Abstract
Background
Skin appearance is a primary indicator of age. During the last decade, substantial progress has been made towards understanding underlying mechanisms of human skin aging. This understanding provides the basis for current use and new development of anti-aging treatments.
Objective
To present state of the art knowledge pertaining to mechanisms involved in skin aging, with specific focus on the dermal collagen matrix.
Results
A major feature of aged skin is fragmentation of the dermal collagen matrix. Fragmentation results from actions of specific enzymes (matrix metalloproteinases), and impairs the structural integrity of the dermis. Fibroblasts that produce and organize the collagen matrix cannot attach to fragmented collagen. Loss of attachment prevents fibroblasts from receiving mechanical information from their support and they collapse. Stretch is critical for normal balanced production of collagen and collagen-degrading enzymes. In aged skin, collapsed fibroblasts produce low levels of collagen and high levels of collagen–degrading enzymes. This imbalance advances the aging process, in a self-perpetuating, never-ending deleterious cycle. Clinically-proven anti-aging treatments such as topical retinoic acid, CO2 laser resurfacing, and intradermal injection of cross-linked hyaluronic acid stimulate production of new undamaged collagen. Attachment of fibroblasts to this new collagen allows stretch, which in turn balances collagen production/degradation and thereby slows the aging process.
Conclusion
Collagen fragmentation is responsible for loss of structural integrity and impairment of fibroblast function in aged human skin. Treatments that stimulate production of new, non-fragmented collagen should provide substantial improvement to the appearance and health of aged skin.
Introduction: Chronological and Sun-Induced Skin Aging
Skin is not only the largest human organ, it is the only organ chronically exposed to the environment and on display. The appearance of ones' skin reflects general health, and communicates ethnicity, lifestyle, and age. These features are largely determined by skin color, texture, firmness, and smoothness. Aging has a large impact on the quality of all of these features. As one ages, skin tends to become uneven in color, roughened, lax, and wrinkled1, 2.
Skin, like all organs of the body, undergoes alterations due to the passage of time. However, because skin is exposed to the environment, it is also subject to direct environmental challenge. By far, the most common source of environmental damage is solar ultraviolet (UV) irradiation 3, 4. The extent of sun damage that one incurs is directly proportional to amount of exposure, and inversely proportional to genetically predetermined amount of skin pigmentation 5. Damage caused by solar UV irradiation, the so-called photoaging, is superimposed on chronological aging.
Thus, as one ages, sun-exposed areas of skin, typically face, neck, upper chest, forearms, and hands, undergo more dramatic alterations than sun-protected areas. These changes are more pronounced in persons who are lightly pigmented. Pigmentation provides substantial protection against damaging effects of acute UV irradiation 6.
The desire of a large proportion of the world's adult population to maintain youthful skin appearance has fueled a multi-billion-dollar industry that includes cosmetics, oral or topical cosmeceuticals, topical prescription drugs, invasive and non-invasive procedures using chemicals, lasers, or abraders, injection of fillers or neurotoxin, and surgery. Increasingly, patients are seeking the help of dermatologists to treat age-related changes in the appearance of their skin. During the last decade, a great deal has been learned regarding the molecular basis of skin aging. Our research has focused on the impact of aging on metabolism, structure, and function of collagen within the dermis 7-13. This article will emphasize these collagen-based findings.
Type I Collagen Supports Skin Structure and Function
The dermis, which provides structural support for the skin's vasculature, appendages, and epidermis, is composed primarily of extracellular matrix. This extracellular matrix is composed of several types of proteins, proteoglycans, and glycosaminoglycans, which are largely produced and secreted by fibroblasts. Type I collagen is by far the most abundant protein in human skin, comprising greater than 90% of its dry weight. The unique physical properties of collagen fibers confer structural integrity to skin 14. Fibrillar collagens type I, III, and V self assemble into larger collagen fibers that form a three dimensional structural network throughout the dermis.
Collagen Network: Mechanical Tension, Stability, and Cross-Linking
The collagen network is organized and maintained by dynamic mechanical tension provided by the fibroblasts responsible for its production 15. As will be discussed below, age related reduction of mechanical tension is a critical driving force for molecular alterations that underlie the appearance of aged skin. Indeed, the physical properties of collagen fibers strongly dictate fibroblast function and vice versa 16, 17. Two inherent properties of collagen fibers appear to be largely responsible for reduced structural integrity that accompanies skin aging. These two properties include an exceedingly long half-life, estimated to be approximately 15 years 18, and intra and inter molecular cross-links that are highly resistant to breakdown 19, 20. These two features conspire to allow accumulation of partially fragmented collagen that compromises structural integrity and impairs fibroblast function in human skin. In order to understand the origin of collagen fragmentation it is necessary to briefly consider collagen metabolism.
