Abstract
Background
It has been shown that colorectal carcinoma is increasing in incidence in African countries. This could be due to change in life style. Molecular pathogenesis of colorectal cancer commonly involves mutation in p53 gene which leads to expression of p53 protein in tumor cells. Expression of p53 protein has been associated with poor clinical outcome and reduced survival in patients.
Objective
This was a retrospective laboratory based study carried out in the Department of Pathology Makerere University, Kampala, Uganda. The aim of the study was to evaluate the expression of p53 protein in colorectal carcinoma in Ugandan patients, specifically its association with histological types, degree of differentiation, sites of the tumor and demographic characteristics of the patients.
Methods
Immunohistochemistry was carried out on 109 patient's paraffin embedded tissue blocks of colorectal carcinoma diagnosed in the Pathology Department, Faculty of Medicine Makerere University Kampala during the period 1995 to 2005. The indirect immunoperoxidase method using monoclonal antibody p53 DO-7 and Envision + Dual link system-HRP to detect p53 expression was used. Haematoxylin and eosin stain was used for evaluation of histological types and degree of differentiation of the tumors. Topography of the tumors and demographic data were obtained from accompanying histological request forms.
Results
Out of 109 patient's tissue blocks that were studied, 61 cases (56%) expressed p53 protein in the nucleus of malignant cells. Right sided colonic tumors were commoner (53.2%) than left sided colonic tumors (46.8%). p53 protein was expressed more in left sided colonic tumors with a significant difference (p<0.05), it was also expressed more in well differentiated tumors and non mucinous adenocarcinomas but with no significant difference (p>0.05). p53 expression was not affected by age or sex.
Conclusion
Frequency of p53 protein expression in Ugandan patients did not differ from that reported in the other parts of the world. It was expressed more in the left sided colonic tumors and this could support the hypothesis that right and left colonic tumors could have different pathogenesis and probably also responsible for difference in prognosis in these two topographic sites.
Keywords: p53, colorectal carcinoma, Immunohistochemistry
Introduction
There is a marked variation in the incidence of colorectal carcinoma worldwide ranking the third common cancer with Western countries having high rate compared to Africa. The incidence (per 100,000 populations) in North America is 40.6 in male and 30.6 in female where in Northern Europe is 34.7 in males and 25.2 in females. In Asian countries for example South Eastern Asia the incidence is 12.6 in males and 10 in females where in Sub Saharan Africa it is 5.7 in male and 4.6 in female per 100,000 populations1. In Uganda it has risen from 3 to 6.8 in females, and from 2.7 to 6.6 per 100,000 in males over the period 1960–19972. Topography of the tumor is also different with low risk areas having the caecum as the commonest site of the tumor2, whereas in high risk areas the tumor commonly affects the left side. These differences are thought to be due to interaction of environmental factors and several genetic alterations. It has been shown that p53 gene mutation is frequent phenomena occurring in about half of the cases of colorectal carcinoma3, 4. The product of p53 gene is 53kd protein (wild type) which is involved in cell cycle regulation. Mutation of p53 gene gives rise to abnormal protein with a long half life rendering it to be easily detected by immunohistochemistry. The mutant form of the p53 gene appears to act as dominant oncogene, but the wild type p53 normally acts as recessive tumor suppressor gene5. It has been reported that in colorectal carcinoma p53 gene mutation causes conversion of late adenomas to carcinoma in adenoma carcinoma sequence6
Detection of p53 protein in malignant cells has also been associated with poor clinical outcome and reduced survival in several tumor types7, 8. This study was therefore designed to assess whether p53 expression do exist in the Ugandan patients, and if the magnitude is the same as compared to that of the other parts of the world. This could provide a clue in the pathogenesis of colorectal carcinoma in low risk populations.
