Abstract
Background
Plants contain relatively few cell types, each contributing a specialized role in shaping plant function. With respect to plant nutrition, different cell types accumulate certain elements in varying amounts within their storage vacuole. The role and mechanisms underlying cell-specific distribution of elements in plants is poorly understood.
Scope
The phenomenon of cell-specific elemental accumulation has been briefly reviewed previously, but recent technological advances with the potential to probe mechanisms underlying elemental compartmentation have warranted an updated evaluation. We have taken this opportunity to catalogue many of the studies, and techniques used for, recording cell-specific compartmentation of particular elements. More importantly, we use three case-study elements (Ca, Cd and Na) to highlight the basis of such phenomena in terms of their physiological implications and underpinning mechanisms; we also link such distributions to the expression of known ion or solute transporters.
Conclusions
Element accumulation patterns are clearly defined by expression of key ion or solute transporters. Although the location of element accumulation is fairly robust, alterations in expression of certain solute transporters, through genetic modifications or by growth under stress, result in perturbations to these patterns. However, redundancy or induced pleiotropic expression effects may complicate attempts to characterize the pathways that lead to cell-specific elemental distribution. Accumulation of one element often has consequences on the accumulation of others, which seems to be driven largely to maintain vacuolar and cytoplasmic osmolarity and charge balance, and also serves as a detoxification mechanism. Altered cell-specific transcriptomics can be shown, in part, to explain some of this compensation.
Keywords: Cell-specific, element, vacuole, transcriptome, ionome, plant nutrition, nutrient storage
INTRODUCTION
Nutrients are often acquired in excess of the immediate needs of the plant and as a result they must be stored within plant tissue until required. Whilst taking-up essential micro- and macronutrients, and despite sophisticated exclusion mechanisms, plants inadvertently acquire elements that are toxic. Therefore, compartmentalization and/or separation of particular elements, especially in their soluble ionic form, are required by the plant for optimal function (Leigh, 1997). This review reports commonly observed elemental distributions within plant tissues, explores the potential reasons for these distributions and examines the processes that may be involved in these organ-, tissue- and cell-type distributions.
Plant vacuoles play a major role in elemental storage (including compartmentation of toxic substances) and in the maintenance of nutrient availability within the cytosol (Leigh, 1997). Well-documented examples include mechanisms for cytosolic homeostasis of calcium (Ca2+) and nitrate (NO3−), and the ability of cells to limit cytosolic sodium (Na+) accumulation (Miller and Smith, 2008; Munns and Tester, 2008; McAinsh and Pittman, 2009). Plant organs may have very different concentrations of elements within tissues which may relate to properties of long-distance transport (xylem vs. phloem), sites of complexation (for many heavy metals or charge-dense ions), or tissue- or cell-specific transport processes such as ion accumulation within vacuoles (Walker and Pitman, 1976; Marschner, 1995; Leigh, 1997; Tester and Leigh, 2001; Britto et al., 2006). In fact, the vacuole of a particular cell may have a very different elemental profile compared with that of a neighbouring cell of the same cell-layer or more commonly of different cell types (Karley et al., 2000a; Karley and White, 2009). As the central vacuole occupies most of the cell volume it is ideally suited for a role in elemental storage (Wagner, 1982; Canut et al., 1993; Andreev, 2001). It is specifically these differences in vacuolar element content on which this review will be focused.
We propose that elemental distributions in plants can be explained by (a) transport pathways that elements take through plants and (b) storage properties of cells that constitute or border the elemental transport corridor. To illustrate this theme the compartmentalization of Ca2+, an essential plant macronutrient, and cadmium (Cd2+) and Na+, two ‘toxic’ cations in plant leaf and root tissues, will be examined in detail. As potassium ions (K+) are the major cationic osmoticum in plants, K+ compartmentation with respect to the aforementioned elements will also be touched upon throughout, but for recent detailed reviews on K+ homeostasis, refer to Shabala and Cuin (2007), Luan et al. (2009) and Karley and White (2009).
There have been over 150 published reports in the past 20 years of cell-type-specific element profiling which have been mainly of a phenomenological nature; however, there has been a resurgence in such reports in the past 5 years (over 50) following technological developments in the molecular interrogation of single cells or cell types. Much of this resurgence has arisen through an interest in bio-fortification of staple foods and phytoremediation/phytoextraction of contaminated sites, which requires a need for an understanding of the physiology of specific-cell types and cell-type compartments in response to enhanced storage of particular ions. By focusing on Ca2+, Cd2+ and Na+, and predominantly plant leaf tissue, other equally important elemental distributions, interactions or relevant processes will not be explored in depth. For instance, seed-loading and seed cell-type-specific nutrient compartmentation is an emerging topic of scientific study but will not be touched upon (refer to Zhang et al., 2007; Kachenko et al., 2009; Punshon et al., 2009; Tauris et al., 2009; White and Broadley, 2009).
The role of particular solute transporters located on the plasma membrane and tonoplast of particular cell types with respect to the accumulation of particular elements within cells will also be discussed. However, the role of transport proteins located on other membranes and indeed membrane processes such as endocytosis or membrane trafficking may play a role in ion compartmentalization but will not be discussed in detail here. For recent reviews on these subjects, the reader is referred to articles such as those by Amtmann and Blatt (2009) and McAinsh and Pittman (2009).
TECHNIQUES FOR PLANT ELEMENTAL PROFILING
The term ‘ionome’ is commonly and perhaps erroneously used to describe the total concentration (i.e. all forms) of selected elements in a sample of plant tissue (Salt et al., 2008). Ionic concentrations (or activities) of elements are more difficult to measure accurately, but are usually considerably less than the total elemental content. For instance, the calcium content of certain Arabidopsis thaliana leaf vacuoles may be as high as 80 mm but the Ca2+ activity (‘free’ Ca2+ not complexed or irreversible bound) has been measured at 12 mm (M. Gilliham and T. Cuin, unpubl. res.). Therefore, the terms ‘ionome’ and ‘ionomics’ (the study of elemental content of plants) should be considered with caution.
Many techniques are available for investigating elemental or ionic content of plant tissues with resolution from the whole plant to the sub-cellular level. Techniques covered here (see Table 1), and used extensively in studies reviewed below, can be broadly separated into those based on: fluorescence or luminesence of indicator macromolecules (e.g. protein ‘biosensors’, chemical-based fluorescent indicators); ionophores (e.g. ion-selective electrodes); X-ray fluorescence detected directly from elements (e.g. X-ray microanalysis); mass-spectroscopy [e.g. inductively coupled plasma–mass spectroscopy (ICP-MS) and stable isotopes] and radioactive emission from tracers. These techniques vary in their throughput, sample preparation, cost, detection limits and ability to discriminate forms of the element. There have been few reviews of each of these techniques in a comparative light (Ortega, 2005; Lobinski et al., 2006), yet each individual method has again been detailed recently in a review by Salt et al. (2008). As a result, we will only address limitations of these with respect to current research findings detailed in the sections below.
Table 1.
Techniques for quantifying elemental (and ionic) content within plant cells: the main spatially resolved analytical techniques for chemical element imaging, quantification and speciation in plant samples
| Basis of measurement | Technique/indicator | Detection limit/range (µg g−1) | Spatial resolution (μm) | Selectivity | Quantification | Analytical depth (μm) | References |
|---|---|---|---|---|---|---|---|
| Protein sensors | Aequorin | 0·004–4·0 (0.1–10 μm) | 5–10 | Calcium | Ratiometric | Tissue depth (<300) | Rutter et al., 1996; Fricker et al., 1999; Plieth, 2006 |
| pHluorin | pH 5·5–7·5 | 5–10 | pH (H+) | Ratiometric | Tissue depth (<300) | Miesenbock et al., 1998 | |
| CAMeleon | 0·0004–0·17 (0.01–4.3 μm) | 5–10 | Calcium (variants with less H+ sensitivity) | Ratiometric | Tissue depth (<300) | Miyawaki et al., 1999 | |
| Fluorescent indicators | Fura-2 | 0·0056 (140 nm) (kDa 0·14) | 5–10 | Calcium | Ratiometric | Cell depth | Mühling and Läuchli, 2002a, b; Halperin and Lynch, 2003; Mazel et al., 2004; Kader and Lindberg, 2005 |
| SBF1 | 87·4 (K. 3·8 mm) | 5–10 | Sodium | Ratiometric | |||
| PBF1 | 199·4 (kDa 5·1 mm) | 5–10 | Potassium (interfering Na+) | Ratiometric | |||
| Ion-selective electrodes | ETH 157 | 46 (2 mm) | 1–2 | Sodium (interfering H+, K+) | Quantitative (ion activity) | Relative to tip dimensions and penetrance (>5) | Fluka Chemicals |
| ETH 129 | 0·0004 (10 nm) | 1–2 | Calcium (interfering K+, H+, Mg2+) | Quantitative (ion activity) | |||
| Valinomycin | 3·9 (100 µm) | 1–2 | Potassium (Ca2+, NH+) | Quantitative (ion activity) | |||
| Measures of total elemental content | Inductively coupled plasma–mass spectroscopy (ICP-MS) | ≥0·001 | N/A | Multi-elemental (all Z) | Quantitative | N/A | |
| X-ray microanalysis (SEM-XRMA) | ∼100 (dehydrated)–1000 (hydrated) | 0·5–5 | Multi-elemental (Z ≥ 6) | Semi-quantitative (commonly, but can be quantitative) | 0·1–1 | Echlin, 1992; Ortega, 2005 | |
| Ion beam microprobe (μ)PIXE | 1–10 | 0·2–2 | Multi-elemental (all Z) | Quantitative | 10–100 | ||
| Synchrotron radiation microprobe (XRF) | 0·1–1 | 0·1–1 | Multi-elemental and isotopic | Semi-quantitative | >100 | ||
| Laser ablation inductively coupled plasma–mass spectroscopy (LA-ICP MS) | 0·01 | 15–50 | Multi-elemental and isotopic | Semi-quantitative | 200 | ||
| Secondary ion mass spectrometry (SIMS) | 0·1 | 0·05 | Multi-elemental and isotopic | Quantitative | 0·1 | Metzner et al., 2008 |
The values shown are representative of a variety of elements ranging from transition metals to macronutrients, within a biological matrix such as an isolated cell or a tissue section (adapted from Ortega, 2005).