Type I Collagen Production and Breakdown
All fibrillar collagens consist of three polypeptide chains wound around each other in a triple helical configuration. Each polypeptide chain is originally synthesized with additional amino acids at both ends that confer solubility 14. The soluble triple helix, which is termed procollagen, is assembled inside fibroblasts. Procollagen is secreted from fibroblasts, and the peptide ends are removed by two enzymes in the extracellular space 21. Removal of the ends produces collagen, which spontaneously assembles (ie, matures) into large fibers that are enzymatically cross-linked 22. This cross-linking is necessary for normal structural support 22. Type I collagen undergoes natural breakdown by enzymatic degradation; however, this degradation in human skin is exceedingly slow 18. Humans express only four enzymes that are capable of initiating breakdown of type I collagen 23.
These collagenases are members of a family of matrix protein-degrading enzymes, referred to as matrix metalloproteinases (MMPs) 24. MMPs are responsible for physiological degradation of various extracellular matrix proteins 23. Of the four collagenases that are expressed in humans, only interstitial collagenase (MMP-1) is involved in normal turnover of skin collagen 8. In healthy young skin, MMP-1 expression is exceedingly low, near the limit of detection by the most sensitive measurement methods. Once cleaved by MMP-1, collagen unravels. Unraveled collagen, called gelatin, then undergoes further degradation by other members of the MMP family, called gelatinases. These gelatinases are also expressed at very low levels in normal skin 8, 9. In addition, skin expresses natural inhibitors of these MMPs. These tissue inhibitors of matrix metalloproteinases (TIMPs) further act to retard collagen breakdown 25. Thus, type I collagen in human skin is very stable, requiring approximately 30 years on average to undergo replacement 18.
Accumulation of Fragmented Collagen and Loss of Mechanical Tension During Aging
This slow rate of type I collagen turnover allows accumulation of age-dependent modifications that impair its functions. These alterations include formation of new cross-links derived from sugars 26. Importantly, these crosslinks are not able to be efficiently broken down and removed during the slow normal process of MMP-mediated turnover, causing accumulation of fragmented collagen within the extracellular matrix as skin ages 27, 28. Cross-links prevent complete removal of collagen fragments. The fragments cannot be repaired or incorporated into newly made collagen fibrils, and therefore cause defects in the three dimensional collagen matrix. These defects impair the structural and mechanical integrity of the dermis and thereby deleteriously alter its function. Accumulation of fragmented collagen lies at the heart of age-related changes in the appearance of human skin (Figure 1).
Figure 1.
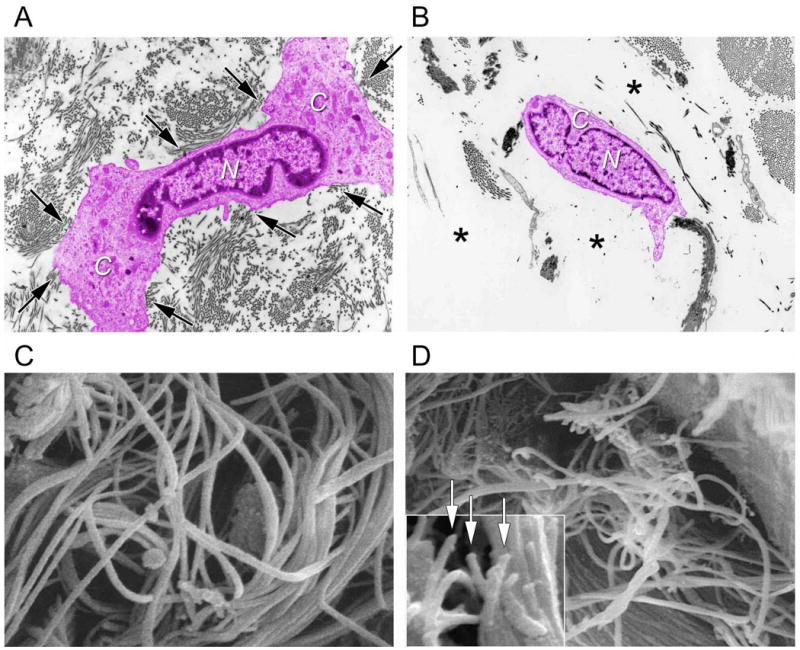
Fragmentation of collagen fibrils within dermis of aged/photoaged skin causes collapse of fibroblasts. A) Transmission electron micrograph of fibroblast (artificially colored pink for clarity) within dermis of young adult, sun-protected human skin. Note elongated, stretched appearance, and extended cytoplasm (C) away from nucleus (N) of fibroblast, which is in close proximity to abundant collagen fibrils (arrows, original magnification ×2,000). The top drawing of Figure 2 depicts a stretched fibroblast attached to intact collagen. B) Transmission electron micrograph of fibroblast (artificially colored for clarity) within dermis of photodamaged human skin. Note collapse of cytoplasm inward towards nucleus (N), and lack of adjacent collagen fibrils, compared