Abstract
Background and Aims
Micronutrient malnutrition, particularly zinc and iron deficiency, afflicts over three billion people worldwide due to low dietary intake. In the current study, wild emmer wheat (Triticum turgidum ssp. dicoccoides), the progenitor of domesticated wheat, was tested for (1) genetic diversity in grain nutrient concentrations, (2) associations among grain nutrients and their relationships with plant productivity, and (3) the association of grain nutrients with the eco-geographical origin of wild emmer accessions.
Methods
A total of 154 genotypes, including wild emmer accessions from across the Near Eastern Fertile Crescent and diverse wheat cultivars, were characterized in this 2-year field study for grain protein, micronutrient (zinc, iron, copper and manganese) and macronutrient (calcium, magnesium, potassium, phosphorus and sulphur) concentrations.
Key Results
Wide genetic diversity was found among the wild emmer accessions for all grain nutrients. The concentrations of grain zinc, iron and protein in wild accessions were about two-fold greater than in the domesticated genotypes. Concentrations of these compounds were positively correlated with one another, with no clear association with plant productivity, suggesting that all three nutrients can be improved concurrently with no yield penalty. A subset of 12 populations revealed significant genetic variation between and within populations for all minerals. Association between soil characteristics at the site of collection and grain nutrient concentrations showed negative associations between soil clay content and grain protein and between soil-extractable zinc and grain zinc, the latter suggesting that the greatest potential for grain nutrient minerals lies in populations from micronutrient-deficient soils.
Conclusions
Wild emmer wheat germplasm offers unique opportunities to exploit favourable alleles for grain nutrient properties that were excluded from the domesticated wheat gene pool.
Keywords: grain quality, iron, macronutrient, micronutrient, protein, Triticum turgidum ssp. dicoccoides, wheat improvement, zinc
INTRODUCTION
Mineral elements play essential roles in the biochemical and physiological functions of any biological system. In plants, appropriate mineral availability is essential for almost every aspect of development, from seed germination and seedling development (Welch, 1999) to yield formation and mineral deposition in grains (Yilmaz et al., 1998; Welch, 1999). Mineral elements are also essential nutrients for animal and human well-being. It is estimated that over three billion people suffer from micronutrient malnutrition worldwide (Bouis, 2003; Welch and Graham, 2004; White and Broadley, 2009), resulting in overall poor health, anaemia, increased morbidity and mortality rates, and low worker productivity (Holtz and Brown, 2004; Sanchez and Swaminathan, 2005; Cakmak, 2008). Currently, mineral malnutrition is considered to be among the most serious global challenges for humans (Copenhagen Consensus 2004; http://www.copenhagenconsensus.com). The availability of sufficient amounts of mineral nutrients in the human diet depends primarily on their composition in higher plants (Grusak and Cakmak, 2005; Cakmak et al., 2010; Sands et al., 2009), particularly on mineral nutrient concentration in staple food crops such as cereal grains. Therefore, enhancement of grain nutrients (biofortification), either agronomically (application of mineral fertilizers) or genetically (breeding), is considered the most promising and cost-effective approach to alleviating malnutrition and related health problems (Welch and Graham, 2004; Bouis, 2007; Cakmak, 2008; Peleg et al., 2009). This solution, however, requires a comprehensive exploration of potential genetic resources and an in-depth understanding of the physiological and genetic basis of nutrient-accumulation processes in grains.
Wheat (Triticum spp.) is the major staple food crop in many parts of the world in terms of cultivated area and food source, contributing 28 % of the world's edible dry matter and up to 60 % of the daily calorie intake in several developing countries (FAOSTAT 2008; http://faostat.fao.org). Therefore, the nutritional quality of wheat grains has a significant impact on human health and well-being worldwide. Although global cereal grain yields have increased dramatically since the Green Revolution (i.e. breeding for semi-dwarf cultivars), a cereal-based diet falls short in providing sufficient nutrients (Welch and Graham, 2004); most agricultural systems in the developing world do not provide enough nutrients from grain for a balanced human diet (Graham et al., 2001; Cakmak et al., 2010). Biofortification of wheat grain through genetic strategies is a powerful approach for changing the nutrient balance in the human diet on a large scale. The genetic diversity of crop plants has been significantly eroded by domestication (i.e. founder effect) and breeding processes (Tanksley and McCouch, 1997; Ladizinsky, 1998). Therefore, a major objective of modern breeding is to identify valuable alleles which have been ‘left behind’ in the wild ancestors of crop plants and to reintroduce them into cultivated crops (Aaronsohn, 1910; Tanksley and McCouch, 1997).
Wild emmer wheat (Triticum turgidum ssp. dicoccoides) is the allo-tetraploid (2n = 4x = 28; genome BBAA) progenitor of cultivated wheat. It is fully compatible with the tetraploid (BBAA) durum wheat and can be crossed with the hexaploid (2n = 6x = 42; BBAADD) bread wheat (Triticum aestivum) (Feldman, 2001). Native stands of wild emmer are distributed throughout the Near Eastern Fertile Crescent, primarily in sporadic and semi-isolated populations (Harlan and Zohary, 1966). Hence, the wild emmer gene pool offers a rich allelic repertoire for the improvement of numerous economically important traits (e.g. Feldman and Sears, 1981; Nevo et al., 2002; Peleg et al., 2005 and references therein). Several studies have reported the existence of substantial variation in grain protein and micronutrients in wild emmer wheat (Avivi, 1979; Cakmak et al., 2004; Peleg et al., 2008a). The current study reports on (1) the genetic diversity for grain protein and mineral nutrient concentrations in wild emmer wheat germplasm and its stability across years, (2) associations among grain nutrients and their relationships with plant productivity, and (3) associations between eco-geographical origin of wild emmer accessions and their grain nutrient concentrations.