Techniques to sample elemental content of plant tissues
Whole plant tissue analysis has been used to identify genetic factors that directly or indirectly influence elemental content of plants. ICP-MS is a common technique for elemental profiling of whole plants or explants (Table 1). Application of ICP-MS to a mutagenized population of 6000 arabidopsis plant lines uncovered 51 mutants with altered elemental profiles (Lahner et al., 2003). Baxter et al. (2008) identified ‘elemental signatures’ which were used as a diagnostic when analysis of the target element alone may be a poor indicator of nutritional status, such as altered iron (Fe) and phosphorous (P) homeostasis. The use of ‘elemental signatures’ reduces the proportion of plants incorrectly identified as having altered elemental accumulation patterns and takes advantage of the many interactions that occur between acquisition, redistribution and storage of mineral elements (see following sections on particular elements). A recent improvement on the throughput of ICP-MS (Table 1) was achieved by Hansen et al. (2009) who developed a miniaturized version (called micro-ICP), involving microwave processing of smaller tissue samples (1–20 mg), in greater number and equivalent accuracy to traditional ICP spectroscopy.
Plants contain approx. 40 different cell types (Martin et al., 2001), each varying in transcript and protein complement and abundance (Kehr, 2003; Roy et al., 2003; Brandt, 2005; Galbraith and Birnbaum, 2006), physiological role (e.g. Tester and Leigh, 2001) and, potentially, individual elemental composition (Karley et al., 2000a). Therefore, whole tissue analysis has its limitations and can blur the individual contribution of each cell type to the elemental profile. As such, methods have been developed to analyse cell-specific element profiles (Table 1).
Except for secondary ion mass spectroscopy (SIMS) and laser ablation ICP-MS (LA-ICP-MS), the multi-element profiling techniques summarized in Table 1 are based on excitation of electrons within ions with measurement of the characteristic and commonly element-specific X-ray emissions. SIMS and LA-ICP-MS involve the physical disturbance of the sample surface to generate charged particles, which are subsequently measured by mass spectroscopy. The variations between methods are the identity of the incident beam (laser, X-ray, electron beam, ion beam), the emission type (X-rays, fluorescent X-rays, plasma/charged ions) and also the detector (EDX or MS). Furthermore, these techniques can be extended by scanning the incident beam across the surface of the sample to generate a two-dimensional ion quantification map, used to great effect in various species looking at numerous ions simultaneously (Storey and Leigh, 2004; Küpper et al., 2000; Kerton et al., 2009). However, X-ray microanalysis (XRMA)-based measurements have a resolution based on electron beam energy which limits the minimum size of compartment that can be assayed without contamination from adjoining compartments. For instance, in cryo-frozen biological tissues with an excitation voltage of 15 keV this approximates to approx. 5 µm2 (Echlin, 1992) so cytoplasmic and cell-wall compartments cannot generally be quantified reliably and depth of the sample must always be considered.
Synchrotron X-ray fluorescence microprobes (μXRF) are becoming a favoured technology for obtaining (semi-) quantitative spatial data in plants and is an improvement on many current ‘mechanical’ means (Metzner et al., 2008; Punshon et al., 2009). These advantages include (with certain modifications) its non-destructive nature, important for precious samples and living material; it permits three-dimensional reconstruction of accumulation patterns and can also distinguish between ionic valencies, critical for accumulation of toxic forms of various ions, including thallium in the hyperaccumulator Iberis intermedia (Scheckel et al., 2007).
Additional dimensions to these techniques have been developed to increase throughput or broaden the scope for analysis of specific samples. One method is single cell sampling and analysis (SiCSA) which involves the insertion of a fine microcapillary into living tissue, for the isolation of cellular sap, which is subsequently dried on membranes (e.g. Pioloform) and analysed by XRMA (Malone et al., 1989). SiCSA has a greater sensitivity and permits a more accurate quantification of elements than hydrated or non-hydrated XRMA. As summarized in Table 1, XRMA requires sufficiently high element concentration to distinguish them from background radiation. Furthermore, as the extracted sap contains all cellular components below 0·5–1 µm (tip diameter) it has been used for the simultaneous detection/quantification of carbohydrates, proteins and gene transcripts by RNA amplification and microarray/qPCR (reviewed in Brandt, 2005). As SiCSA does not require the extensive preparation required with fixation/freezing of laser microdissection, LMD or protoplasting, for cell sorting, SiCSA is both rapid and facilitates repeated measurements on the same individual plant throughout the lifecycle, or to following responses to and recovery from stresses (Fricke et al., 1994b; Tomos and Sharrock, 2001). However, although arguably more invasive and suffering from a greater number of potential artefacts, protoplasting and LMD can sample a greater number of cells and therefore provide a better average signal across cell or tissue types (Meyers et al., 2004; Galbraith and Birnbaum, 2006; Kralj et al., 2009).
Techniques for measuring ion activities or transport
A greater number of techniques exist for the profiling, not total element concentrations, but solute or ion activities within tissue, and transport of ions across membranes. Such information is useful in indicating elemental content of individual cells or subcellular compartments or interrogating ionic homeostasis mechanisms. These include the use of indicator molecules, complementation of yeast, electrophysiological measurements of current through native membranes or of proteins expressed in heterologous systems and spectrophotometer-based measurements of transport across isolated membranes (Gilliham, 2007).
Processes with degrees of invasiveness are used to introduce light-emitting or -absorbing indicators such as chemical probes or recombinant biomolecular indicators [e.g. for the measurement of pH 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) vs. green fluorescent protein (GFP) derivative pHluorin]; once present kinetic and spatial information regarding ion distributions in living specimens can be obtained (Plieth, 2001; Fricker et al., 2006). Both luminescent and [single or dual excitation (or emission) wavelength] fluorescent indicators are commonly used. However, use of such indicators to measure ion activities relies on the binding and therefore ‘buffering’ of the target species so can directly interfere with the intended measurement (Fricker et al., 1999).
Single wavelength excitation chemical-based indicators are available for many ions, including Na+ (e.g. CoroNa green) and Ca2+ (e.g. Ca-green, Fluo-3), but studies using these indicators often fail to include loading controls so data cannot be interpreted quantitatively (Fricker et al., 2006; Oh et al., 2009). Dual-excitation ratiometric indicators (which can be quantitative) are shown in Table 1; however, disadvantages of these indicators still remain, including a poor specificity in targeting the indicator to particular compartments or the inability to discriminate between the target species and inteferring ions (Plieth, 2001). Bioluminescent recombinant aequorin can be used to quantify changes in [Ca]cyt within plant tissues in response to various stimuli or following circadian rhythms (Knight, 2000; Welch, 2002; McDowell, 2003; Plieth, 2006; Dodd et al., 2007; Morris et al., 2008). The ability to target recombinant proteins easily to discrete intra- or extra-cellular compartments, membranes or specific cell types along with recent improvements in indicator proteins to detect a whole range of metabolites and ions including H+, Ca2+ and glutamate to sensitivities equivalent to traditional chemical indicators, means these biosensors are a powerful tool for dynamic, sub-cellular ionic quantification at varying concentrations in plants with a capacity for stable transformation (Kiegle et al., 2000; Lalonde et al., 2005). Ion activities can also be measured directly within plant tissues using ion-selective electrodes which have excellent spatial and temporal resolution but are extremely low throughput having only been used successfully by a handful of research groups (Miller and Wells, 2006; see review by Miller et al., 2001).