Materials and methods
109 patients were recruited for the study from the years 1995–2004 where their paraffin embedded tissue blocks were retrieved from the repository of the Department of Pathology, Faculty of Medicine Makerere University. During this time there was 150 cases of colorectal carcinoma investigated at Mulago hospital, the ones with incomplete clinical data, demography and tissue samples which was not adequate were ruled out so remaining with 109 cases. For the patients with more than one blocks all the blocks were examined and the one which represent the tumor best (with no necrosis, no much mesenchymal tissue) was selected for the study. Haematoxylin and Eosin sections were prepared to establish histological types and degree of differentiation of the tumor. Request forms accompanied these biopsies were retrieved and topography of the tumor and demographic data was obtained.
Tissue sections of 3ì m thickness were prepared and mounted on sialinized slides. Immunohistochemical staining was done following standard procedures as described by a manufacturer. Following incubation overnight at 56°C, the sections were dewaxed and rehaydrated. Antigen retrieval was done by immersing the slides in Tris-Edta solution pH 9.0 and heated for 10 min at high power and then 15 min at low power in the domestic microwave rated 800W. Sections were then transferred to phosphate buffered saline (PBS) pH 7.6 after cooling. This buffer was used for washing in all steps. Endogenous peroxidase activity of the tissue was blocked with 3% hydrogen peroxide for 10 min (Dako Denmark K4065) and sections were washed three times. Sections were incubated with monoclonal antibody p53 DO-7 (Dako Denmark) for 60 min followed by wash in PBS.
Antibody binding was detected by using Envision + Dual link system-HRP (Dako Denmark) for 45 min, followed by 3 3′-Diaminobenzidine in chromogen diluted in the ratio of 1: 50 with DAB substrate buffer after wash. Sections were counterstained by Meyer's haematoxylin for 10 min and then mounted with DPX. A section of esophageal carcinoma known to stain positive for p53 was included in each run as positive control and N- Universal negative control mouse (Dako) which does not recognize p53 protein as negative control. A tumor was classified as p53 positive when nuclear staining was observed in 5% or more of the cells counted in 10 high power fields[9, 10]. Right sided tumors were classified as those originating proximal to the splenic flexure and left side tumor as those located distal to or at this site including rectal tumors9, 11.
SPSS for Windows 10.0 software was used to analyze the data and X2 test was used to assess the association between p53 expression and histopathological features and demographic characteristics of the patient. For statistical significance, the cut off point for p-value was set at 0.05.
Approval was sought from Department of Pathology and Makerere Faculty of medicine research and ethics committee. No names of patients were used; biopsy numbers were used to identify the specimens and searching demographic data from the request form.
Results
A total of 109 biopsy specimens were studied and of these 61 cases (56%) expressed p53 in the nuclei of the malignant cells (95% confidence limits 46%–65%). Fifty one cases (56.8%) were males of whom (56.9%) expressed p53, and 58 (53.2%) were females of whom (55.2%) expressed p53. Patient's age ranged from 13–85 years with mean of 48.4 and standard deviation of 15.9. Most of the patients belonged to the local Ganda group while the remaining came from other tribal groups from different parts of Uganda. Expression of p53 by age groups is shown in table 1 and the age group 51–60 years expressed p53 more than the other age groups (75%) with no significant difference (p> 0.05). This trend remains the same even after stratifying the population in two age groups, young age (below 50 years) and old group (above 50 years). When p53 was analyzed by the degree of differentiation of the tumor, 65.9% of well differentiated tumors expressed it, 54.3% in moderately differentiated and 45.5% in poorly differentiated tumors. The trend shows decrease in p53 expression with worsening histological grade with no significant difference (p >0.05). This trend was also the same when p53 was analyzed by histological types of the tumor; whereas non mucinous adenocarcinoma expressed more p53 (59%) compared to the mucinous adenocarcinoma (45.8%) and signet ring cell carcinoma 57.9% (p>0.05). Tumors of the left colon expressed p53 (55.7%) more than the right side (44.3%) as shown in the table 2, with significant difference (p< 0.05).