MATERIALS AND METHODS
Plant material and growing conditions
A total of 154 genotypes, including wild emmer (Triticum turgidum ssp. dicoccoides (Körn.) Thell.) accessions from across the Near Eastern Fertile Crescent and diverse wheat cultivars (Supplementary Data Table S1, available online), were characterized in this 2-year field study for grain protein and mineral nutrient concentrations. In the 2006/7 (hereafter 2007) experiment, 128 wild emmer accessions, seven durum wheat and ten bread wheat cultivars were studied (145 genotypes in total). In the 2007/8 (hereafter 2008) experiment, 73 wild emmer accessions, five durum wheat and four bread wheat cultivars, representing a wide range of grain mineral concentrations, were re-examined and supplemented with nine wild emmer accessions to support population analyses (91 genotypes in total). Both trials were conducted at the experimental farm of The Hebrew University of Jerusalem in Rehovot, Israel (34°47′N, 31°54′E; 54 m asl). The soil at this location is brown-red degrading sandy loam (Rhodoxeralf) comprising 83 % sand, 3 % silt and 14 % clay. Seeds were disinfected (in 3·6 % sodium hypochloric acid for 10 min) and placed for vernalization on a moist germination paper for 5 weeks in a cold room (4 °C) in the dark, followed by 3 d of acclimation at room temperature. Seedlings were then transplanted into an insect-proof screen-house in mid to late December. A randomized block design, with five or six replicates (2007 and 2008, respectively), was employed. Each plot consisted of five plants planted 8 cm apart in a 40-cm-long single row. The two plants at the edges of each plot served as borders, and the other three were harvested. Plants were treated routinely with pesticides to prevent the development of pathogens or insect pests and weeded manually once a week.
Phenotypic measurements
Wild emmer wheat has a brittle rachis. To minimize seed dispersal, in 2007, harvest was conducted when 50 % of the plants in an individual plot had reached maturity (dry peduncle and awns), whereas in 2008, each spike was individually harvested at maturity. The harvested spikes were oven-dried (35 °C for 48 h) and weighed to obtain spike dry matter (DM). As wild emmer wheat is difficult to thresh, spike DM [which is highly correlated with grain yield (r > 0·93; Z. Peleg, unpubl. res.)] was used as an indicator of yield potential. The vegetative tissues were harvested, oven-dried (80 °C for 48 h) and used to calculate total DM (vegetative DM + spike DM). A sub-sample of the harvested spikes from each plot (approx. 20 g) was threshed manually by rubbing between two rough rubber surfaces and the grain was subjected to mineral analyses. Nitrogen concentration in the grain was determined by using a C/N analyser (TruSpec CN, Leco Co., St Joseph, MI, USA). Grain nitrogen concentration was multiplied by 5·83 to obtain grain protein concentration (GPC) (Merrill and Watt, 1973). Grain macronutrients (calcium, Ca; magnesium, Mg; potassium, K; phosphorus, P; sulphur, S) and micronutrients (zinc, Zn; iron, Fe; copper, Cu; manganese, Mn) concentrations were determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES; Vista-Pro Axial; Varian Pty Ltd, Canberra, Australia), after digesting the samples in a closed microwave system. Measurements of mineral nutrients were checked by using the certified values of the related minerals in reference leaf and grain samples obtained from the National Institute of Standards and Technology (NIST; Gaithersburg, MD, USA).
Soil samples were collected from 0–30 cm depth in 12 habitats of Israeli wild emmer populations and analysed at the Ministry of Agriculture laboratory in Hadera, Israel (Supplementary Data Table S2, available online). Three samples from each site were analysed for soil composition (percentage of clay, silt, sand and CaCO3), chemical traits (pH, EC), and concentrations of nitrogen (NH4, NO3), micronutrients (Zn, Fe, Mn, Cu, Cl) and macronutrients (P, K, Ca, Mg).
Statistical analysis
The JMP® ver. 7·0 statistical package (SAS Institute, Cary, NC, USA) was used for statistical analyses. All phenotypic variables were tested for normal distribution. Correlation analyses were used to assess the association among the various grain mineral concentrations in both experiments. Analysis of variance was employed to test the genetic diversity between wild emmer geographical groups, using a nested block design model, with geographical group and accession (nested within group) as fixed effects and blocks as a random effect. Student's t-test was used to compare means of geographical groups at a probability level of 5 %. Analysis of variance was employed to test the genetic diversity among and within populations, using a nested block design model, with population and accession (nested within population) as fixed effects and blocks as a random effect. LSMeans Tukey test was used to compare population means at a probability level of 5 %. Principal component analysis (PCA) was used to determine the associations among the grain protein and nine mineral nutrient concentrations. PCA was based on a correlation matrix and was presented as biplot ordinations of populations (PC scores). Two components were extracted using Eigenvalues > 1 to ensure meaningful implementation of the data by each factor.
RESULTS
Genetic diversity for grain nutrients and its stability across years
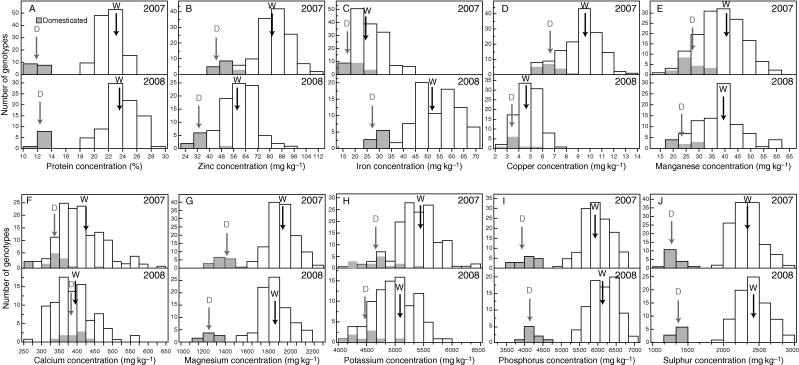
The genetic diversity for grain protein and mineral nutrient concentrations was studied across two years in a large collection of wild emmer wheat accessions and a set of durum wheat and bread wheat cultivars. Frequency distributions of grain nutrients, with domesticated wheat indicated in grey, are presented in Fig. 1 (for detailed data see Table S1, available online). The wild emmer accessions exhibited normal distributions for most variables in both years, with the exception of S, Ca and Fe, which did not distribute normally in 2007, and Ca and Mn, which showed nearly significant normal distributions in 2008.
Fig. 1.
Frequency distribution of 145 wheat accessions in the 2007 experiment and 91 accessions in the 2008 experiment, for grain concentrations of the following nutrients: (A) protein, (B) zinc, (C) iron, (D) copper, (E) manganese, (F) calcium, (G) magnesium, (H) potassium, (I) phosphorus and (J) sulphur. The domesticated wheat lines are marked in grey. Arrows indicate mean values of wild emmer (W) and domesticated (D) wheat.