Radioactive and non-radioactive tracers have been used for over 50 years to measure elemental fluxes or distribution within plant compartments using analogues or tracers (e.g. 45Ca, Sr and 90Sr for Ca; 15N and 13N for N; 22Na for Na) in combination with radiography for plant distribution (Britto et al., 2004; Loveland et al., 2005; Kiser et al., 2008; Kronzucker et al., 2008) or chromatography for flux measurements (MacRobbie, 1971; Sherma and Van Lenten, 1971; Hirsch et al., 1998; Fernie et al., 2005) or SIMS (Metzner et al., 2008). Compartmental analysis by tracer efflux (CATE) has been useful in estimating vacuolar and cytoplasmic pool sizes of elements and fluxes across membranes (Walker and Pitman, 1976; Britto et al., 2006). But this technique has been questioned with regard to solutes with pool sizes that rapidly turnover (such as Ca2+) and also gives different results from techniques that measure ion activity leading to different interpretations of results (for further discussion, see Carden, 2003; Kronzucker et al., 2006).
In summary, compartmentation of elements and transcripts has been detected by multiple techniques, each with their own caveats or limitations. The only way to overcome such problems is by using techniques in combination, or several techniques in parallel (Gilliham, 2007). The following section summarizes data from these many different techniques, drawing together consistent data but highlighting differences that may be due to both technical and biological issues.
CELL-SPECIFIC ELEMENTAL COMPARTMENTATION IS COMMONLY OBSERVED
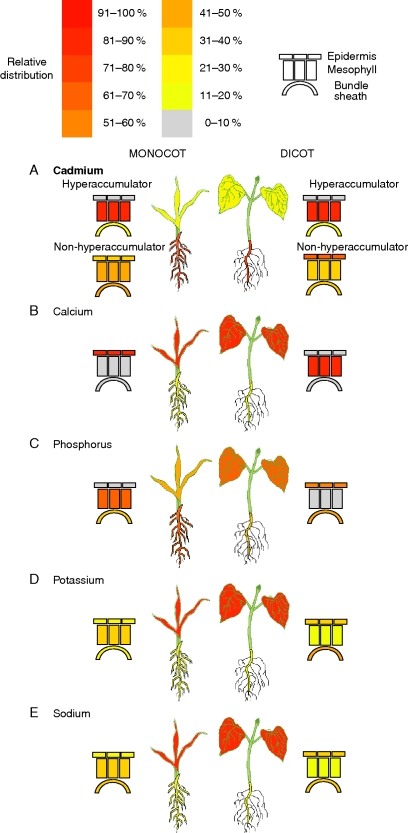
Organ (leaf and root) and cell-specific vacuolar concentrations of leaf elemental distributions (Ca, K, P, Cd and Na) between the Poales and the majority of eudicot species studied have been summarized in Fig. 1, with a broader dataset presented in Table 2. Generalizations can be drawn which will be expanded on below in case studies, including: (a) elements that are transported exclusively in the transpiration stream will accumulate to greater concentrations in highly transpiring organs, e.g. Mn and Ca; (b) Ca and P are never co-localized together when soluble; (c) many heavy metals are excluded from the shoot by complexing in the root apoplast; (d) K+ is a major osmoticum and compensates for, among others ions, Ca2+, Cd2+ and Na+ accumulation in conditions of deprivation or excess; (e) expression profiles of specific genes (particular ion transporters) can be correlated with elemental content.
Fig. 1.
Relative accumulation profiles of the elements Cd (both hyperaccumulators and non-hyperaccumulators), Ca, Cd, K, P and Na at the organ level and cell-specific levels within the leaf. Model monocot (left side of each figure) and dicot (right) shown with shoot and root concentrations (independent of root/shoot mass) converted into proportion within the plant and then averaged for n = 2–12 plant species. Stylized leaf cross-section shown for both monocot and dicot, again represented as a heat map showing relative proportion of [ion] within the leaf. Despite prevalence of all ions within trichomes, they are excluded from images due to lack of reporting on trichome prevalence, or dimensions.
Table 2.
Ion distribution within plants reveals distinct, cell-specific patterns that contrast between monocot (Poales*) and eudicot† orders
| Ion concentration (mm) |
||||
|---|---|---|---|---|
| Element | Epidermis | Mesophyll | Bundle sheath | References |
| Poales | ||||
| Ca | 27·5 ± 5·0 (15–37) | 4·0 ± 0·8 (2–6) | 3·2 ± 0·5 (2–5) | Malone et al., 1989, 1991; Dietz et al., 1992b; Fricke et al., 1994a, b, c, 1996; Brune et al., 1995; Koroleva et al., 2000 |
| Na (2 mm) | 31 ± 12·8 (1–51) | 18 ± 3·1 (3–29) | 17 ± 1·8 (5–19) | |
| K | 211 ± 18·8 (169–249) | 258 ± 11·8 (216–299) | 185 ± 23·8 (180–225) | |
| Mg | 5 ± 1·5 (2–10) | 17·1 ± 4·3 (12–28) | N/A | |
| S | 0·8 | 2·4 | N/A | |
| P | 3·8 ± 1·8 (0–10) | 54·3 ± 8·4 (25–75·5) | 28 | |
| Cl | 106 ± 20 (61·8–160) | 26 ± 7 (16–40) | 34 | |
| NO3 | 161 ± 36 (55–220) | 151 ± 46 (47–226) | 160 | |
| Mn | High | Undetectable | Undetectable | |
| Ni (100 µm) | 0·0021 ± 0·0006 | 0·0094 ± 0·0013 | N/A | |
| Cd (100 µm) | 0·0303 ± 0·0057 | 0·0423 ± 0·0106 | 0·040 ± 0·0077 | |
| Malate | 160 ± 31 | 30 ± 14·1 | 32 ± 17·2 | |
| Eudicots | ||||
| Ca | 10·1 ± 8·2 (0–45) | 68 ± 14 (15–88) | 5·2 ± 2·6 (∼2–9·6) | Mesjasz-Przybylowicz et al., 1994, 2001a, b; Küpper et al., 1999, 2000, 2001; Zhao et al., 2000; Ager et al., 2002, 2003; Bidwell et al., 2004, Epimashko et al., 2004; Fernando et al., 2006; Kerton et al., 2009 |
| Na | 15 | 5 | 14 | |
| K | 106 ± 34 (40–154) | 86 ± 25 (10–120) | 128 ± 22 (75–180) | |
| Mg | 25 | 8 | N/A | |
| S | 20 | 30 | N/A | |
| P | 60·2 ± 20·9 (20–101) | 20·2 ± 8·5 (0–42) | 47 ± 15 (20–80) | |
| Cl | 60·6 ± 10·4 (31–82) | 11·6 ± 1·4 (5–21) | N/A | |
| NO3 | n.d. | n.d. | N/A | |
| Mn | Undetectable | High | Undetectable | |
| Ni (100 µm) | 185 ± 36 (154–213) | 59 ± 12 (25–82) | 26 | |
| Cd (100 µm) | 0·0502 ± 0·010 | 0·0202 ± 0·008 | 0·0252 ± 0·009 | |
| Malate | N/A | 30·9 ± 2·3 (midday) | N/A | |
* Poales includes barley and wheat, with data presented for older leaves, ≥4 d after full expansion.
† Eudicots include tomato, tobacco, arabidopsis, poplar, coriander and Mesambranthemum with Ni data presented for the Ni hyperaccumulators Berkheya coddii, Hybanthus floribundus and Senecio anomalochrous.
Data are presented as mean ± standard error (n = 5–16 reports for each ion) for all reports under their standard growth conditions (concentration range among all species shown in brackets).
N/A, data was not available.
CALCIUM
Calcium nutrition
To plants, Ca2+ has both pivotal structural and essential signalling roles (Hirschi, 2004). Therefore, control of its distribution is of vital importance to physiological function (White and Broadley, 2003; McAinsh and Pittman, 2009). Plant productivity and tolerance to biotic and abiotic stresses is also linked to plant Ca status; plants deficient in Ca are often more susceptible to pathogens and stresses such as salinity (Bangerth, 1979; Marschner, 1995). Ca deficiencies in humans (e.g. osteoporosis, rickets, eclampsia) and animals (e.g. milk-fever, lambing sickness) are also common and may be reduced by maximizing Ca intake in diets (Welch, 2002; McDowell, 2003; Morris et al., 2008). In populations with staple diets containing only cereals or low dairy intakes, increasing crop Ca bioavailability may be an important part of this strategy (Morris et al., 2008). Therefore, understanding the mechanisms of Ca transport and storage in plants is fundamental to maintaining plant productivity and animal health.