to (A). Fibroblast is surrounded by amorphous material (*) The lower drawing of Figure 2 depicts a collapsed fibroblast surrounded by broken collgen. (C) Scanning electron micrograph of collagen fibrils in young adult human skin. Note that long fibrils are closely packed and fill space. Also note no apparent breaks in the fibrils (original magnification ×10,000). (D) Scanning electron micrograph (original magnification ×8,000) of collagen fibrils in photodamaged human skin. Note large gaps and numerous fragmented fibrils, compared to (C). Inset shows higher magnification of fragmented ends of fibrils (arrows, original magnification ×12,5000). Panels C and D are from Fligiel, S et al. Journal Investigative Dermatology, 120: 842-848, 2003.
The central role that collagen fragmentation plays in skin aging is a consequence of the physical and functional interactions between dermal fibroblasts and matrix. Fibroblasts are developmentally programmed to produce collagen matrix, which is the main structural component of connective tissue. Fibroblasts have cell surface receptors, called integrins, which specifically attach to proteins in the matrix including type I collagen. Integrins span the cell membrane, and attachment to the matrix causes them to cluster and form complexes with the actin cytoskeleton inside the cell 29. Thus integrins connect the matrix (outside the cell) to the cytoskeleton (inside the cell) to form focal adhesions. Focal adhesion complexes serve both regulatory and mechanical functions, which are inexorably connected 30.
Formation of focal adhesion complexes activates intracellular signal transduction cascades that regulate fibroblast metabolism, including the balance between production of type I collagen and its breakdown by MMPs. Focal adhesions also provide attachment sites that allow fibroblasts to spread, which is necessary for growth, survival and function 31. Attachment to the matrix allows fibroblast intracellular cytoskeletal machinery (microfilaments) to apply mechanical traction on intact collagenous matrix 32. The cytoskeletal machinery, which resides on the inner side of the fibroblast surface membrane and throughout the cytoplasm, is physically coupled to integrins, and utilizes this coupling to pull on the collagen fiber network 32, 33. Inherent structural properties of the intact collagen network in young skin offers resistance to the pull, thereby creating mechanical tension within the fibroblast and collagen matrix. The inward “pull” of the actin-myosin microfilaments promotes assembly of intracellular scaffolding (microtubules and intermediate filaments), which causes an outward “push” that mediates normal cellular stretch. Counter balancing inward “pull” and outward “push”, both originating from attachment of fibroblasts to intact collagen, establishes dynamic tension within fibroblasts and the collagenous matrix 34. This dynamic mechanical tension controls fibroblast shape (i.e., stretch), and function 15, 17, 35, 36.
Reduced Mechanical Tension Decreases Collagen Production and Increases Collagen Breakdown
Fibroblasts have evolved to regulate their synthesis of collagen and other extracellular matrix proteins in response to mechanical tension. Increased mechanical tension stretches fibroblasts, which coordinately increases collagen production and decreases collagenase production (Figure 2). This regulation of connective tissue content by mechanical tension is easily observed during development, when organ growth is accompanied by concomitant connective tissue expansion. Also, expansion of skin for certain surgical procedures relies on the ability of fibroblasts to produce more extracellular matrix in response to mechanical stretch.
Figure 2.