A comparison between the wild emmer accessions and the domesticated lines by Student's t-test revealed significantly greater values for the wild accessions for all minerals in both years (data not shown), with the exception of Ca in 2008. For example, the average values of GPC were 12·0 and 22·2 % in 2007 and 12·5 and 23·9 % in 2008 for the domesticated and wild genotypes, respectively (Fig. 1A). The highest values of grain protein, Zn and Fe concentration in the wild accessions were about two-fold greater than the highest values in the domesticated genotypes (Fig. 1A–C).
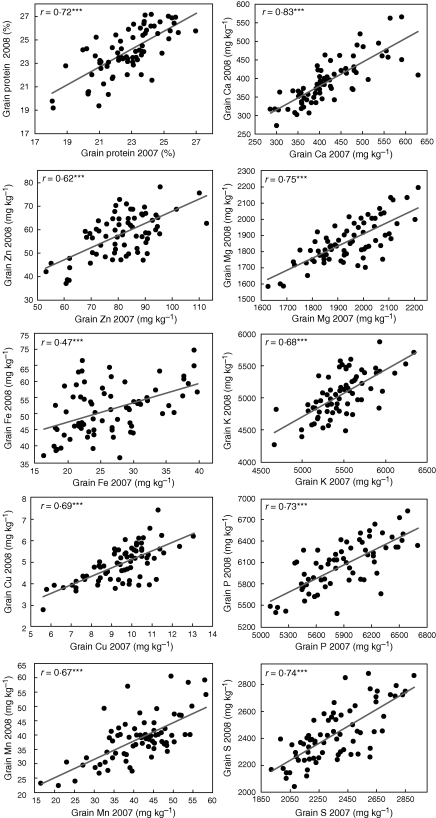
Grain protein and macronutrients (Ca, Mg, K, P and S) as well as one micronutrient (Mn) distributed across similar ranges in both years, whereas most micronutrient (Zn, Fe and Cu) concentrations showed different ranges in each year (Fig. 1). Regardless of the range of distribution, the 73 wild emmer accessions tested in both experiments exhibited a significantly positive correlation (P ≤ 0·001) between years for each of the grain nutrients tested (Fig. 2), with correlation coefficients being 0·62 ≤ r ≤ 0·83, with the exception of Fe (r = 0·47).
Fig. 2.
Correlations between grain nutrient concentrations in the 2007 vs. the 2008 experiments.
Associations among grain nutrients and productivity traits
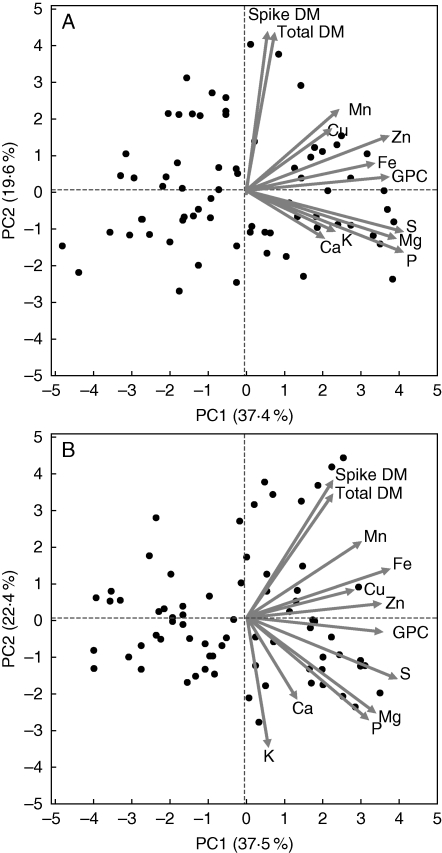
As grain nutrient concentrations of the domesticated wheat cultivars were significantly lower than those of the wild emmer accessions, only the latter were used to study the association between grain nutrients. PCA of the 73 wild emmer accessions, tested across the 2 years, extracted two major principal components (Eigenvalues > 1), which collectively accounted for 57·0 and 59·9 % of the variation for 2007 and 2008, respectively (Fig. 3). In 2007 (Fig. 3A), principal component 1 (PC1, x-axis) explained 37·4 % of the dataset variation, and was loaded positively with GPC, Zn, Fe, P, Mg and S; principal component 2 (PC2, y-axis) explained 19·6 % of the variation, and was positively loaded with spike DM and total DM. In 2008 (Fig. 3B), PC1 explained 37·5 % of the dataset variation, and was loaded positively with GPC, Zn, Fe, P, Mg, S and Mn; PC2 explained 22·4 % of the variation, and was positively loaded with spike DM and total DM and negatively loaded with Mg, K, P and Ca.
Fig. 3.
Principal components analysis (based on correlation matrix) of continuous plant traits recorded on 73 wild emmer wheat accessions in (A) 2007 and (B) 2008 field experiments. Biplot vectors are trait factor loadings for principal component (PC) 1 and PC2.
A closer look at the PCAs (Fig. 3) shows that in both years, there were two major clusters of minerals: one consisting of the micronutrients (Zn, Fe, Cu and Mn) and the other consisting of the macronutrients (Ca, Mg, K, P and S), with the GPC located between the two groups, somewhat towards the micronutrients. This was further supported by significant positive correlations among all the micronutrients (except Cu and Fe in 2007), and among all macronutrients (except K and Ca in 2007), but fewer significant correlations between macro- and micronutrients, usually involving Zn and Fe (Table 1). Also in line with the PCA, GPC exhibited significant positive correlations with all micronutrients, but only with three (Mg, P, and S) of the five macronutrients.
Table 1.