Calcium ions (Ca2+) are carried through the plant mainly through the apoplastic (extracellular) pathway from where they are taken into cells via protein-mediated transport (Marschner, 1995; White and Broadley, 2003). Ca2+ is transferred from root-to-shoot in the xylem, with the rate of deposition driven by transpiration rate and soil Ca2+ concentration ([Ca2+]) (Smith, 1991; White, 2001). Ca2+ is ostensibly phloem immobile; once deposited in the shoot it is not readily redistributed (White and Broadley, 2003). Debate surrounds whether the endodermis within the root forms an impermeable barrier to the continuous extracellular movement of Ca2+ to the shoot and whether two membranes must be traversed, once prior to the endodermis and once within the stele, before Ca2+ can enter the xylem and be delivered to the shoot (Clarkson, 1991; White, 2001; Cholewa and Peterson, 2004). The differences detected in the permeability of the endodermis to Ca2+ appear to be species-specific. For instance, in arabidopsis most Ca2+ follows an apoplastic-dominated pathway (White, 2001; Hayter and Peterson, 2004; Baxter et al., 2009) whereas in other species, such as onion, suberization of the endodermis requires that most Ca2+ transport to the xylem has a symplastic component (Cholewa and Peterson, 2004). It has been proposed that monocots have a tighter control apoplastic loading of Ca2+ through the presence of an intact endodermis (Chino, 1981), but there are obvious exceptions to this with rice which has a significant proportion of delivery in the shoot through the apoplast-dominated pathway (Yadav et al., 1996). Regardless, once loaded into the xylem there is a substantial flux of Ca2+ from plant roots to shoots carried in the transpiration stream which is then distributed through the apoplast, taken up by cells and deposited in vacuoles for long-term storage (Clarkson, 1984; Leigh, 1997; White and Broadley, 2003; Storey and Leigh, 2004; Karley and White, 2009).
Accumulation of Ca in different organs
The majority of Ca2+ taken into the root system is transported to the shoots (Fig. 1). Given the relative phloem immobility of Ca2+, the levels accumulated within lowly transpiring organs, such as fruits or seeds, are very low, generally <1 % of the total plant Ca (Starck et al., 1994; Bermudez et al., 2008). Broadley et al. (2003) performed a literature survey on over 200 angiosperm species regarding shoot Ca accumulation, summarizing the variance within and across plant species and orders. It was shown that, while much variance was at the level of the plant orders, a highlight was the divergence between commelinoid monocots, accumulating relatively less shoot calcium than most eudicots. This may be in part due to the lower cation exchange capacity of roots or the greater endodermal barrier to apoplastic loading of Ca (Chino, 1981; Marschner, 1995). However, it may also be related to the lower Ca storage capacity of most commelinoid monocots, as elaborated on later.
Within transpiring leaves, cellular uptake of Ca2+ or assimilation into cell wall pectate complexes is crucial to control free Ca2+ in the apoplast, [Ca2+]apo, which must be kept below approx. 0·05 mm to prevent Ca2+-induced stomatal closure (Smith, 1991; de Silva et al., 1996). This may also be achieved by sequestration into trichomes, which accumulate large quantities of Ca2+ (and phosphorous), into crystal-containing idoblasts and/or into Ca oxalate crystals, or by secretion into cells close to guard cells (de Silva et al., 1996; Franceschi and Nakata, 2005; Broadley et al., 2003; Sanders et al., 2002). In contrast, lowly transpiring organs may suffer from Ca deficiencies and this has major horticultural implications in terms of crop spoilage as susceptible plant parts include fruits and leaf tips [e.g. blossom end-rot in tomato (Ho and White, 2005) and leaf burn in lettuce (Barta and Tibbitts, 2000)].
Cell-specific accumulation of Ca
Within cells, concentrations of free Ca ions [Ca2+] are spatially regulated (Sanders et al., 2002; Hetherington and Brownlee, 2004). This appears, in part, due to the presence of particular transporters present on each (sub-) cellular membrane with varying affinities for Ca2+, which can be regulated by a host of transcriptional and post-translational factors. Transport of Ca2+ across membranes regulates [Ca2+]cyt between nm and low μm levels temporally during ‘resting’ and signalling events that can be specific to particular physiological responses (reviewed in McAinsh and Pittman, 2009). Despite its ubiquitous functions, Ca appears to be stored differentially between vacuoles of different cell types, with only certain cell types exceeding [Ca]vac >5 mm.
Root apoplastic [Ca2+] is consistently greater than root vacuolar [Ca2+], XRMA studies of root cell vacuoles from a range of species have found Ca to be below the level of detection [for cryo-scanning electron microscopy (cryo-SEM)/XRMA, i.e. <10 mm] in all root cell types, while other ions were found to be measurably and disproportionately accumulated (Storey et al., 2003a). These species include Arabidopsis thaliana (M. Gilliham, unpubl. res.), Thlaspi caerulescens (Wójcik et al., 2005), Lactuca sativa (lettuce) (Lazof and Läuchli, 1991); Zea mays (maize) (Chino, 1981), Phaseolus vulgare (kidney bean) (Chino, 1981), Populus alba (poplar) (Cocozza et al., 2008), Triticum aestivum (wheat) (Hodson and Sangster, 1989) and Hordeum vulgare (barley) (Huang and Van Steveninck, 1988). Few studies have looked at cell specificity in Ca accumulation along the length of the root. However, one study in poplar showed similar cellular levels in all root cells across a transverse section, yet a longitudinal reduction in total vacuolar Ca from the highest level in the root tip (Cocozza et al., 2008). Meristematic cells of Vitis vinifera (grapevine) roots accumulated Ca in raphide crystals (proposed to be calcium oxalate) in specialized cortical cells which are hypothesized to play a role in regulating Ca delivery to the xylem stream (Storey et al., 2003b). In contrast to roots, Ca is frequently observed to be compartmentalized within leaf vacuoles.
In barley, Ca has been found in the leaf epidermis but is below detection limits within the mesophyll (<10 mm; see Table 1 and Fig. 1) (Dietz et al.., 1992b; Fricke et al.., 1994a; Karley et al., 2000a). Furthermore, different epidermal cells vary in their vacuolar [Ca], with interstomatal cells (those epidermal cells next to stomata) accumulating much greater Ca (>300 mm) than epidermal ridge or trough cells further away from the peristomatal cavity (Fricke et al., 1995, 1996; Fricke, 2004). All commelinoid monocots so far studied, including wheat and Sorghum bicolor (millet) (Boursier and Läuchli, 1989; Läuchli and Boursier, 1989; Dietz et al., 1992b; Leigh and Storey, 1993; Williams et al., 1993; Hodson and Sangster, 1998; Karley et al., 2000a, b) accumulate Ca within the epidermis.
In contrast, most eudicots studied, accumulate Ca predominantly in the mesophyll, as observed in Vicia faba (broad bean), Lupinus luteus (yellow lupin), Leontodon hispidus, Citrus jambhiri (rough lemon), Thalapsi precox and Coriandrum sativum (coriander) (Fig. 1) (Outlaw et al., 1984; Treeby et al., 1987; de Silva et al., 1996; Storey et al., 2003a; Vogel-Mikuš et al., 2008a, b; Kerton et al., 2009). Given that mesophyll cells generally contribute >50 % of the total leaf area, and the epidermis <15 % in eudicots and ≤28 % in barley (Pyke et al., 1991; Dietz et al., 1992a; Klich, 2000) the distinct patterns of Ca accumulation between commelinoid monocots and eudicots is likely to, at least in part, underpin the lower leaf [Ca] in the monocots (see above; Broadley et al., 2003).
The role, and mechanism, for preferential accumulation of elements is currently unknown but may be, in part, linked to maximizing elemental availability to the plant. For instance, Ca and P are never localized within the same compartment in high concentrations when soluble and bioavailable (Fig. 1 and Table 1) (Hepler and Wayne, 1985; Leigh and Tomos, 1993; Grusak and DellaPenna, 1999; Brinch-Pedersen et al., 2002). Another consequence of cell-preferential vacuolar ion accumulation is that only certain ion combinations contribute to osmotic balance in specific cell types. For instance, vacuolar concentrations of total Ca ([Ca]vac) can be enhanced over that reported under standard growth conditions (Table 2), increasing from approx. 20 mm to 100 mm in shoots of K-deficient barley, suggesting a role of Ca2+ in charge and/or osmotic balance (Leigh et al., 1986). Ca/K interplay is observed to occur in a cell-specific manner in barley plants grown under low [Ca] conditions, where epidermal K compensates for the loss of Ca (Fricke et al., 1994b). Furthermore, an arabidopsis T-DNA insertion line with enhanced root suberization, termed esb1, was shown to have a 50 % reduction in shoot Ca, but a 30 % increase in K+ and a 40 % increase in Na+ (Baxter et al., 2009). Interestingly, Ca2+-deficiency down-regulates expression of vacuolar-targeted Na+/H+ antiporters of the NHX family, including AtNHX1, AtNHX2 and AtNHX5 in roots (Maathuis et al., 2003). This appears to correlate with the increased Na+-content of shoots under such conditions which may result from the reduced ability of roots to sequester Na+ (also see the section entitled ‘Cell-specific compartmentation of Na in leaves’).
Does differential expression of Ca2+-transporters underpin cell-specific Ca distribution?
Previously it has been suggested that it is the transport routes that Ca2+ takes that determines where Ca2+ is stored; in cereals, vein extensions from the xylem are proposed to expose the epidermis to Ca2+ first, and in most eudicots the xylem unloads to the mesophyll first (Karley et al., 2000a). However, if this is the predominant mechanism determining leaf cell-specific accumulation, why is Ca not accumulated within the bundle sheath cells of species that accumulate Ca in the mesophyll? Furthermore, leaves that have been fed unphysiological levels of Sr2+ (50 mm), which is a reliable tracer for Ca2+, accumulate Sr2+ in the apoplast around all cells but only within vacuoles of cells that normally take up Ca2+ (Storey and Leigh, 2004). However, it was also shown in barley that the influx of 45Ca2+ across the plasma membrane of mesophyll and epidermal protoplasts was equal despite the clear differences in storage capacity of the vacuole (Karley et al., 2000b). As a result, it is likely that the different transport characteristics of the epidermal and mesophyll tonoplast control differential Ca accumulation.