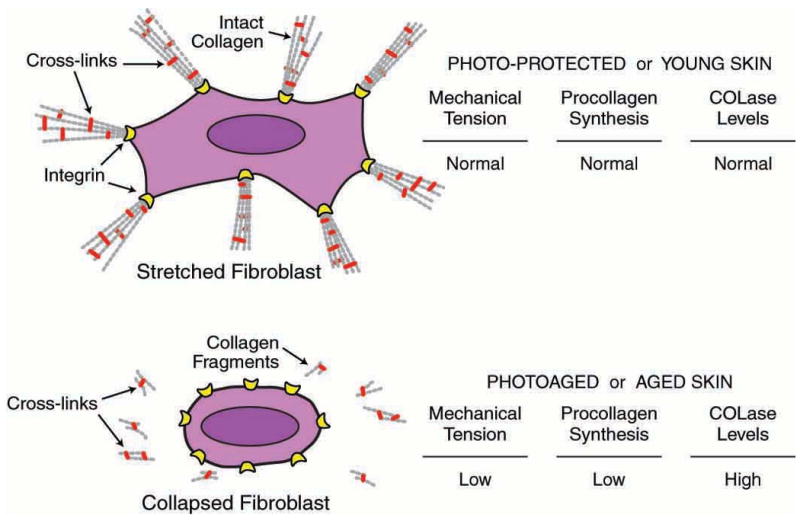
Model depicting relationship between mechanical tension, and collagen production and fragmentation in human skin. In sun-protected young adult human skin, intact type I collagen fibrils in the dermis provide mechanical stability and attachment sites for fibroblasts. Receptors (integrins) on the surface of fibroblasts attach to collagen (and other proteins in the dermal extracellular matrix). Cytoskeletal machinery (actin-myosin microfilaments, not shown) within fibroblasts pulls on the intact collagen matrix, which in turn offers mechanical resistance. Dynamic mechanical tension that is created promotes assembly of intracellular scaffolding (microtubules/intermediate filaments, not shown), which pushes outward to cause fibroblasts to stretch. This stretch is required for fibroblasts to produce normal levels of collagen and MMPs. In photodamaged/aged human skin, attachments of fibroblasts to integrins are lost, and fragmented collagen fibrils fail to provide sufficient mechanical stability to maintain normal mechanical tension. Reduced mechanical tension causes fibroblasts to collapse, and collapsed fibroblasts produce less procollagen and more collagenase (COLase). Reduced collagen production and increased collagenase-catalyzed collagen fragmentation result in further reduction of mechanical tension, thereby causing continual loss of collagen.
Unfortunately, the converse is also true. Reduced mechanical tension causes fibroblasts to collapse within the dermis, which coordinately reduces collagen production and increases production of MMPs 9, 11, 13, 37. This state of reduced mechanical tension, which causes fibroblasts to collapse, arises as a direct result of collagen fragmentation 13, 38. Collagen fragmentation causes loss of attachment sites for integrins and impairs the ability of collagen fibers to provide mechanical resistance against cytoskeletal contractile forces (i.e., “pull”, exerted by actin-myosin microfilaments) by fibroblasts (Figure 2). With loss of matrix attachment and mechanical resistance, focal adhesions fail to form and fibroblasts are unable to generate inward “pull”. In the absence of “pull”, counterbalancing scaffolding (microtubules and intermediate filaments) that exerts “push” does not assemble, and the fibroblast collapses. Thus, slow accumulation of cross-linked collagen fragments, which comes about during the aging process, compromises the structural integrity of the collagenous matrix thereby creating an environment in which fibroblasts lose mechanical tension (i.e., collapse) and thereby produce less collagen and more MMPs 12. Once initiated, this shift in balance towards net collagen degradation is self-perpetuating and never-ending leading to the thin, fragile collagen-deficient skin observed in the elderly.
Sunlight Accelerates Collagen Fragmentation
The mechanisms of collagen fragmentation and the physiological consequences on fibroblast function, described above, apply to both photoaging and chronological aging. What then, distinguishes these two processes? In simple terms, photoaging can be considered to represent accelerated chronological aging. Acceleration occurs because UV irradiation acutely induces collagen-degrading MMP activities and suppresses collagen production 8, 9, 39. Thus, UV irradiation, like chronological aging, shifts the balance towards net collagen degradation. The negative impact on collagen metabolism by UV irradiation can be quite dramatic. A moderate UV irradiation exposure, which produces mild pinkness, but not overt sunburn, increases MMP levels hundreds of times compared to non-irradiated skin 8. UV irradiation simultaneously reduces collagen production by approximately 80% 9, 39. These changes occur within 24 hours following a single acute exposure and then subside to normal during the following 48-72 hours. However, daily minimal to moderate sun exposure maintains MMP levels and suppression of collagen production throughout the course of exposure 9. Of course, UV irradiation causes a multitude of alterations in skin other than collagen degradation. Among the most prominent of these are: increased pigment formation, resulting in tanning; DNA damage, which can cause mutations leading to skin cancer; and formation of pre-vitamin D3, which leads to increased vitamin D3 synthesis that is required for bone health and may protect against various autoimmune diseases.