Table 1.
p53 protein expression in different age groups
| Age group | p53 expression | |
| Positive (n %) | Negative (n %) | |
| 11–20 | 7 (58.3%) | 5 (41.7%) |
| 21–30 | 9 (56.3%) | 7 (43.8%) |
| 31–40 | 7 (58.3%) | 6 (46.2%) |
| 41–50 | 7 (58.3%) | 5 (41.7%) |
| 51–60 | 12 (75.0%) | 4 (25%) |
| 61–70 | 3 (33.3%) | 6 (66.7%) |
| 71–80 | 11 (61.1%) | 7 (38.9%) |
| 81–90 | 5 (38.5%) | 8 (61.5%) |
X2 = 6.111, p = 0.527
Table 2.
p53 expression in right colon and left colon
| Site of tumor | p53 expression | |
| Positive (n %) | Negative (n %) | |
| Right colon | 27 (44.3%) | 31 (64.6%) |
| Left colon | 34 (55.7%) | 17 (35.5%) |
X2 = 4.455 p = 0.035
Discussion
In this study, p53 protein expression was seen in 56% of colorectal carcinomas. This prevalence is similar to what have reported and ranged from 52.5% to 61.4%11–17. These figures are lower than that reported by Banu Lebe et al18 but higher than those which were found in some studies which varied from 43% to 48%10, 19, 20. The over expression of p53 seen in this study and other previous studies, support the hypothesis that p53 gene mutations are important in colorectal carcinogenesis. This is also supported by results from a number of studies showing that p53 expression is positively correlated with p53 gene mutation14, 20–22 . These findings suggest that a subset of colorectal carcinomas in both low and high incidence groups is due to p53 gene mutation.
A study from Israel showed that the prevalence of p53 in high risk, intermediate risk and low risk groups for colorectal cancer was similar23. This could be due to a common pathway in p53 mutation in colorectal carcinogenesis. Two common agents, alcohol and tobacco smoke have been implicated as possible factors in p53 alterations in the pathogenesis of colorectal cancer in both low risk and high risk populations21, 24. These two agents could also be important in Ugandan cases.
There have been reports about clinical and pathological differences between right and left side colorectal tumors15, 17, 23, 25. Our findings of a higher proportion of p53 positive tumors on the left colon compared to the right colon tend to support these views. This implies that the pathogenesis of left and right sided colonic tumors are different, and possibly the reasons for differences in their prognosis. In sporadic colorectal carcinomas mutation of the p53 gene is common finding in left colon tumors but less in right colon tumors10, 22; this can infer that most of colorectal cancers in our setting are sporadic.
It is known that adenomatous polyposis syndrome predispose to the tumors occurring in the left colon while right colonic tumors are commonly associated with hereditary non polyposis colorectal cancer (HNPCC)26, 27. In our setting adenomatous polyposis syndrome has rarely been reported. Environmental factors and particularly dietary are thought to play key roles in pathogenesis of colorectal cancer especially in the left colon22, 26. Change in the life style of Ugandans with introduction of fast foods could be one of the mechanisms for the pathogenesis of the left colon cancers. This could explain the increase in incidence of colorectal carcinoma which has been reported byWabinga et al28. On the other hand, hereditary non polyposis colorectal cancer syndrome produces few adenomas with carcinoma occurring mainly in the right side. In this study there were more cases of the right side tumors which could be due to a high prevalence of carcinoma of the caecum in Ugandan29. Another suggestion is that hereditary non polyposis syndrome could be involved in the pathogenesis of colorectal cancer in our setting.