There are several gene families reported as capable of transporting Ca2+ within plants, including Ca2+-permeable ion channels (GLRs, CNGCs, annexins and TPC), Ca2+-transporting P-type-ATPases (ACA and ECA) and divalent cation-H+ antiporters (CAX, CCX and CHX) (Maser et al., 2001; Shigaki and Hirschi, 2006; McAinsh and Pittman, 2009). A study by Carter et al. (2004), on the tonoplast proteome of arabidopsis mesophyll cells, identified key members of these families. Whilst channels will move Ca2+ passively down its electrochemical gradient (into the cytoplasm), only tonoplast-localized transporters capable of catalysing Ca2+ uptake against its electrochemical gradient into vacuoles could perform the role of preferential Ca2+ accumulation under physiological conditions (Pottosin and Schönknecht, 2007; McAinsh and Pittman, 2009).
‘Calcium channels’ do not directly control calcium compartmentation within arabidopsis vacuoles
Although non-selective cation channels (NSCC) have frequently been characterized using radioactive tracers and electrophysiology, the molecular identity of NSCCs is not clear (Demidchik and Maathuis, 2007). As related proteins encode ion channels in other kingdoms, likely NSCC candidates include members of both cyclic-nucleotide gated channel-like proteins (CNGCs) and glutamate receptor-like proteins (GLRs) (Demidchik and Maathuis, 2007; Roy et al., 2008). Misexpression of individuals from both gene families have been shown to alter Na+, K+, Cs+ or Ca2+ fluxes into and accumulation within tissues, or the sensitivity of plants to Na+, K+ or Ca2+ (Hampton et al., 2005; Gobert et al., 2006; Ali et al., 2007; Guo et al., 2008).
Most arabidopsis plants so far characterized with misexpression of AtGLR have Ca-related phenotypes. For instance, AtGLR3·3 and AtGLR3·4 have been implicated Ca2+-entry into cells gated by selected amino acids, and while the latter is speculated to have a role in abiotic stress signalling both have been implicated in sensing soil N status (Meyerhoff et al., 2005; Stephens et al., 2008). AtGLR3·1 is important in stomatal guard cell closure mediated by apoplastic Ca2+ (Cho et al., 2009). Over-expression of AtGLR3·2 did not alter total shoot [Ca], but induced symptoms of Ca deficiency (leaf tip necrosis, hypersensitivity to Na+ and K+), that could be alleviated by increasing [Ca2+] in the growth medium (Kim et al., 2001). Whereas, antisense AtGLR1·1 plants were hypersensitive to supplemental Ca2+ but not K+ or Na+ (Kang and Turano, 2003). Due to its vascular localization, it was proposed that AtGLR3·2 has a role in xylem unloading of Ca2+ (Kim et al., 2001; Turano et al., 2002). However, in general, it is not entirely clear how misexpression of any AtGLR can cause Ca-2+-sensitive phenotypes. Furthermore, in arabidopsis no T-DNA insertion line for the GLRs have a perturbed Ca2+ accumulation phenotype from the public PiiMS database (http://www.ionomicshub.org/home/PiiMS) and there is no clear expression pattern of the 20 members of the GLR family in the leaf epidermis or mesophyll (Ca-poor and Ca-rich cell types, respectively) except that AtGLR3·7 was present in all cell types (Roy et al., 2008). Whether this was due to stoichastic variation in detection of transcripts (Raser and O'Shea, 2004) or a flexible complementation between family members is unknown. However, with such information it is difficult to delineate the precise role of AtGLRs in cell-specific Ca accumulation in arabidopsis.
The channel properties of AtCNGCs appear to be a little more secure than those of AtGLRs. Heterologous expression of certain members resulted in detection of Na+-, K+- and Ca2+-permeable ion-channel activity and in planta study of mutants lacking expression of AtCNGC1, AtCNGC2, AtCNGC11 and AtCNGC12 revealed Ca2+-related phenotypes (Hampton et al., 2005; Chin et al., 2009). For instance, cngc1 mutants show reduced Ca2+-uptake into plants, while the cngc2 lines are hypersensitive to Ca2+ in the growth medium (Chan et al., 2003; Ma et al., 2006) and in standard growth medium are transcriptionally similar to high Ca2+-grown plants (Chan et al., 2008). Mining the PiiMS database, indicated that two independent mutations in AtCNGC2 showed a 5–15 % increase in shoot [Ca] and a 10 % decrease in shoot [K] compared with the wild-type background. The cell type in which this additional Ca is stored is unknown and needs to be established to see if the poor growth phenotype of these plants is related to defective Ca2+-signalling or -storage (Chan et al., 2008). In summary, the roles for AtCNGC and AtGLR in cell-specific Ca accumulation are not well established and it is likely, from current evidence, that many of these genes play more of a role in Ca2+-signalling than in Ca storage.
Richardson et al. (2007), in the search for epicuticular wax genes compared the transcriptomes of barley leaf epidermis (the Ca-accumulating cell type) against that of the remaining leaf. Mining their dataset, we found almost seven times the number of transcripts for Ca2+-transporters and Ca2+-binding proteins enriched within the Ca-rich epidermis. Among these included homologues of the auto-inhibited Ca-ATPase (ACA) family, the cation exchanger (CAX) family, the annexins and numerous calcium-dependent/calmodulin interacting protein kinases (CDPK/CIPK) and calmodulins. Homologues have also been found to be enriched in the mesophyll (where Ca is accumulated) in the eudicot arabidopsis by transcriptomic (Yang et al., 2008) and proteomic methods (Carter et al., 2004). Those candidates detected in all three studies and reported to be tonoplast targeted will be discussed here.
The role of ACA in cell-type-specific Ca accumulation
There are ten members in the ACA type-P2A Ca2+-ATPase family in arabidopsis. In general, ACA proteins are likely to be high-affinity Ca2+ pumps (KM = 0·4–10 µm; Hayter and Peterson, 2004), stimulated by calmodulin, and have been implicated in adjusting [Ca2+]cyt within the nanomolar range (Shigaki and Hirschi, 2006). As a result, these have been suggested to be critical for control of the [Ca]cyt signature by returning [Ca]cyt to the pre-signalling level (Maeshima, 2001; Anil et al., 2008). However, only AtACA4 and AtACA11 are both leaf and tonoplast specific (Geisler et al., 2000; Baxter et al., 2003; Carter et al., 2004; Lee et al., 2007a).
A mutant lacking both AtACA4 and AtACA11 was reported to have reduced shoot [Ca2+] and a reduction in transpiration rate compared with parental plants in a wild-type background. Whether the reduction in shoot [Ca2+] is a consequence or cause of the reduced transpiration rate is unknown. In addition, whilst ACA4 and ACA11 have been found in the Ca-rich mesophyll tonoplast of arabidopsis (Carter et al., 2004; Yang et al., 2008), it is not known whether the mesophyll cells of aca4/aca11 plants are compromised in capacity to load Ca. Numerous hypersensitive response-like lesions were also associated with aca4/aca11 mutation but could be suppressed by abolishing expression of the sid2 gene (isochorismate synthase essential for SA biosynthesis), or over-expression of a salicylic acid-degrading enzyme, NahG (Boursiac et al., 2007). Such a phenotype in aca4/aca11 plants hints at a perturbed regulation of [Ca]cyt which is likely to include a delayed return to steady-state [Ca]cyt following any elevation.
The role of cation/H+ antiporters (CAX) in cell-specific Ca accumulation
CAX proteins include low-affinity (CAX1; KM = 10–15 µm) Ca2+-transporters that use the proton-motive force generated by the vacuolar proton-pumping ATPase and pyrophosphatase to remove Ca2+ from the cytosol when elevated significantly above resting concentrations (i.e. after a signalling event or as a result of high Ca influx into the cell) (Hirschi et al., 1996). In arabidopsis there are six CAX genes and four have been functionally characterized to some degree (Shigaki and Hirschi, 2006; Shigaki et al., 2006). CAX proteins have also been implicated with a role in Ca accumulation within the leaf and given the lower expression of AtCAX1 in roots and localization of the protein in mesophyll tonoplast preparations (Carter et al., 2004); this may contribute to the very low root cell vacuolar [Ca2+] and the higher accumulation of Ca2+ (and Mn2+) in the mesophyll (Table 2) (Hirschi, 1999; Hirschi et al., 2000; Cheng et al., 2005).