Aging Fibroblasts Versus Aging Collagen Matrix
Processes described above emphasize the importance of structure, composition, and organization of the collagenous matrix as a primary determinant of age-related changes resulting in the wrinkled appearance of human skin. This emphasis on cellular environment rather than on genetically inherent age-related/UV–mediated fibroblast alterations is unique. Without going into detail, it is important to point out that most of the effort attempting to understand biology of aging has focused on genetics and changes that occur within cells, rather than in their external environment 40. Research into age-dependent cellular changes has described senescence and the free radical theory of aging. Replicative senescence involves exhausting the genetically-predetermined proliferative capacity of a cell through multiple rounds of cell division 41, 42. Although senescent cells remain viable, they undergo irreversible functional alterations associated with an aged phenotype. For human skin fibroblasts, senescence results in reduced collagen and increased MMP-1 production. Although senescence is readily observable in cell culture, it has been difficult to substantiate senescence in human skin in vivo.
The free radical theory of aging posits that chemically-reactive free radicals, mainly reactive forms of molecular oxygen (reactive oxygen species-ROS) are the primary cause of aging. ROS generated as a consequence of aerobic energy metabolism, within mitochondria, oxidize cellular constituents thereby impairing cell function 43. Accumulation of oxidative damage over time results in irreversible cellular functional impairment and the aged phenotype. Interestingly, oxidative damage can also lead to functional, oxidative stress-induced senescence 44. Although a wealth of studies convincingly support the free radical theory of aging, especially in simple model organisms, the contribution of irreversible oxidative damage to aging in humans remains an area of intensive research. One way to assess the relative contribution of extracellular environment versus intra-cellular alterations is to determine functional capacities of cells that have been removed from their tissue environment.
Old Fibroblasts Have Substantial Capacity to Produce Collagen
Such studies have been conducted on fibroblasts with both photoaged and chronologically-aged human skin. Fibroblasts cultured from severely photoaged forearm skin were found to be indistinguishable from fibroblasts cultured from subject-matched sun-protected skin, with respect to collagen and MMP-1 production 11, 37, 38. In contrast, fibroblasts cultured from sun-protected skin of individuals greater than 80 years of age displayed only a modest age-related reduction in their capacity to produce collagen, compared to fibroblasts cultured from sun-protected skin of individuals under the age of 30 13. Intrinsic differences in fibroblasts could not account for the marked alterations to collagen production or MMP-1 activity observed in aged or photoaged skin. These data support the concept that fragmented collagen in the fibroblast environment is a predominant determinant of reduced collagen production in both photoaged and chronologically aged human skin fibroblasts.
Treatments for Aged Skin that Stimulate Collagen Production
The finding that fibroblasts in both photoaged and chronologically-aged human skin possess substantial capacity to produce new collagen when removed from their fragmented extracellular matrix provides a foundation for therapeutic intervention. Although there exists a multitude of treatments that claim to improve appearance of aged skin, few have been rigorously evaluated. Retinoic acid was the first topical treatment rigorously shown to improve appearance of photoaged human skin. Retinoic acid acts through well-characterized intra-cellular receptors that function to regulate gene expression 45. Through complex, and not fully understood molecular pathways, retinoic acid or its metabolic precursors retinol or retinal, are able cause deposition of new, undamaged collagen in both photoaged and chronologically aged human skin 46-48. Accumulation of new collagen can result in marked improvement in appearance of aged skin 49.
Another well-documented treatment for improving appearance of aged skin is CO2 laser resurfacing. This procedure thermally ablates epidermis (with thermal damage to superficial dermis) and thereby stimulates a robust wound healing response. The natural course of wound healing involves remodeling of dermal collagen and other matrix molecules. This remodeling involves an initial inflammatory phase, characterized by massively high levels of MMPs that degrade the fragmented collagenous matrix, followed by substantial and extended production of new undamaged collagen 50. CO2 laser resurfacing regenerates both the epidermis and dermis thereby improving both appearance and health of aged skin 51.
Less invasive laser procedures, employing different types of lasers, have recently been developed. However, these procedures appear to have minimal ability to remodel the dermal extracellular matrix 52. Thus, it remains questionable whether these minimally invasive laser procedures can significantly improve the appearance of aged skin. Future research may show that to be effective a laser must cause at least sufficient damage to induce MMPs and collagen to remodel the dermis.