Coefficients of correlation (r) between plant productivity (spike DM and total DM per plant) and concentrations of grain protein (GPC) and mineral nutrients in a collection of 73 wild emmer wheat accessions tested across two years
| Spike DM | Total DM | GPC | Zn | Fe | Cu | Mn | Ca | Mg | K | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total DM | 0.94*** | ||||||||||
| 0.96*** | |||||||||||
| GPC | 0·09 | 0·09 | |||||||||
| 0·28* | 0·23 | ||||||||||
| Zn | 0·28* | 0·31** | 0·41*** | ||||||||
| 0·25* | 0·29* | 0·41*** | |||||||||
| Fe | 0·16 | 0·12 | 0·38** | 0·67*** | |||||||
| 0·55*** | 0·49*** | 0·61*** | 0·40*** | ||||||||
| Cu | 0·14 | 0·19 | 0·50*** | 0·42*** | 0·08 | ||||||
| 0·13 | 0·13 | 0·29* | 0·59*** | 0·43*** | |||||||
| Mn | 0·24* | 0·30** | 0·45*** | 0·45*** | 0·37** | 0·44*** | |||||
| 0·44*** | 0·46*** | 0·45*** | 0·52*** | 0·52*** | 0·35** | ||||||
| Ca | −0·16 | −0·05 | −0·01 | 0·36** | 0·29* | 0·05 | 0·14 | ||||
| −0·05 | −0·05 | 0·07 | 0·13 | 0·09 | 0·06 | −0·09 | |||||
| Mg | −0·07 | −0·05 | 0·59*** | 0·35** | 0·37** | 0·25* | 0·27* | 0·30** | |||
| −0·01 | 0·00 | 0·65*** | 0·25* | 0·40*** | 0·16 | 0·17 | 0·39*** | ||||
| K | 0·00 | −0·01 | 0·19 | 0·27* | 0·34** | 0·04 | 0·09 | 0·22 | 0·45*** | ||
| −0·23 | −0·24* | 0·05 | −0·08 | −0·07 | −0·12 | −0·16 | 0·24* | 0·34** | |||
| P | −0·10 | −0·09 | 0·54*** | 0·43*** | 0·44*** | 0·13 | 0·23 | 0·33** | 0·80*** | 0·58*** | |
| 0·00 | 0·02 | 0·50*** | 0·29* | 0·33** | 0·17 | 0·24* | 0·39*** | 0·78*** | 0·60*** | ||
| S | −0·01 | 0·00 | 0·66*** | 0·45*** | 0·41*** | 0·19 | 0·17 | 0·41*** | 0·74*** | 0·33** | 0·75*** |
| 0·17 | 0·20 | 0·64*** | 0·46*** | 0·49*** | 0·36** | 0·30** | 0·40*** | 0·66*** | 0·26* | 0·67*** |
The upper and lower numbers refer to the 2007 and 2008 experiments, respectively.
Asterisks indicate significance at *P < 0·05, **P < 0·01 and ***P < 0·001.
Another interesting aspect is the association between grain nutrients and plant productivity. It is of interest that spike DM and total DM (indicators of the plant's reproductive and overall productivity, respectively) did not show any significant negative correlation with grain nutrient constituents, with only one exception: K vs. total DM in 2008 (Table 1). Moreover, plant productivity exhibited positive and significant correlations with grain Zn and Mn (consistent across the two years), as well as with Fe in 2008.
Association between eco-geographical origin and grain nutrients
To investigate the relationships between the origin of the wild emmer accessions and their grain protein and mineral concentrations, a collection of 128 wild emmer wheat accessions, tested in 2007, was divided into two geographical groups as suggested by Luo et al. (2007). These included the southern Fertile Crescent (Israel, Jordan, Lebanon and Syria) with 101 accessions and the northern Fertile Crescent (Turkey, Iran and Iraq) with 27 accessions. Analysis of variance revealed significant effects of geographical group and accessions (nested within geographical group) for GPC, Zn and Fe (Table 2), as well as for most other mineral nutrients (data not shown). Grain Zn and Fe showed significantly higher values for the southern group, whereas GPC was higher for the northern group (Table 2). Nevertheless, for all mineral nutrients, differences between groups were of small magnitude (≤5 %).
Table 2.
Analysis of variance for the effect of geographical origin in the Fertile Crescent and accessions (nested within geographical group) on concentrations of grain protein (GPC), zinc (Zn) and iron (Fe) in 128 wild emmer wheat accessions grown in the 2007 experiment
| Geographical group | GPC (%) | Zn (mg kg−1) | Fe (mg kg−1) | |
|---|---|---|---|---|
| North | 22·6 a | 76·5 b | 24·8 b | |
| South | 22·1 b | 80·4 a | 25·9 a | |
| Source of variance† | d.f. | Sum of squares | ||
| Geographical group (αi) | 1 | 19·1*** | 1,194*** | 99·8** |
| Accessions (group) (Lj(αi)) | 126 | 1,412*** | 46,087*** | 13,516*** |
| Block (βk) | 4 | 32·8*** | 530·4* | 154·2* |
| Experimental error | 353 | 359 | 361 |
The accessions are divided into two geographical groups: South (Israel, Jordan, Lebanon and Syria) and North (Turkey, Iran and Iraq).
Within each trait, means of geographical groups followed by different letters are significantly different (P < 0·05; Student's t-test).
Asterisks indicate significance at *P ≤ 0·05, **P ≤ 0·01 or ***P ≤ 0·001.
† Tested model: Yijk = μ+ αi + Lj(αi) + βk + eijk.
Dissecting the overall genetic diversity into its components (between and within populations) is of critical importance when devising an optimal strategy for further exploration of potential genetic resources. The genetic diversity between and within populations was examined using ten and 12 (2007 and 2008, respectively) wild emmer populations from Israel. Analysis of variance revealed highly significant (P ≤ 0·001) effects of populations and accessions (nested within populations) for grain protein, Zn and Fe concentrations (Table 3), as well as for most other mineral nutrients (data not shown).
Table 3.