Furthermore, over-expression of an N-terminally truncated form of AtCAX1 (called sCAX1) which is constitutively active in tobacco (Hirschi, 1999) and tomato (Park et al., 2005) was associated with an increased leaf [Ca], although this vacuolar sequestration seemed to be irreversible, isolating Ca away from the apoplast, signalling pathways and pectin cross-linking (Park et al., 2005) as plants also had symptoms of Ca-deficiency. As with the ACA genes, an arabidopsis mutant lacking two CAX family members, CAX1 and CAX3, sharing 77 % protein identity (Maser et al., 2001; Cheng et al., 2003) resulted in a significant reduction in shoot Ca, while the single CAX mutants have insignificant reduction in shoot [Ca2+], but do exhibit other growth phenotypes (Cheng et al., 2003; Zhao et al., 2008). Studies on the cell-specific expression of these Ca2+-transporters in arabidopsis and homologues in other plant species will be required to ascertain if their differential expression or regulation is involved in cell-specific Ca2+ compartmentation.
Calcium binding proteins can also affect Ca accumulation in specific cells and may help in biofortification efforts
Calcium-binding proteins (CaBP) such as calreticulin located in the endoplasmic reticulum, help retain Ca2+ within the endoplasmic reticulum and buffer [Ca2+]cyt (Akesson et al., 2005). CaBP are also on vacuolar membranes and are thought to reduce vacuolar Ca2+ activity (aCa2+) and thereby increase Ca-storage capacity of the vacuole (Yuasa and Maeshima, 2001). Furthermore, while tomato plants overexpressing CAX1 result in a Ca-deficiency phenotype, this is alleviated and [Ca] is further increased by co-expression of calreticulin. Whether the storage capacity of both the endoplasmic reticulum and vacuole is increased remains to be seen. Further indicating their potential role in buffering Ca2+-availability, CaBP found in arabidopsis have so far only been localized to the plasma membrane and so are not likely to play a significant role in Ca-accumulation (Nagasaki et al., 2008).
The role of signalling and transpiration in Ca accumulation
Although it is likely that the vacuolar Ca2+-transporter complement may influence where Ca is stored in the leaf, the role of transpiration is not disputed in the supply of Ca; when transpiration was inhibited, Ca (or Sr) did not accumulate in leaves (Storey and Leigh, 2004). Lower transpiration was also shown to reduce the accumulation of Ca (Storey and Leigh, 2004; Kerton et al., 2009; Fricke, 2004). Nevertheless, Ca still preferentially accumulates in the same cell types as under higher transpiration. This contrasts with nutrients such as Cl and K that do not require transpiration to accumulate to high levels in tissues, presumably due to the redistribution that occurs via symplast or the phloem (Fricke, 2004; Storey and Leigh, 2004).
We propose the importance of vacuolar Ca transporters in cell-specific accumulation patterns based on evidence from transcriptional profiling and the phenotypes of mutant plants, yet many of these transporters are also post-translationally regulated. However, as with the transporters, it is clear that protein kinases (CDPKs or CIPKs), and their interacting partners – calmodulins or calcineurin B-like proteins (CBLs) – may be expressed in a cell-specific manner (EFP browser; Winter et al., 2007). As they are known to interact with, and regulate the activity of various ion transporters, including ACAs, transcriptomics may still provide a powerful tool in deciphering elements important in determining Ca accumulation (Hwang et al., 2000a, b; Kim et al., 2007; Luan et al., 2009),
We have shown, and as will be seen in the following section on sodium, that Ca levels in cells can be perturbed under various conditons. The CBL-CIPK and calmodulin–CDPK networks have been implicated in signal transduction, thus providing a medium for bringing about the observed ion perturbations in a cell-specific manner (Luan et al., 2009). While influencing transporter activity, these protein kinase networks may also contribute to wider transcriptional control, with leaf expressed members of the CDPK family known to bind and activate transcription factors (Choi et al., 2005; Milla et al., 2006; Yu et al., 2007).
SODIUM COMPARTMENTATION IS AN IMPORTANT MECHANISM OF SALINTY TOLERANCE
Compartmentation of Na+ maintains low [Na+]cyt
Sodium is a micronutrient for C4 and CAM plants, where it plays a role in CO2 fixation, but is not generally required for the growth of C3 plants (Brownell and Crossland, 1972, 1974). Some halophytic land plants require high concentrations of Na+ surrounding their roots (100–200 mm) for optimum growth but to most plants such concentrations would terminally interfere with growth and metabolism (Flowers and Dalmond, 1992; Tester and Davenport, 2003; Shabala and Cuin, 2007). For halophytes and glycophytes alike, compartmentalization of Na+ away from the apoplasm and cytoplasm is thought to be one of a series of important mechanisms to tolerate high soil Na+ (Flowers et al., 1977; Flowers and Colmer, 2008; Munns and Tester, 2008). Cytosolic Na+ is believed to interfere with cellular processes which require K+ and high apoplastic [Na+] will reduce turgor and the driving force for leaf growth (Flowers et al., 1991). As Na+ is carried in the transpiration stream, and there is limited evidence for Na+ redistribution in the phloem leaves are particularly prone to injury by Na+ (Munns and Tester, 2008).
Technical limitations have resulted in few measurements of plant tissue aNacyt and, to our knowledge, leaf aNacyt has never been measured directly. Debate remains surrounding the reliability of such measures which is not helped by comparison of measurements of total ion concentration ([i]) to activity (ai) (Kronzucker et al., 2006). However, there is agreement that when aNacyt increases beyond 30–40 mm, or [Na+]cyt beyond 100 mm, cellular function will be impared (even in halophytes) (Hajibagheri et al., 1988; Halperin and Lynch, 2003; Flowers and Colmer, 2008; Munns and Tester, 2008). As such, more salt-tolerant cultivars have often been observed to minimize aNacyt, and retain greater aKcyt, compared with less-tolerant cultivars (Flowers and Hajibagheri, 2001; Carden, 2003; Kader and Lindberg, 2005; Anil et al., 2007).
Retention of vacuolar K may contribute toward the regulation of cytosolic [K+] and [Na+] under saline conditions (Flowers and Hajibagheri, 2001; Carden, 2003; Läuchli et al., 2008). At high external aNa+, Ca and K deficiency is induced by reducing membrane aCa2+ which reduces selectivity of Na+ transport into cells and reduces K+ influx (Reid and Smith, 2000). Membrane Ca2+ also reduces Na+-induced K+ efflux (Shabala et al., 2006). Both of these can be partially ameliorated by adding additional Ca2+ to salinized plants (Munns and Tester, 2008). So mechanisms to control compartmentation of K, Ca and Na are all important to salinity tolerance.
Roots compartmentalize Na in a specific cell type to reduce shoot Na load
Cell-specific and whole-organ compartmentation of Na within plant roots has been observed in many species, but variation exists between the cell type that accumulates the highest total Na according to the species or variety, root type, composition of the root bathing medium, the growth conditions and sampling time. Retention of Na within roots has been muted as a key Na tolerance mechanism of glycophytes which enables them to lower their turgor and continue to grow in the face of an increasingly negative soil water potential (Tester and Leigh, 2001; Munns and Tester, 2008). However, in barley and wheat, sensitive varieties have been observed to accumulate more Na in roots than tolerant varieties (Flowers and Hajibagheri, 2001; Läuchli et al., 2008), although in tolerant varieties of barley the amount of root Na was greater in younger plants (Flowers and Hajibagheri, 2001; Carden, 2003).
XRMA studies of salinized arabidopsis, grapevine and wheat roots show interesting parallels (Storey et al., 2003b; Läuchli et al., 2008). In 2-week-old durum wheat (and arabidopsis) seedlings exposed to 50 mm NaCl the [Na] in seminal (or primary) roots is relatively high in the epidermal vacuoles and decreases towards the endodermis (Läuchli et al., 2008). The vacuoles of root cortical cells are much like leaf mesophyll cells in that they have the highest volume and hence have the greatest storage capacity of each respective organ. It is hypothesized that the epidermis and cortex ‘mops up’ apoplastic Na+ prior to reaching the inner part of the root. In all species and root types (e.g. seminal or lateral), the pericycle appears to play a major role in Na compartmentation in more tolerant varieties. Although xylem [Na] concentrations are likely to be higher in the less-tolerant varieties, the Na content of the xylem parenchyma vacuoles can differ (Läuchli et al., 2008; Møller et al., 2009). In durum wheat, [Na] increased in the pericycle vacuoles in both varieties when exposed to 50 mm NaCl but, in the less-tolerant durum variety, [Na] was also significantly higher in the xylem parenchyma cell vacuoles – possibly reflecting the higher xylem [Na]. This contrasts with the result with more salt-tolerant arabidopsis where xylem parenchyma cells had higher [Na] than the more Na-sensitive arabidopsis genotype (Møller et al., 2009). Whether XRMA is the best technique to estimate ion concentrations in small cells or cells with small vacuoles such as xylem parenchyma cells, or the pericycle, is debatable (refer to ‘Techniques for plant elemental profiling’ section; Wegner and Raschke, 1994). In addition, positional and developmental effects are likely in different plant species or even across the same organ; therefore sections taken at different distances up the root may yield different results and, when it is not known if the whole root or only part of the root shares the same accumulation profile, [Na] results are difficult to interpret physiologically.