Several studies have shown that p53 over expression is associated with poorer prognosis in patients with colorectal cancer19, 21, 30, 31, while others has shown that p53 expression has prognostic implication on left side tumors only with short survival11, 15, 22. With regard to grade Kapiteijn et al 200122 found that in poorly differentiated colorectal carcinomas, over expression of p53 occurred in more than 70% of tumors compared to less than 30% in those with well or moderately well differentiated tumors. In this study, p53 expression was more common in well differentiated tumors than in poorly differentiated tumors. Although the differences were not significant, the trend showed a decreased in p53 expression with worsening histological grade, suggesting a possible association. This could be due to the fact that there were more cases of well differentiated tumors in this study; this also can infer that expression of p53 in Ugandan patients is not influenced by degree of tumor differentiation, and this can be linked by poor prognosis of this cancer in Ugandan patients as five year survival rate is very low (8.3%) compared to 54.2% for Black American patients32.
Lack of p53 nuclear staining does not always rule out absence of p53 mutation. p53 staining is common in missense mutations which increase stability of the protein rendering it easily to be detected by immunohistochemisry. Mutations which produce deletion, truncation or no protein can not be detected by immunohistochemistry but only by molecular methods10, 12, 14. It is possible that in our setting as the grade of the tumor advance the mutations patterns are different and does not lead to expression of the p53 protein, this require molecular studies to confirm. One study found that patients with mutations due to deletion had advanced disease and tumor stage10, it is possible that most of poorly differentiated tumors in our area has this type of mutations. In contrast to our findings and those of Kapiteijn et al 200122, Cameiro et al33 found no relationship between p53 expression and histological differentiation. These divergent results could be due to differences in the various populations which need to be further investigated.
In this study, p53 expression was used as a marker of p53 gene mutation. Previous studies have shown that p53 expression is an approximate measure of p53 gene mutation since it can detect up to 75% of tumors with real gene mutation, while other studies have shown up to 94% agreement between p53 expression and mutation status of p53 gene by molecular studies22, 34. For routine purposes, detection of p53 mutation using immunohistochemistry is more feasible and cheaper than molecular analysis which is more expensive and difficult to apply when using archival material12.
Conclusion
p53 expression in Ugandan patients appear to be similar to those from the other parts of the world where such studies has been done, it was expressed more on the left sided colonic tumors. This could support the hypothesis that right and left colonic tumors could have different pathogenesis and reasons for different prognosis in these tumors. There is need to conduct further studies to ascertain the types of genetic mutations and also to find out if some cases could be due to epigenetic factors.
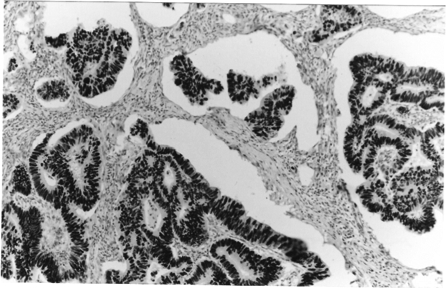
Figure 1.
Photomicrograph showing uniform nuclear staining for p53 protein in a well differentiated adenocarcinoma x100
Acknowledgement
We thank Bugando University College of Health Sciences (BUCHS) for funding the research and all the support when the work was being done. We are also grateful to Dr Lynnette Tumwine, Dr. Hawa Nalwoga, Dr. Sam Kalungi and Dr Stephen Chiwuwa for their fruitful advice, and lastly to Betty Namwase and Ms F. Otwoda for their technical work