Analysis of variance for the effect of population and accessions (nested within population) on concentrations of grain protein (GPC), zinc (Zn) and iron (Fe) in populations of wild emmer wheat from Israel, grown in the 2007 and 2008 experiments
| GPC (%) |
Zn (mg kg−1) |
Fe (mg kg−1) |
|||||
|---|---|---|---|---|---|---|---|
| Population | 2007 | 2008 | 2007 | 2008 | 2007 | 2008 | |
| Ammiad | 22·10 c | 24·41 ab | 84·43 b | 62·96 abcd | 23·35 c | 56·85 abc | |
| Beit-Oren | 22·67 bc | 23·58 bc | 70·06 c | 57·84 bcde | 18·65 d | 49·95 cde | |
| Givat Koach | 24·43 a | 25·35 a | 84·26 b | 64·55 abc | 29·22 ab | 53·91 bcd | |
| Kokhav Hayarden | 23·23 abc | 25·46 a | 84·03 b | 58·41 bcde | 31·76 a | 62·76 a | |
| Mt. Gilbboa | 22·98 abc | 25·38 ab | 79·18 bc | 47·48 e | 31·50 ab | 52·88 abcde | |
| Mt. Hermon | 22·13 bc | 21·83 d | 76·46 bc | 50·98 e | 23·08 cd | 44·40 e | |
| Rosh Pinna | 22·32 bc | 22·92 cd | 81·61 b | 56·49 cde | 26·66 bc | 49·81 de | |
| Tabigha (Basalt) | 21·90 c | 23·89 abc | 85·25 b | 61·91 abcd | 23·73 c | 54·50 bcd | |
| Tabigha (Terra rossa) | 23·49 ab | 24·78 ab | 94·66 a | 67·97 ab | 28·33 ab | 55·99 abcd | |
| Yehudiyya | 24·30 a | 25·54 a | 79·17 bc | 68·05 a | 23·06 cd | 59·76 ab | |
| Upper Ma'ale Merav | - | 25·27 ab | - | 64·08 abc | - | 57·82 abc | |
| Lower Ma'ale Merav | - | 24·92 ab | - | 52·64 de | - | 59·13 ab | |
| Source of variance† | d.f.‡ | Sum of squares | |||||
| Between populations (αi) | 9, 11 | 101*** | 301*** | 6800*** | 7419*** | 2665*** | 6025*** |
| Within population (Lj(αi)) | 35, 40 | 156*** | 469*** | 12013*** | 14266*** | 2978*** | 5275*** |
| Block (βk) | 4, 5 | 14·79 (P = 0·05) | 143*** | 789* | 2,607*** | 138 (P = 0·09) | 40897*** |
Within each nutrient, means followed by different letters are significantly different (P < 0·05; Student's t-test).
Asterisks indicate significance at *P ≤ 0·05, ***P ≤ 0·001; n.s., non-significant effect.
† Tested model: Yijk = μ+ αi + Lj(αi) + βk + eijk.
‡ Number of degrees of freedom corresponding to 2007 and 2008, respectively
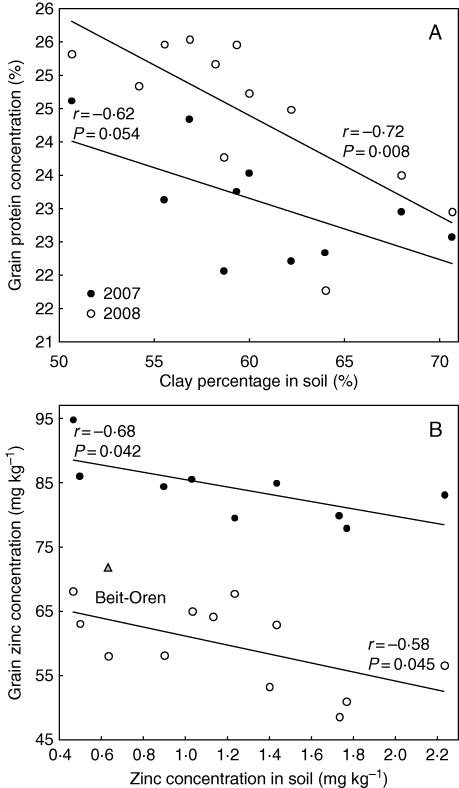
Soil samples collected from the populations' natural habitats (Table S2) were analysed to examine the association between their characteristics and mineral nutrient concentrations in grain produced under our experimental conditions. Among all of the soil and grain variables tested, associations between any two pairs of variables were consistent across the two years. Negative associations were found between soil clay percentage and GPC (%), with the 2008 data showing high significance (r = –0·72, P < 0·01) and the 2007 data being nearly significant (r = –062, P = 0·054) (Fig. 4A). Significant negative correlations (r = –0·68, P = 0·042, and r = –0·58, P= 0·045, for 2007 and 2008, respectively) were also found between soil Zn concentration in the habitats and grain Zn concentration, with the exception of a single population in 2007 (Fig. 4B).
Fig. 4.
Correlation between soil properties of wild emmer wheat collection sites (= populations) and grain nutrient compositions. (A) Soil clay percentage vs. grain protein concentration and (B) zinc concentration in soil vs. grain, in the 2007 (excluding Beit-Oren population, indicated) and 2008 experiments.
DISCUSSION
Ever since the dawn of agriculture, cereals have been a major source of calories for mankind. The demand for cereal continues to grow (for wheat, approx. 2 % per year; Skovmand et al., 2001) concurrent with the global expansion of human populations. Although the primary objective of plant breeding programmes focuses on increased yield, an equally important quest that remains a principal concern but is largely overlooked in breeding programmes is the nutritional value of staple food crops (Welch and Graham, 1999; Cakmak, 2008; Cakmak et al., 2010). Nutritionally enhanced cereal can contribute to health improvement, both directly by enhancing micronutrient availability and indirectly through improved agronomic performance and crop yields (Welch, 1999; Gómez-Galera et al., 2010). However, breeding programmes directed towards increased yield have narrowed the genetic basis of modern crop plants. Therefore, it is vital to explore the genetic resources of the wild relatives of crop plants as they harbour a vast allelic richness of desirable genes.
Genetic diversity for grain nutrients in wild emmer wheat
The current study presents a comprehensive analysis of grain protein (N), micronutrient (Zn, Fe, Cu and Mn) and macronutrient (Ca, Mg, K, P and S) concentrations for a large germplasm collection representing the eco-geographical distribution of wild emmer wheat across the Near Eastern Fertile Crescent. The wild emmer accessions exhibited wide variations in grain concentrations of all nutrients in the two years investigated (Fig. 1). Moreover, wild emmer showed significantly higher concentrations of most grain nutrients across the two years relative to the domesticated wheat cultivars, demonstrating a huge potential for wheat improvement. For example, the highest values of GPC, Zn and Fe in the wild accessions were about two-fold greater than in the domesticated lines in both years (Fig. 1A–C). Previous studies have consistently shown the advantage of wild emmer over cultivated wheat for higher grain nutrient concentrations (e.g. Avivi, 1979; Cakmak et al., 2004; Peleg et al., 2008a). Recently, it has been suggested that in order to have a measurable biological impact on human health, grain concentrations of Zn and Fe should be increased by at least 10 and 25 mg kg−1, respectively (Graham et al., 2007; Cakmak 2008). The higher values found in wild emmer germplasm suggest that it could serve as a potential genetic source to face this challenge.