The role of HKT1;5-type genes in reducing shoot [Na] by retrieving Na+ from the xylem is now well established (reviewed in Munns and Tester, 2008). Both the HKT1;5 homologous proteins in rice [OsHKT1;5 (SKC1)] and arabidopsis (AtHKT1;1) are expressed in the stele (Sunarpi et al., 2005). The homologues from bread wheat (TaHKT1;5D) and durum wheat line 149 (TmHKT1;5A) have also been proposed to share such a localization (Byrt et al., 2007). By over-expressing AtHKT1;1 selectively within stelar cells, Na+-influx into such cells was increased over non-HKT1-expressing stelar cells, as was their [Na] content, whilst shoot [Na] was reduced (Møller et al., 2009). Furthermore, highlighting the importance of such a mechanism in plants, transgenic plants over-expressing AtHKT1 within stelar cells were also more salt tolerant.
In wheat, Nax1 (TaHKT1;4) is the proposed candidate for another mechanism contained within durum wheat line 149 that keeps lower laminar [Na] by retrieving Na+ into the leaf sheath (and the root) (Byrt et al., 2007). However, although Nax1 is likely to encode a Na+-selective transporter, all attempts to clone and functionally characterize HKT1;4 genes have so far failed.
A number of other genes, which may have a role in the accumulation of Na in particular root cells or in the regulation of Na+ transport to the shoot, have been implicated from existing datasets (Birnbaum et al., 2003; Dinneny et al., 2008). In arabidopsis, AtNHX4, AtNHX5 and AtNHX6 all decrease in expression from epidermis to inner cortex, then peak again in the stele at various segments (stages I, II, III as per Birnbaum et al., 2003) along the primary root (Birnbaum et al., 2003; Nawy et al., 2005) and increase in total expression levels along the vertical axis. Both AtNHX1 and AtNHX2, which are tonoplast located, and AtNHX5, which is of unknown membrane location, have all been implicated in Na compartmentation in the root vacuole (Yokoi et al., 2002). However, arabidopsis mutants lacking expression of NHX4 have recently been shown to increase salt tolerance and this enhanced tolerance was associated with reduced root and shoot [Na] (Li et al., 2009). As such, it would be important to identify the membrane location of AtNHX4. It is possible that pleiotropic effects were also at work masking the true function of the gene (as occurs with many vacuolar Ca transporters, see above). However, little functional compensation has so far been reported between members of the NHX family; when AtNHX1 is knocked out, no other members change in expression (Sottosanto et al., 2007). Interestingly, salt-tolerant varieties of wheat have greater transcript abundance of NHX transporters and the majority of other arabidopsis NHX T-DNA insertion lines have resulted in greater sodium sensitivity (Apse et al., 2003; Saqib et al., 2005). In addition, over-expression of members of the NHX family increases [Na] of most tissues and Na+-tolerance in other species [AtNHX1 in cotton (He et al., 2005a), brassica (Zhang et al., 2001) and in tomato (Zhang and Blumwald, 2001); OsNHX1 in ryegrass (Wu et al., 2005); AtNHX3 in sugarbeet (Liu et al., 2008); and AtNHX7/SOS1 in arabidopsis (Shi et al., 2003)]. Therefore, it is likely that many of these genes do contribute to cell-specific accumulation of Na in the root or shoot; however, definitive information is limited on which particular gene or suite of genes is active in each cell type.
In arabidopsis, SOS1 (AtNHX7), an Na+/H+ antiporter, is located on the plasma membrane of root-tip meristematic cells and xylem parenchyma cells of the root and shoot (Shi et al., 2002). SOS1 has been demonstrated to catalyse the movement of Na+ out of cells and by doing so SOS1 has recently been proposed to protect cells of the elongation zone from accumulating Na+ (Guo et al., 2009; Oh et al., 2009). In addition SOS1 activity is thought to keep the pericycle [Na] low which may be important in reducing symplastic flow toward the xylem (Oh et al., 2009). Higher activity (i.e. not only salt-inducible but also in a cell-specific manner) of SOS1 in Thellungeiella halophila, a salt-tolerant relative of arabidopsis, is also thought to be a mechanism that allows the plant to grow in higher salinities than arabidopsis (Oh et al., 2009).
Although HKT2;1 has been implicated in Na+ entry into some plants under low K+ (Laurie et al., 2002), the major entry point for Na+ into the root cells (and the root symplast) is thought to be through NSCCs on the plasma membrane. Most of these NSCC have been characterized electrophysiologically in the cortical or epidermal cells and so would co-incide with sites of accumulation. However, it is not known whether similar conductances are present in pericycle cells where lower [Na]vac are found. Several AtGLRs may encode Na- (and Ca2+-) permeable channels (Roy et al., 2008) and several AtGNGCs such as AtCNGC3 have been shown to be permeable to Na+ (Demidchik and Maathuis, 2007). Also members of both families have altered expression during salinity (Maathuis, 2006; Maathuis et al., 2003). The entry of Na+ through NSCC depolarizes the membrane which precludes K+-influx through inward K channels, such as AKT1, and can lead to substantial K+-loss from cells through depolarization-activated K+-channels, such as GORK, in less-tolerant species or varieties contributing to reduced K : Na ratios in cells (Shabala et al., 2006; Chen et al., 2007; Shabala and Cuin, 2007; Cuin et al., 2008). A diagnostic to test for the NSCCs involved in Na+ entry into cells may be their apparent block by polyamines (Shabala et al., 2007); polyamines appear to reduce Na+ entry into cells by a dual role of directly blocking NSCCs and increasing activity of H+-ATPases in the plasma membrane which would increase membrane potentials and hence the driving force for K+-entry through proteins such as AKT or high-affinity K+/H+ exchangers such as HAK/KUP or Na+ removal through plasma membrane Na+/H+ exchangers such as SOS1 (Shabala and Cuin, 2007; Shabala et al., 2007). Interestingly, another mechanism that T. halophila employs that results in higher Na+ tolerance than arabidopsis appears to be a higher selectivity of K+ over Na+ in its root cell NSCCs which results in a smaller depolarization upon Na exposure of T. halophila allowing continued K+ uptake, unlike in arabidopsis (Amtmann et al., 2005; Volkov and Amtmann, 2006). Such a mechanism could be conferred by amino acid substitutions or regulatory differences of the same protein or the presence of completely different proteins catalysing Na+ and K+ influx.
Cell-specific compartmentation of Na in leaves
In grasses, there are contradictory data in regard to whether specific cell types differentially compartmentalize Na within leaves (Fricke et al., 1994a, b, 1996; Karley et al., 2000a). Depending on the techniques used, preferential accumulation of Na was detected in vacuoles of epidermal cells of barley grown in [Na]ext of <1 mm (using ICP of isolated protoplasts) or ≤150 mm Na (SiCSA/XRMA), but no preferential accumulation was observed using cryo-SEM/XMRA at low (25 mm NaCl) or high Na (≥200 mm NaCl) (Leigh and Storey, 1993; James et al., 2006). Na appears to be accumulated at the expense of both Ca and K in all cell types of the leaf (Fricke et al., 1996). Although XRMA is not very sensitive to changes in [Na] due to high background Bremsstrahlung radiation it is mystifying why preferential accumulation is reliably detected in roots but not the shoots as [Na] is usually greater in shoots than in roots of barley. It is possible, as the number of studies looking at leaf cell-specific [Na] are relatively few, that differences in growth conditions or genotypes are the explanation for the differences between studies; clearly more work would be needed to confirm the overriding pattern.
The accepted paradigm for many cereal crops (excluding bread wheat which shows some degree of tissue tolerance to Na) is that the severity of salinity (NaCl) stress is proportional to leaf [Na] (Munns and Tester, 2008). However, declines in photosynthetic rates are not necessarily associated with increases in [Na] in leaf mesophyll cells in plants grown at high [NaCl]ext (Fricke et al., 1996; James et al., 2006). Based on measurements of aK+cyt (Cuin et al., 2003) and calculated [K+]cyt (James et al., 2006) both papers speculated that redistribution of K+ from epidermal vacuoles to mesophyll cytoplasm was a more important mechanism in salinity tolerance than excluding Na from the mesophyll per se. Cuin et al. (2003) further speculated that unlike the mesophyll, the epidermis can maintain viability with a very low aK+cyt, as epidermal cells are not highly metabolically active, as opposed to mesophyll cells.
In contrast to Na+, Cl− does show cell-specific compartmentation in cereals accumulating preferentially within the epidermis (Fricke et al., 1994a). Only above 100–150 mm [NaCl]ext does Cl begin to accumulate to appreciable levels within the mesophyll vacuoles and these increases are still only proportional to those within the epidermis. However, no strong or consistent link has been found with accumulation of Cl in the mesophyll with a reduction in photosynthesis and a much stronger correlation holds for Na (Leigh and Storey, 1993; Fricke et al., 1996; James et al., 2006). It appears that mesophyll vacuoles are also able to compartmentalize toxic [Cl−] away from the cytoplasm when cells start accumulating Cl. Furthermore, the distribution of nitrate (NO3−) mimics that of Cl− and as many nitrate-permeable channels and/or transporters are permeable to both ions it is likely that both ions are secreted into the vacuoles through the same mechanism. The identity of that transporter which is likely to be more active in epidermal cells than the mesophyll and is located on the tonoplast has not yet been definitively determined but is likely to be CLCa (De Angeli et al., 2009). A complicating factor in correlating nitrate transporters with nitrate accumulation is that the activity of nitrate reductase will also determine nitrate distribution; as nitrate reductase is more active in mesophyll cells compared with the epidermis this may contribute toward the greater epidermal [NO3−], but not [Cl], within leaf epidermal cells (Roy et al., 2003). In arabidopsis, both Na and Cl are selectively accumulated in the leaf epidermis under NaCl stress (M. Gilliham, unpubl. res.), opening the way for a more rapid identification of the molecular determinants of both Na and Cl storage in leaves.