Hyaluronic Acid Dermal Filler Stretches Fibroblasts and Stimulates Collagen Production
Finally, dermal fillers have gained recent popularity. These materials are injected into the dermis underlying surface irregularities to stretch skin into a smoother appearance. Until recently, cross-linked collagen has been the most commonly used filler material, but cross-linked hyaluronic acid is now preferred, primarily because it does not require allergy testing. Fillers by their volume actually stretch the dermis. As described above, fibroblasts respond to stretch by producing more collagen and less MMPs, prompting the hypothesis that the benefit from fillers may partially result from deposition of new collagen.
This hypothesis was recently examined in photoaged forearm skin following injection of cross-linked hyaluronic acid 53. Interestingly, collagen production was substantially increased at sites of filler injection, compared to vehicle. Increased collagen production was observed within one month of cross-linked hyaluronic acid injection and remained elevated for at least three months. Numerous fibroblasts were observed surrounding sites of hyaluronic acid deposition within the dermis (Figure 3). These fibroblasts had a distinct elongated stretched appearance, and expressed high levels of type I procollagen, as revealed by immunohistology. Increased collagen production by these stretched fibroblasts was associated with increased levels of signaling molecules, transforming growth factor-beta, and connective tissue growth factor, which are known to stimulate collagen production. Similar results have been obtained in the sun-protected skin of elderly (greater than 80 years old) individuals (unpublished data).
Figure 3.
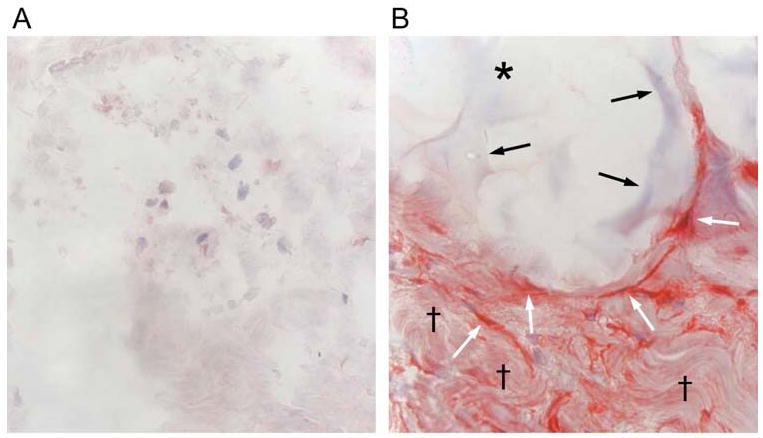
Mechanical stretch induced by dermal injection of cross-linked hyaluronic acid filler stimulates collagen production in photodamaged human skin. (A) Saline vehicle (control) or (B) cross-linked hyaluronic acid filler (CLHA) was injected into photodamaged forearm skin. Skin biopsies were obtained four weeks post injection and analyzed for type I procollagen expression by immunohistochemistry. (A) Fibroblasts (nuclei stained blue) in saline-injected skin display barely-detectable procollagen expression (red stain). Also note amorphous space and fragmented thin appearance of collagen extracellular matrix (original magnification ×400). (B) In CLHA–injected skin, fibroblasts display intense type I procollagen immunostaining (red). CLHA appears as light blue material (black arrows) that occupies space adjacent to stretched fibroblasts (white arrows). Note also densely packed collagen fibrils (black daggers), not seen in saline-injected skin (A). These dense collagen fibrils are likely derived from CLHA-induced type I procollagen (i.e., conversion of type I procollagen into type I collagen) (original magnification ×400).
These results are important for at least two reasons: 1) they provide direct evidence that application of mechanical tension to the dermal extracellular matrix stimulates collagen production in vivo, and 2) they provide in vivo confirmation of the in vitro finding that fibroblasts in photoaged and chronologically-aged skin have substantial capacity to produce new collagen when removed from their in vivo damaged collagen matrix.
Summary
Taken together, the information presented above support the concept that collagen deficit in both photoaged and chronologically-aged human skin derives primarily from altered mechanical properties of the fragmented collagenous extracellular matrix of dermis, rather than from time and/or UV irradiation-derived genetic damage to fibroblasts themselves. Understanding molecular mechanisms by which mechanical tension regulates fibroblasts function, and exploiting this knowledge to maintain optimal levels of collagen production throughout an individual's lifetime provide exciting opportunities for future treatments to improve the health and appearance of skin.
Acknowledgments
Ms Laura VanGoor for graphics.
Funding Sources: National Institutes of Health (AG019364 [GJF] and AG025186 [GJF])
Footnotes
Conflict of Interest: Regents of the University of Michigan hold patents regarding the use of matrix metalloproteinase inhibitors for treatment and prevention of skin aging.