Associations between grain nutrients
Correlation between grain concentrations of different mineral nutrients may indicate the existence of one or more common genetic–physiological mechanisms involved in mineral absorption or uptake by the root system, translocation and redistribution within the plant tissues, remobilization to the grain and accumulation in the developing grain. Numerous significant correlations were found between the different grain nutrient concentrations, with particularly close associations among all four micronutrients (Zn, Fe, Cu and Mn) and GPC on the one hand, and among the macronutrients (Ca, Mg, K, P and S) on the other (Table 1, Fig. 3).
Grain Zn and Fe concentrations were positively correlated with each other in the current study (Table 1, Fig. 3), similar to previous reports on wild emmer wheat (e.g. Cakmak et al., 2004; Peleg et al., 2008a) and domesticated wheat (Morgounov et al., 2007; Zhao et al., 2009). Positive correlations between GPC and both Zn and Fe, as found in the current study, have been reported for several cereals including: wild emmer wheat (Cakmak et al., 2004; Peleg et al., 2008a), emmer wheat, Triticum dicoccun (Gregorio, 2002), bread wheat (Peterson et al., 1986; Raboy et al., 1991) and Triticale (Feil and Fossati, 1995). Furthermore, genetic mapping in a recombinant inbred line population derived from a cross between durum wheat and wild emmer revealed significant co-localization of quantitative trait loci (QTLs) conferring high GPC, high Zn and high Fe (Peleg et al., 2009). Likewise, co-localization of QTLs for Zn and Fe concentrations has been reported in rice (Stangoulis et al., 2007). Recently, the Gpc-B1 locus from wild emmer was found to encode an NAC transcription factor (NAM-B1) inducing accelerated senescence and greater grain concentrations of protein, Zn and Fe (Uauy et al., 2006; Distelfeld et al., 2007). Positive correlations were also noted in the current study between GPC and three macronutrients, Mg, P and S (Table 1). Similarly, a strong positive association between GPC and total P has been reported in winter wheat (Raboy et al., 1991).
Associations between grain nutrients and eco-geographical origin of wild emmer wheat
Wild emmer grows in a discontinuous arc from the southern Levant to north-western Syria, south-eastern Turkey, northern Iraq and north-west Iran, known as the ‘Fertile Crescent’. In the current study, wild emmer accessions originating from across the Fertile Crescent, with greater availability of accessions from various habitats in Israel, were tested. Wild emmer has been classified into two major groups, southern and northern (Luo et al., 2007). In agreement with this, differences in grain nutrient concentrations between these two groups were significant, albeit of negligible magnitude (Table 2). A nested analysis of variance of the wild emmer populations (= habitats) from Israel revealed significant genetic diversity both within and between populations. Differences between populations were of considerable magnitude (e.g. population averages for grain Zn ranged from 70·06 to 94·66 and 47·48 to 68·05 mg kg−1 for 2007 and 2008, respectively; Table 3). These wild populations were collected in Israel across a relatively small geographical area (approx. 55 × 150 km) that is nevertheless quite diverse in eco-geographical conditions (Peleg et al., 2005). Wide genetic diversity, both between and within wild emmer populations, has been reported previously for numerous characteristics and genetic markers (Feldman and Sears, 1981; Nevo et al., 2002; Peleg et al., 2005, 2008b).
The characteristics of wild emmer populations are assumed to reflect different adaptations to their habitats, acquired throughout their long evolutionary history. In previous studies, allelic diversity (microsatellite markers) and drought resistance were associated with various environmental characteristics (i.e. rainfall, temperature and soil type) (Peleg et al., 2005, 2008b). Different soil mineral compositions in natural habitats have been suggested to trigger evolutionary adaptations which may affect the ability of native accessions to absorb minerals from the soil (e.g. Burgess, 1911; Bonfil and Kafkafi, 2000). The current study investigated the association between soil properties of the habitats from which the wild emmer accessions had been collected (Table S2) and grain nutrient concentrations obtained under the test environments (Table S1). Negative correlations were found in both years between soil clay percentage and GPC (Fig. 4A). Low clay percentage was associated with lower soil water content (and more severe terminal drought under Mediterranean conditions). Accelerated senescence (i.e. drought escape), which is associated with greater GPC (Uauy et al., 2006), would be beneficial under conditions of low soil water content, which could have enhanced GPC in low clay soils. Negative correlations were also found in both years between soil-extractable Zn and grain Zn concentrations (Fig. 4B), suggesting that populations originating from Zn-deficient soils have evolved a better capacity to accumulate the deficient mineral in their grains, which may be of critical importance for seedling germination and establishment. Similarly, in a study of wild emmer, Bonfil and Kafkafi (2000) suggested that when a certain nutrient is naturally deficient in the soil, genotypes which store a higher concentration of that nutrient in the seed will have an advantage under such conditions. The amount of minerals in the seed depends on a plethora of processes, including absorption from the soil, uptake by the roots, translocation and redistribution within the plant tissues and remobilization to the seed (Grusak and Cakmak, 2005; Cakmak et al., 2010). The mechanisms, reported here and elsewhere, that underlie adaptations to nutrient-deficient soils, whether they rely on enhanced capacity of root absorbance, translocation or deposition in grain, have yet to be studied.
Prospects for wheat improvement
The stability of grain nutrient constituents across environments is of interest for both crop breeding and commercial production. High genotype × environment interactions for grain nutrient concentrations have been reported in cultivated wheat (e.g. Oury et al., 2006; Morgounov et al., 2007), as well as for wild emmer under contrasting conditions of water availability (Peleg et al., 2008a; Gómez-Becerra et al., 2010). Therefore, selection of donor wild accessions for breeding programmes should take into account stability across environments. The wild emmer accessions tested in the current study showed significant genotype × year interactions for most nutrients, with the exception of Fe (data not shown); nevertheless, they also exhibited a significant positive correlation (P ≤ 0·001) between the two years (Fig. 2). The current results, as well as previous studies (Peleg et al., 2008a; Gómez-Becerra et al., 2010), suggest that genotype × environment interactions for grain mineral nutrients are non-cross-over interactions, and therefore reasonable advances in selection and breeding can be expected.