References
- 1.Bernard W, Stewart PK, editors. World Cancer burden. Lyon: IARC Press; 2003. pp. 16–17. [Google Scholar]
- 2.Parkin DM, Ferlay J, Hamdi-Cherif H, Sitas F, Thomas JO, Wabinga H, Whelan SL. Cancer in Africa. IARC Press Lyon; 2003. pp. 277–280. [Google Scholar]
- 3.Kressner U, Inganas M, Blicstand I, Påhlam L, Glimelius B, Lindmark G. Prognostic value of p53 genetic change in colorectal cancer. J Clin Oncol. 1999;17:593–599. doi: 10.1200/JCO.1999.17.2.593. [DOI] [PubMed] [Google Scholar]
- 4.Mueller RF, Yung LD. Elements of medical genetics. 9 ed. Churchill; p. 172. [Google Scholar]
- 5.Harris Curtis C., Hollstein M. Clinical implications of the p53 tumor suppressor gene. New Eng J of Med. 1993;329:1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- 6.Fearon ER, Vogelstan B. Genetic model for colorectal Tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Dignam JJ, Ye Y, Colangelo L, Smith R, Mamouncs EP, Wie HS, Wolmark N. Prognosis after rectal cancer in blacks and whites participating in adjuvant therapy. Clin Oncol. 2003;21:413–420. doi: 10.1200/JCO.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Levine AJ, Perry ME, Chang A, Silver A, Dittmer D, Wu M, Welsh D. The role of p53 tumor suppressor genes in tumorigenesis. Br J Cancer. 1994;57:1–9. doi: 10.1038/bjc.1994.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsaleh H, Powell B, McCaul K, Grieu F, Grant Joseph D, Iacopeta B. p53 Alteration and microsatellite instability have predictive value for survival benefit from chemotherapy in stage III colorectal carcinoma. Clin Cancer Res. 2001;7:1343–1349. [PubMed] [Google Scholar]
- 10.Bazan V, Migliavacca M, Tubiolo C, et al. Have p53 gene mutations and Protein expression a different biological significance in colorectal cancer? Journal of Cellular Physiology. 2002;191:237–246. doi: 10.1002/jcp.10088. [DOI] [PubMed] [Google Scholar]
- 11.Paluszkiewicz P, Berbeæ H, Pawlowska-Wakowicz B, Cybusk M, Paszkowsca A. p53 protein accumulation in colorectal cancer tissue has prognostic value only in left sided colon tumors. Cancer detection and prevention. 2004;28:252–259. doi: 10.1016/j.cdp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Nassierowsken-Guttmejer A, Treeciale L, Nowacki MP, Ostrowsk J. p53 protein accumulation and p53 gene mutation in colorectal cancer. Path Oncol Res. 2000;6:275–279. doi: 10.1007/BF03187331. [DOI] [PubMed] [Google Scholar]
- 13.Cotran R, Kumar V, Collins T. Pathological basis of disease. 7 ed. Philadelphia: W.B and Saunders; 2004. p. 864. [Google Scholar]
- 14.Colomer A, Erill N, Verdm M, et al. Lack of p53 nuclear immunostaining is not indicative of absence of TP53 gene mutation in colorectal adenocarcinomas. Appl immunohistochemistry and Molecular Morphology. 2003;11:130–137. doi: 10.1097/00129039-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Rosati G, Chiacchio R, Reggiardo G, De Sanctis D, Manzione L. Thymidylate synthase expression, p53, bcl-2, Ki-67 amd p27 in colorectal cancer: Relationship with tumor recurrence and survival. Tumor Biol. 2004;25:258–263. doi: 10.1159/000081389. [DOI] [PubMed] [Google Scholar]
- 16.Zhau X, Yu J, Chen H, Yu H, Luo H. Expression of cellular FLICE- inhibitory protein and its association with p53 mutation in colon cancer. World Gastroenterol. 2005;11:2482–2485. doi: 10.3748/wjg.v11.i16.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jong K, Gouw Annete SH, Peeters P, et al. 53 mutation analysis of colorectal liver metastases. Relation to actual survival, angiogenic status and p53 overexpression. Clin Cancer Res. 2005;11:4067–4073. doi: 10.1158/1078-0432.CCR-04-2389. [DOI] [PubMed] [Google Scholar]