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study Concept and design: Fisher, Varani, Voorhees. Acquisition of data: Fisher, Varani, Voorhees. Analysis of and interpretation of data: Fisher, Varani, Voorhees. Drafting of the manuscript: Fisher. Critical revision of the manuscript for important intellectual content: Fisher, Varani, Voorhees. Administrative, technical, and material support: Fisher, Varani, Voorhees
References
- 1.Kligman AM. Early destructive effects of sunlight on human skin. JAMA. 1969;210:2377–2380. [PubMed] [Google Scholar]
- 2.Yaar M, Eller M, Gilchrest B. Fifty years of skin aging. J Invest Dermatol Sympos Proc. 2002;7:51–58. doi: 10.1046/j.1523-1747.2002.19636.x. [DOI] [PubMed] [Google Scholar]
- 3.Lavker R. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol. 1979;73:559–566. doi: 10.1111/1523-1747.ep12532763. [DOI] [PubMed] [Google Scholar]
- 4.Scharffetter-Kochanek K. Skin aging. Clin Exp Dermatol. 2001;26(7):561. doi: 10.1046/j.1365-2230.2001.00906.x. [DOI] [PubMed] [Google Scholar]
- 5.Miyamura Y, Coelho SG, Wolber R, Miller SA, Wakamatsu K, Zmudzka BZ, Ito S, Smuda C, Passeron T, Choi W, Batzer J, Yamaguchi Y, Beer JZ, Hearing VJ. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20(1):2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 6.Kollias N, Sayre RM, Zeise L, Chedekel MR. Photoprotection by melanin. J Photochem Photobiol B. 1991;9(2):135–160. doi: 10.1016/1011-1344(91)80147-a. [DOI] [PubMed] [Google Scholar]
- 7.Talwar HS, Griffiths CEM, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- 8.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 9.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Eng J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 10.Fisher G, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees J. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 11.Fligiel S, Varani J, Datta S, Kang S, Fisher G, Voorhees J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- 12.Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 13.Varani J, Dame M, Rittie L, Fligiel S, Kang S, Fisher G, Voorhees J. Decreased collagen production in chronologically aged skin. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uitto J. Collagen. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedbrg IM, Austen KF, editors. Dermatology in General Medicine. New York: McGraw-Hill; 1993. pp. 299–314. [Google Scholar]
- 15.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13(5):264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 16.Grinnell F. Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10(9):362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- 17.Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol. 2003;9(1):3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- 18.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275(50):39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 19.Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16(7):387–398. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- 20.Reiser KM, Last JA. Biosynthesis of collagen crosslinks: in vivo labelling of neonatal skin, tendon, and bone in rats. Connect Tissue Res. 1986;14(4):293–306. doi: 10.3109/03008208609017472. [DOI] [PubMed] [Google Scholar]
- 21.Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- 22.Siegel RC. Lysyl oxidase. Int Rev Connect Tissue Res. 1979;8:73–118. doi: 10.1016/b978-0-12-363708-6.50009-6. [DOI] [PubMed] [Google Scholar]
- 23.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapiere Ch M. Tadpole collagenase, the single parent of such a large family. Biochimie. 2005;87(3-4):243–247. doi: 10.1016/j.biochi.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Gomez D, Alonso H, Yoshiji H, Thorgeirsson U. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–112. [PubMed] [Google Scholar]
- 26.Monnier VM, Mustata GT, Biemel KL, Reihl O, Lederer MO, Zhenyu D, Sell DR. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: an update on “a puzzle nearing resolution”. Ann N Y Acad Sci. 2005;1043:533–544. doi: 10.1196/annals.1333.061. [DOI] [PubMed] [Google Scholar]
- 27.Rittie L, Berton A, Monboisse JC, Hornebeck W, Gillery P. Decreased contraction of glycated collagen lattices coincides with impaired matrix metalloproteinase production. Biochem Biophys Res Commun. 1999;264(2):488–492. doi: 10.1006/bbrc.1999.1519. [DOI] [PubMed] [Google Scholar]
- 28.Vater C, Harris E, Siegel R. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J. 1979;181:639–645. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brakebusch C, Fassler R. The integrin-action connection, an eternal love affair. EMBO J. 2003;22:2324–2333. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langevin H. Connective tissue: A body-wide signaling network? Med Hypoth. 2005;66:1074–1077. doi: 10.1016/j.mehy.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Rusoslathi E. Stretching is good for a cell. Science. 1997;276:1345–1346. doi: 10.1126/science.276.5317.1345. [DOI] [PubMed] [Google Scholar]