High yield capacity is a major objective of any crop breeding programme. Therefore, when selecting for high grain nutrients, the impact on plant productivity should be of major concern. An inverse relationship between grain yield and grain nutrient concentrations (especially GPC) is a well-documented phenomenon in cultivated wheat (Löffler et al., 1983; Gauer et al., 1992; Feil, 1997; Ortiz-Monasterio et al., 1997; Oury et al., 2006; Murphy et al., 2008). However, in wild emmer there were generally no associations between plant productivity (spike DM and total DM) and grain nutrient concentrations, previously under two irrigation regimes (Peleg et al., 2008a) and in the current study across two years. Although the above data refer to associations within collections of either wild or domesticated wheat, the comparison between these diverse germpasms is more complicated. Under both years of the current study, the average spike DM and grain weight were, respectively, approx. 20 and approx. 30 % lower in the wild emmer accessions than the domesticated cultivars. If a given amount of minerals was allocated to reproductive organs, such reductions could account for up to approx 40 % increase in grain nutrient concentrations. Therefore, the approx. 100 % increase in GPC Zn and Fe in the wild emmer relative to domesticated wheat suggests a greater capacity of mineral accumulation in the former.
CONCLUSIONS
During its long evolutionary history under wide ecological amplitude, wild emmer germplasm has accumulated high genetic diversity for various biotic and abiotic stress adaptations. High genetic diversity was found among the wild emmer accessions for all grain nutrients, with considerable potential for improvement of domesticated wheat. Among the wild emmer populations studied, the Tabigha (Terra rossa) population exhibited consistently higher GPC, Zn and Fe concentrations, offering the greatest potential for wheat breeding. The significant within-population diversity suggests that promising populations warrant extensive exploration of their genetic potential for high grain nutrients. The associations between the three compounds of major interest, GPC, Zn and Fe, suggest that all three nutrients can be improved concurrently. These results exemplify unique opportunities to exploit favourable alleles that were excluded from the domesticated gene pool as a result of the genetic bottleneck involved in domestication processes.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the HarvestPlus biofortification challenge programme (www.harvestplus.org). We thank the National Small Grains Collection (NSGC), USA, Institute of Plant Genetics and Crop Plant Research (IPK) Genebank, Gatersleben, Germany, and Prof. F. Salamini, Max-Plank Institute, Germany, for providing some of the germplasm used in this study. We gratefully acknowledge V. Barak, A. Brodutch and U. Uner for excellent technical assistance in the field.
LITERATURE CITED
- Aaronsohn A. Agricultural and botanical exploration in Palestine. Bulletin Plant Industry, U.S. Department of Agriculture, Washington. 1910;180:1–63. [Google Scholar]
- Avivi L. Utilization of Triticum dicoccoides for the improvement of grain protein quantity and quality in cultivated wheats. Monografia Genetica Agraria. 1979;4:27–38. [Google Scholar]
- Bonfil DJ, Kafkafi U. Wild wheat adaptation in different soil ecosystems as expressed in the mineral concentration of the seeds. Euphytica. 2000;114:123–134. [Google Scholar]
- Bouis HE. Micronutrient fortification of plants through plant breeding: can it improve nutrition in man at low cost? Proceedings of the Nutrition Society. 2003;62:403–411. doi: 10.1079/pns2003262. [DOI] [PubMed] [Google Scholar]
- Bouis HE. The potential of genetically modified food crops to improve human nutrition in developing countries. Journal of Developmental Studies. 2007;43:79–96. [Google Scholar]
- Burgess JL. The influence of the soil type on the plant variety. Agronomy Journal. 1911;3:58–72. [Google Scholar]
- Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant and Soil. 2008;302:1–17. [Google Scholar]
- Cakmak I, Torun A, Millet E, et al. Triticum dicoccoides: an important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Science and Plant Nutrition. 2004;50:1047–1054. [Google Scholar]
- Cakmak I, Pfeiffer WH, McClafferty B. Biofortification of durum wheat with zinc and iron. Cereal Chemistry. 2010;87:10–20. [Google Scholar]
- Distelfeld A, Cakmak I, Peleg Z, et al. Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiologia Plantarum. 2007;129:635–643. [Google Scholar]
- Feil B. The inverse yield–protein relationship in cereals: possibilities and limitations for genetically improving the grain protein yield. Trends in Agronomy. 1997;1:103–119. [Google Scholar]
- Feil B, Fossati D. Minerals composition of Triticale grains as related to grain yield and grain protein. Crop Science. 1995;35:1426–1431. [Google Scholar]
- Feldman M. The origin of cultivated wheat. In: Bonjean AP, Angus WJ, editors. The world wheat book. Paris: Lavoisier Tech & Doc; 2001. pp. 3–56. [Google Scholar]
- Feldman M, Sears ER. The wild gene resources of wheat. Scientific American. 1981;244:102–112. [Google Scholar]
- Gauer L, Grant C, Gehl D, Bailey L. Effects of nitrogen fertilization on grain protein content, nitrogen uptake, and nitrogen use efficiency of six spring wheat (Triticum aestivum L.) cultivars, in relation to estimated moisture supply. Canadian Journal of Plant Science. 1992;72:235–241. [Google Scholar]
- Gómez-Becerra HF, Yazici A, Ozturk L, et al. Genetic variation and environmental stability of grain mineral nutrient concentrations in Triticum dicoccoides under five environments. Euphytica. 2010;171:39–52. [Google Scholar]
- Gómez-Galera S, Rojas E, Sudhakar D, et al. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Research. 2010 doi: 10.1007/s11248-009-9311-y. in press. doi:10.1007/s11248-009-9311-y. [DOI] [PubMed] [Google Scholar]