- 18.Lebe B, Sariodlu S, Sökmen S, Hülya E, Füzün M, Küpeliodlu A. The clinical significance of p53, p21, and p27 expression in rectal carcinoma. Appl Immunohistochem Mol Morphol. 2005;13:38–44. doi: 10.1097/00129039-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Bauzoorene H, Gervas P, Cerottini JP, et al. p53 and k-ras as prognostic factor for Dukes' stage B colorectal cancer. European Journal of Cancer. 2000;36:1008–1015. doi: 10.1016/s0959-8049(00)00036-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhao D, Ding X, Peng J, Zheng Y, Zhang S. Prognostic significance of bcl-2 and p53 expression in colorectal carcinoma. Journal of Zhejiang University. 2005;6:1163–1169. doi: 10.1631/jzus.2005.B1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Cheng Y, Chen M, Shyang Lin, Chou M, Lee H. Different p53 mutation patterns in colorectal tumors from smokers and nonsmokers. Environmental and Molecular mutagenesis. 2006;47:527–532. doi: 10.1002/em.20222. [DOI] [PubMed] [Google Scholar]
- 22.Kapiteiijn E, Liefers G, Los L, et al. Mechanisms of oncogenesis in colon versus rectal cancer. J Pathol. 2001;195:171–178. doi: 10.1002/path.918. [DOI] [PubMed] [Google Scholar]
- 23.Darwish H, Trejo I, Shapira I, et al. Fighting colorectal cancer: Molecular epidemiology differences among Ashkenazi and Sephardic Jews and Palestinians. Annals of Oncology. 2002;13:1497–1501. doi: 10.1093/annonc/mdf230. [DOI] [PubMed] [Google Scholar]
- 24.Terry M, Neugut A, Mansukhani M, Waye J, Harpaz N, Hibshoosh H. Tobacco, alcohol and p53 overexpression in early colorectal neoplasia. BMC Cancer. 2003;3:29. doi: 10.1186/1471-2407-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth EF, Sharma A, Sivarajasingham N, Hartley J, Monson J. Prognostic implication of hMLH1 and p53 immunohistochemical status in Right-sided colon cancer. Dis Colon Rectum. 2004;47:2086–2092. doi: 10.1007/s10350-004-0710-0. [DOI] [PubMed] [Google Scholar]
- 26.Bufill JA. Colorectal cancer: Evidence for distinct genetic categories based on proximal or distal tumor location. Ann Int Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 27.Nagorni A, Kutic V, Milanovic J, Zivkovic V, Bjelakovic G. The Lynch Syndrome: Report on family “S”. Arch Gastroenterohepatol. 2002;21:1–2. [Google Scholar]
- 28.Wabinga HR, Parkin DM, Wabwire-Maugen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda 1960–1997. Br J Cancer. 2000;83:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owor R. Carcinoma of colon and rectum in Ugandan Africans. East Afr Med J. 1983;60:228–231. [PubMed] [Google Scholar]
- 30.Zhang H. Evaluation of four antibodies in detecting p53 protein for predicting clinicopatological and prognostic significance in colorectal adenocarcinoma. Clin Cancer Res. 1999;5:4126–4132. [PubMed] [Google Scholar]
- 31.Lashner Bret A, Bauer William M, Rybicki Lisa A, Goldblum John R. Abnormal p53 immunohistochemistry is associated with an increased colorectal cancer-related mortality in patients with ulcerative colitis. Amer J of gastroenterol. 2003;98:1423–1427. doi: 10.1111/j.1572-0241.2003.07573.x. [DOI] [PubMed] [Google Scholar]
- 32.Gondos A, Brenner H, Wabinga H, Parkin DM. Cancer survival in Kampala, Uganda. Br J Cancer. 2005;92:1808–1812. doi: 10.1038/sj.bjc.6602540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameiro Fabian P, Ramalho Leandra NZ, Britto-Garcia Ribeiro-Silva A, Zucoloto S. Immunohistochemical Expression of p16, p53 and p63 in Colorectal Adenomas and Adenocarcinomas. Dis Colon Rectum. 2006;49:588–594. doi: 10.1007/s10350-006-0515-4. [DOI] [PubMed] [Google Scholar]
- 34.Servomaa K, Kosma V-M, Hirvikoski P, Rytömaa T. p53 and K-ras mutations in carcinoma of the rectum among Finish women. J Clin Pathol: Mol Pathol. 2000;53:24–30. doi: 10.1136/mp.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]