- 32.Lambert Ch A, Nusgens BV, Lapiere Ch M. Mechano-sensing and mechano-reaction of soft connective tissue cells. Adv Space Res. 1998;21(8-9):1081–1091. doi: 10.1016/s0273-1177(98)00031-3. [DOI] [PubMed] [Google Scholar]
- 33.Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19(1):43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Ingber DE. The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst. 2004;1(1):53–68. [PubMed] [Google Scholar]
- 35.Lapiere CM. The ageing dermis: the main cause for the appearance of “old skin”. Brit J Dermatol. 1990;122(35):5–11. doi: 10.1111/j.1365-2133.1990.tb16119.x. [DOI] [PubMed] [Google Scholar]
- 36.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20(7):811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 37.Varani J, Spearman D, Perone P, Fligiel S, Datta S, Wang Z, Shao Y, Kang S, Fisher G, Voorhees J. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Amer J Pathol. 2001;158:931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varani J, Schuger L, Dame M, Leonard C, Fligiel S, Kang S, Fisher G, Voorhees J. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photoaged skin. J Invest Dermatol. 2004;122:1471–1479. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- 39.Fisher G, Datta S, Wang Z, Li X, Quan T, Chung J, Kang S, Voorhees J. c-Jun dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoid acid. J Clin Invest. 2000;106:661–668. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40(12):1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- 41.Hornsby PJ. Short telomeres: cause or consequence of aging? Aging Cell. 2006;5(6):577–578. doi: 10.1111/j.1474-9726.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 42.Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40(6):466–472. doi: 10.1016/j.exger.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Harman D. Free radical theory of aging. Mutat Res. 1992;275(3-6):257–266. doi: 10.1016/0921-8734(92)90030-s. [DOI] [PubMed] [Google Scholar]
- 44.Passos JF, Von Zglinicki T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res. 2006;40(12):1277–1283. doi: 10.1080/10715760600917151. [DOI] [PubMed] [Google Scholar]
- 45.Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. Faseb J. 1996;10(9):1002–1013. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 46.Griffiths CEM, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) New Eng J Med. 1993;329:530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- 47.Cho S, Lowe L, Hamilton TA, Fisher GJ, Voorhees JJ, Kang S. Long-term treatment of photoaged human skin with topical retinoic acid improves epidermal cell atypia and thickens the collagen band in papillary dermis. J Am Acad Dermatol. 2005;53(5):769–774. doi: 10.1016/j.jaad.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 48.Kafi R, Kwak HSR, Schumacher WE, Cho S, Hanft VN, Hamilton TA, King AL, Neal JD, Varani J, Fisher GJ, Voorhees JJ, Kang S. Improvement of Naturally Aged Skin With Vitamin A (Retinol) Arch Dermatol. 2007;143:606–612. doi: 10.1001/archderm.143.5.606. [DOI] [PubMed] [Google Scholar]
- 49.Ellis CN, Weiss JS, Hamilton TA, Headington JT, Zelickson AS, Voorhees JJ. Sustained improvement with prolonged topical tretinoin (retinoic acid) for photoaged skin. J Am Acad Dermatol. 1990;23(4 Pt 1):629–637. doi: 10.1016/0190-9622(90)70265-j. [DOI] [PubMed] [Google Scholar]
- 50.Orringer JS, Kang S, Johnson TM, Karimipour DJ, Hamilton T, Hammerberg C, Voorhees JJ, Fisher GJ. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch Dermatol. 2004;140(11):1326–1332. doi: 10.1001/archderm.140.11.1326. [DOI] [PubMed] [Google Scholar]
- 51.Orringer JS, Johnson TM, Kang S, Karimipour DJ, Hammerberg C, Hamilton T, Voorhees JJ, Fisher GJ. Effect of carbon dioxide laser resurfacing on epidermal p53 immunostaining in photodamaged skin. Arch Dermatol. 2004;140(9):1073–1077. doi: 10.1001/archderm.140.9.1073. [DOI] [PubMed] [Google Scholar]
- 52.Orringer JS, Voorhees JJ, Hamilton T, Hammerberg C, Kang S, Johnson TM, Karimipour DJ, Fisher G. Dermal matrix remodeling after nonablative laser therapy. J Am Acad Dermatol. 2005;53(5):775–782. doi: 10.1016/j.jaad.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 53.Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, Voorhees JJ. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143(2):155–163. doi: 10.1001/archderm.143.2.155. [DOI] [PubMed] [Google Scholar]


