers (levels 1–4) and assigning these to a definitive zone gives it a significant advantage in a clinical and research setup. This classification has been uniformly adopted by surgeons, neurologists, and radiologists at the author's institution (Chang Gung Memorial Hospital, Taipei-Linkou, Taiwan) for ease of communication and avoidance of confusion.
Table 5.
Different Classifications for Different Sites of BPI
| Narakas | Millesi | Alnot | Terzis | |
|---|---|---|---|---|
| Level 1 | Supraganglionic root | Supraganglionic root | Preganglionic root | Root |
| Level 2 | Infraganglionic spinal nerve | Infraganglionic root | Postganglionic root | Supraclavicular postganglionic |
| Level 3 | Infraganglionic trunk | Trunks (supraclavicular) | Supraclavicular and retroclavicular (trunk) | |
| Level 4 | Retroclavicular (division and cord) | Cords (infraclavicular) | Infraclavicular | Infraclavicular |
| Level 5 | Terminal branches |
Nerve Transfer versus Neurotization
These two terms are often confused with each other: nerve transfer and neurotization. Nerve transfer implies an active process. However, the term neurotization offers an active and passive option: “A” neurotizes “B” or “A” is neurotized by “B.” When using the term neurotization, the “neurotizer” can sometimes be indistinct from the target. In nerve reconstruction, most procedures are active ones. Therefore, nerve transfer is a more precise term than neurotization.
Evaluation of Results and Outcomes
It is difficult and impractical to provide patients and their families with expectations about the results of treatment. Spontaneous recovery in adult BPI, especially in high-energy injury, is rare but still possible, in particular in lower plexus root injuries. In BPI after either nerve reconstruction or FFMT, motor function recovery assessment is recommended based on joint movement strength, not on individual muscles, using the modified British Medical Research Council (MRC) grading system. It is simple and practical. In successful level 4 BPI cases after nerve grafting, M4 with 180-degree shoulder elevation, M4 or M4+ elbow flexion and extension, M3+ or M3 finger flexion and extension, and M2 or less of hand intrinsic muscles can usually be expected. In postreconstruction cases of total root avulsion with multiple nerve transfers, a successful outcome is usually characterized by 60-degree shoulder abduction, M4 elbow flexion, and M2–4 finger flexion. In addition to this, M2–3 wrist and finger extension can be obtained after FFMT in the late reconstructions.
REFERENCES
- Narakas A. Brachial plexus surgery. Orthop Clin North Am. 1981;12:303–323. [PubMed] [Google Scholar]
- Millesi H. Surgical management of brachial plexus injuries. J Hand Surg [Am] 1977;2:367–378. doi: 10.1016/s0363-5023(77)80046-4. [DOI] [PubMed] [Google Scholar]
- Alnot J Y. In: Terzis JK, editor. Microreconstruction of Nerve Injuries. Philadelphia, PA: WB Saunders; 1987. Traumatic paralysis of the brachial plexus: preoperative problems and therapeutic indications. pp. 325–345.
- Terzis J K, Liberson W T, Maragh H A. In: Terzis JK, editor. Microreconstruction of Nerve Injuries. Philadelphia, PA: WB Saunders; 1987. Motorcycle brachial plexopathy. pp. 361–384.
- Chuang D CC. In: Mathes SJ, Hentz VR, editor. Plastic Surgery. Vol. 7. Philadelphia, PA: Saunders Elsevier; 2006. Adult brachial plexus injuries. pp. 515–538.
- Chuang D CC. Functioning free muscle transplantation for brachial plexus injury. Clin Orthop Relat Res. 1995;314:104–111. [PubMed] [Google Scholar]
- Chuang D CC. In: Gilbert A, editor. Brachial Plexus Injuries. London, UK: Martin-Dunitz Ltd; 2001. Palliative surgery: forearm and hand deformities. pp. 293–302.
- Carlstedt T, Grane P, Hallin R G, Norén G. Return of function after spinal cord implantation of avulsed spinal nerve roots. Lancet. 1995;346:1323–1325. doi: 10.1016/s0140-6736(95)92342-x. [DOI] [PubMed] [Google Scholar]
- Fournier H D, Mercier P, Menei P. Repair of avulsed ventral nerve roots by direct ventral intraspinal implantation after brachial plexus injury. Hand Clin. 2005;21:109–118. doi: 10.1016/j.hcl.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Mackinnon S E, Novak C B. Nerve transfers. New options for reconstruction following nerve injury. Hand Clin. 1999;15:643–666, ix. [PubMed] [Google Scholar]
- Chuang D CC. Nerve transfers in adult brachial plexus injuries: my methods. Hand Clin. 2005;21:71–82. doi: 10.1016/j.hcl.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Cardenas-Mejia A, O'Boyle C P, Chen K T, Chuang D CC. Evaluation of single-, double-, and triple-nerve transfers for shoulder abduction in 90 patients with supraclavicular brachial plexus injury. Plast Reconstr Surg. 2008;122:1470–1478. doi: 10.1097/PRS.0b013e3181881fc5. [DOI] [PubMed] [Google Scholar]
- Doi K. Management of total paralysis of the brachial plexus by the double free-muscle transfer technique. J Hand Surg Eur Vol. 2008;33:240–251. doi: 10.1177/1753193408090140. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Brachial plexus injuries. Clin Neurol Neurosurg. 1993;95(Suppl.):S1–S2. doi: 10.1016/0303-8467(93)90026-d. [DOI] [PubMed] [Google Scholar]
- Narakas A O. Lesions found when operating traction injuries of the brachial plexus. Clin Neurol Neurosurg. 1993;95(Suppl):S56–S64. doi: 10.1016/0303-8467(93)90037-h. [DOI] [PubMed] [Google Scholar]
- Millesi H. In: Tubiana R, editor. The Hand. Philadelphia, PA: WB Saunders; 1988. Brachial plexus lesions: classification and operative technique. pp. 645–655.
- Alnot J Y. In: Tubiana R, editor. The Hand. Philadelphia, PA: WB Saunders; 1988. Traumatic brachial plexus palsy in adults. pp. 607–644.
- Terzis J K, Vekris M D, Soucacos P N. Outcomes of brachial plexus reconstruction in 204 patients with devastating paralysis. Plast Reconstr Surg. 1999;104:1221–1240. doi: 10.1097/00006534-199910000-00001. [DOI] [PubMed] [Google Scholar]
- Boome R S. In: RS Boome, editor. The Brachial Plexus. NY: Churchill Livingstone; 1997. Practical anatomy, clinical assessment, and surgical exposure. pp. 9–17.
- Leffert R D. In: Green D, editor. Operative Hand Surgery. 3rd ed. New York, NY: Churchill Livingstone; 1993. Brachial plexus. pp. 1483–1516.