- Graham RD, Welch RM, Bouis H. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Advances in Agronomy. 2001;70:77–142. [Google Scholar]
- Graham RD, Welch RM, Saunders DA, et al. Nutritious subsistence food system. Advances in Agronomy. 2007;92:1–74. [Google Scholar]
- Gregorio GB. Progress in breeding for trace minerals in staple crops. Journal of Nutrition. 2002;132:500S–502S. doi: 10.1093/jn/132.3.500S. [DOI] [PubMed] [Google Scholar]
- Grusak MA, Cakmak I. Methods to improve the crop-delivery of minerals to humans and livestock. In: Broadley MR, White PJ, editors. Plant nutritional genomics. Oxford: Blackwell Publishing; 2005. pp. 265–286. [Google Scholar]
- Harlan JR, Zohary D. Distribution of wild wheat and barley. Science. 1966;153:1074–1080. doi: 10.1126/science.153.3740.1074. [DOI] [PubMed] [Google Scholar]
- Holtz C, Brown KH. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutrition Bulletin. 2004;25:94–204. [PubMed] [Google Scholar]
- Ladizinsky G. Plant evolution under domestication. Dordrecht: Kluwer Academic Press; 1998. [Google Scholar]
- Löffler C, Bush R, Wiersma J. Recurrent selection for grain protein percentage in hard red spring wheat. Crop Science. 1983;23:1097–1101. [Google Scholar]
- Luo MC, Yang ZL, You FM, Kawahara T, Waines JG, Dvorak J. The structure of wild and domesticated emmer wheat populations, gene flow between them, and the site of emmer domestication. Theoretical and Applied Genetics. 2007;114:947–959. doi: 10.1007/s00122-006-0474-0. [DOI] [PubMed] [Google Scholar]
- Merrill AL, Watt BK. Energy value of food—basis and derivation. Washington, DC: US Department of Agriculture Handbook No. 74; 1973. [Google Scholar]
- Morgounov A, Gómez-Becerra HF, Abugalieva A, et al. Iron and zinc grain density in common wheat grown in Central Asia. Euphytica. 2007;155:193–203. [Google Scholar]
- Murphy KM, Reeves PG, Jones SS. Relationship between yield and mineral nutrient concentrations in historical and modern spring wheat cultivars. Euphytica. 2008;163:381–390. [Google Scholar]
- Nevo E, Korol AB, Beiles A, Fahima T. Evolution of wild emmer and wheat improvement: population genetics, genetic resources, and genome organization of wheat's progenitor, Triticum dicoccoides. Berlin: Springer-Verlag; 2002. [Google Scholar]
- Ortiz-Monasterio JI, Sayre KD, Rajaram S, McMahon M. Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Science. 1997;37:898–904. [Google Scholar]
- Oury F-X, Leenhardt F, Rémésy C, et al. Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat. European Journal of Agronomy. 2006;25:177–185. [Google Scholar]
- Peleg Z, Fahima T, Abbo S, et al. Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant, Cell and Environment. 2005;28:176–191. [Google Scholar]
- Peleg Z, Saranga Y, Yazici A, Fahima T, Ozturk L, Cakmak I. Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. Plant and Soil. 2008a;306:57–67. [Google Scholar]
- Peleg Z, Saranga Y, Krugman T, Abbo S, Nevo E, Fahima T. Allelic diversity associated with aridity gradient in wild emmer wheat populations. Plant, Cell and Environment. 2008b;31:39–49. doi: 10.1111/j.1365-3040.2007.01731.x. [DOI] [PubMed] [Google Scholar]
- Peleg Z, Cakmak I, Ozturk L, et al. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat × wild emmer wheat RIL population. Theoretical and Applied Genetics. 2009;119:353–369. doi: 10.1007/s00122-009-1044-z. [DOI] [PubMed] [Google Scholar]
- Peterson CJ, Johnson VA, Mattern PJ. Influence of cultivar and environment on mineral and protein concentrations of wheat flour, bran, and grain. Cereal Chemistry. 1986;63:183–186. [Google Scholar]
- Raboy V, Noaman MH, Taylor GA, Pickett SG. Grain phytic acid and protein are highly correlated in winter wheat. Crop Science. 1991;31:631–635. [Google Scholar]
- Sanchez PA, Swaminathan MS. Cutting world hunger in half. Science. 2005;307:357–359. doi: 10.1126/science.1109057. [DOI] [PubMed] [Google Scholar]
- Sands DC, Morris CE, Dratz EA, Pilgeram A. Elevating optimal human nutrition to a central goal of plant breeding and production of plant-based foods. Plant Science. 2009;177:377–389. doi: 10.1016/j.plantsci.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovmand B, Reynolds MP, Delacy IH. Searching genetic resources for physiological traits with potential for increasing yield. In: Reynolds MP, Ortiz-Monasterio JI, McNab A, editors. Application of physiology in wheat breeding. Mexico: CIMMYT; 2001. pp. 17–28. [Google Scholar]
- Stangoulis JCR, Huynh BL, Welch RM, Choi EY, Graham RD. Quantitative trait loci for phytate in rice grain and their relationship with grain micronutrient content. Euphytica. 2007;154:289–294. [Google Scholar]
- Tanksley SD, McCouch SR. Seed banks and molecular maps: unlocking genetic potential from the wild. Science. 1997;277:1063–1066. doi: 10.1126/science.277.5329.1063. [DOI] [PubMed] [Google Scholar]
- Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science. 2006;314:1298–1301. doi: 10.1126/science.1133649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RM. Importance of seed mineral nutrient reserves in crop growth and development. In: Rengel Z, editor. Mineral nutrition of crops: fundamental mechanisms and implications. New York: Food Products Press; 1999. pp. 205–226. [Google Scholar]
- Welch RM, Graham RD. A new paradigm for world agriculture: meeting human needs: productive, sustainable nutritious. Field Crops Research. 1999;60:1–10. [Google Scholar]
- Welch RM, Graham RD. Breeding for micronutrients in staple food crops from a human nutrition perspective. Journal of Experimental Botany. 2004;55:353–364. doi: 10.1093/jxb/erh064. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Ekiz H, Gultekin I, Torun B, Karanlik S, Cakmak I. Effect of seed zinc content on grain yield and zinc concentration of wheat grown in zinc-deficient calcareous soils. Journal of Plant Nutrition. 1998;21:2257–2264. [Google Scholar]
- Zhao FJ, Su YH, Dunham SJ, et al. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. Journal of Cereal Science. 2009;49:290–295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.