CADMIUM COMPARTMENTATION DIFFERS BETWEEN CELL TYPES WITHIN AND BETWEEN HYPERACCUMULATOR AND NON-ACCUMULATOR PLANTS
Cadmium nutrition
The sensitivity of plants to heavy metals depends on an interrelated network of molecular and physiological mechanisms. These include; uptake and accumulation of metals through binding to extracellular exudates and cell wall; complexation of ions inside the cell by organic acids, amino acids, ferritins, phytochelatins (PCs) and metallothioneins; general biochemical stress defence responses such as the induction of antioxidative enzymes and activation or modification of plant metabolism to allow adequate functioning of metabolic pathways; rapid repair of damaged cell structures; and compartmentalization in the vacuole (Verkleij et al., 1990; Rauser, 1999; Hall, 2002; Cho et al., 2003). Cadmium (Cd) translocation and accumulation within the plant is intimately linked with transport through a variety of proteins with greater specificity for ions other than Cd2+, including Ca2+, Zn2+, Mg2+, Mn2+, Pb2+, Cu2+ and Ni2+ (Broadley et al., 2001; Shigaki et al., 2003; Yang et al., 1996). As a result, it is not surprising that there are correlations in shoot content of Cr, Pb, Cu, Ni and Zn (Broadley et al., 2001), yet the cell-specific accumulation patterns are not universally consistent and, as such, Cd is a good candidate to elaborate on in this review. The terms Cd hyperaccumulator and Cd-tolerant lines will refer to species able to accumulate large amounts of Cd in the shoot and able to grow in Cd-rich environments with limited effects on growth, while non-accumulators and sensitive lines are those that are significantly affected by high levels of Cd.
In plants, cadmium disrupts the homeostasis of essential metals by displacing them from metal-binding proteins and transcription factors (Clemens, 2006). This affects root growth and biomass production by inhibiting photosynthesis, respiration and mineral uptake and by disturbing the plant water status (Perfus-Barbeoch et al., 2002). In addition, genotoxicity and ecotoxicity of food-borne Cd in animals is well-documented, so its accumulation in the edible portion of plants is disadvantageous (Peralta-Videa et al., 2009; Chaney et al., 2000). There is substantial genotypic variation underlying Cd accumulation in leaf and grain in maize (Zhang and Song, 2008), suggesting that genetic factors underlie differences in Cd accumulation. But, the ability to redistribute Cd and/or limit its uptake in agricultural crops to reduce the adverse effects to the plant and to human health are important strategies being adopted for the traditionally higher Cd-accumulating tobacco leaf (Wagner and Yeargan, 1986) and durum wheat grain (McLaughlin et al., 1998a; Ozkutlu et al., 2007).
Cadmium is preferentially partitioned in plant roots
A number of reports have demonstrated the preferential accumulation of Cd and other non-essential heavy metals within the root as opposed to the shoot (Puig and Peñarrubia, 2009; Verbruggen et al., 2009). Work in maize and pea grown at a range of [Cd] within the soil demonstrates consistently 12–17 % of the total Cd in the plant is partitioned to the shoot, with the majority retained within the roots (Fig. 1) (Lozano-Rodríguez et al., 1997). As Cd is phloem-mobile (Herren and Feller, 1997), this evidence implicates root-to-shoot translocation as a major hurdle to the accumulation of Cd within the aerial tissues (leaf and grain). This theory is supported by reports in rice correlating Cd allocation in the shoot and grain across a genetic diversity set (69 accessions) with Cd concentration in the xylem (Uraguchi et al., 2009) and identifying associated QTL (quantitative trait loci) (Xue et al., 2009). Given the toxicity of Cd2+ to plants and animals, it is critical to determine how Cd partitioning within the leaf is correlated with Cd tolerance and how it can be enhanced to reduce accumulation in the edible portion of plants (Hu et al., 2009; Xue et al., 2009).
Given that Cd does not accumulate to very high intracellular levels in most plants (20–50 µm), often being conjugated in the apoplast, a detailed investigation of methods of Cd tolerance, and optimization of plant-based strategies for phytoremediation/phytoextraction (Ishikawa et al., 2006), has focused research on Cd hyperaccumulators, including Potentilla griffithii (Hu et al., 2009), Arabidopsis halleri (Küpper et al., 2000), black oat (Avena strigosa) (Uraguchi et al., 2009) and Thlaspi caerulescens (Lombi et al., 2000; Wójcik et al., 2005; Küpper et al., 2007a). Once internalized, it has been shown that the vacuole is the primary site of accumulation of a number of heavy metals including Cd (Ernst et al., 1992; Hall, 2002).
Cadmium is distributed differently between cell types in hyperaccumulator and non-accumulator plant species
Cells accumulating Cd vary widely between and within plants. Within certain ecotypes of T. caerulescens, Cd is accumulated in the root apoplast (bound to cell wall components) and vacuoles of cortex parenchyma cells, endodermis, parenchyma cells of the central cylinder and xylem vessel, while accumulation within the leaf is predominantly in the leaf mesophyll vacuole, with less in the epidermis and none found in the apoplast by X-ray microanalysis (Fig. 1) (Wójcik et al., 2005). This may be linked to the probable forms of Cd complexed with PCs, or organic acids, which can be taken into the xylem and then rapidly taken-up by leaf cells – which may contribute to the mesophyll cells (being the initial cell type exposed to the Cd–PC complexes from the xylem) accumulating more than epidermal cells (Hu et al., 2009). Arabidopsis halleri accumulates 1500 mg kg−1 Cd in the shoot, predominantly in the trichome base (Hokura et al., 2006) and mesophyll cells (Küpper et al., 1999, 2000; Sarret et al., 2002; Küpper and Kroneck, 2005; Ueno et al., 2008).
It was shown recently, by ion-selective electrode measurement, that the majority of Cd in the xylem sap is in the ionic form, but the remaining Cd appears to be complexed in an organic, sulphur-rich form, e.g. Cd–PC complexes (Ueno et al., 2008). We propose that free Cd2+ is not rapidly taken up by leaf cells and as a result accumulates at the base of the trichomes (as CaPO4 fills the branches of the trichomes and the upper stem, restricting additional extracellular nutrients into the branch of the trichomes), while complexed Cd is taken up rapidly into the mesophyll. This is supported by evidence in the Cd-sensitive A. thaliana, which accumulates fewer PCs than A. halleri, where Cd is only found in the trichome base and stem (Lee et al., 2003). Increasing the levels of PCs by over-expression of AtPCS1 caused an increase in shoot Cd and Cd tolerance within A. thaliana (Ager et al., 2002, 2003; Lee et al., 2003).
Using X-ray microanalysis, Wójcik et al. (2005) also demonstrated mesophyll accumulation of Cd as electron-dense deposits in Thlaspi caerulescens with some localized in the epidermis, in a decreasing manner following the route of water flow. Vogel-Mikuš et al. (2008b) showed the slightly less-tolerant Thlaspi praecox accumulates less Cd in the mesophyll than in the epidermis. This evidence supports a partitioning role of Cd into the mesophyll vacuole to enhance Cd tolerance. The mesophyll cells were also shown to accumulate elevated concentrations of Mg2+, interpreted as a defence against the substitution of Mg2+ in chlorophyll by Cd2+ (Küpper et al., 2001, 2007a).
Thlaspi caerulescens can accumulate 25 000–30 000 mg Zn kg−1 dry weight and 2000 mg Cd kg−1 dry weight in the shoot without showing any toxicity symptoms or reduction in growth (Brown et al., 1996; Shen et al., 1997; Küpper et al., 1999). The increase in [Cd2+], given it is primarily targeted to the mesophyll, is found to be associated with a proportionate reduction in other cations within the same cell, primarily K+, and Ca2+ in Thlaspi (Wójcik et al., 2005) and A. halleri (Küpper et al., 2000) by comparison with closely related non-hyperaccumulators and by analysis of response to altered levels of Cd in the growth solution used. It has been shown that when Trifolium repens (Wang and Song, 2009) and barley (Elenany, 1995) plants are exposed to higher Ca : Cd ratios, plants show an enhanced tolerance to Cd. Given the differential accumulation profile of Ca and Cd the protective influence of higher [Ca] has been proposed to result from reduced absorption of Cd (McLaughlin et al., 1998b; He et al., 2005b), ability to form Ca/Cd crystals intracellularly and extracellularly (Choi et al., 2001; Choi and Harada, 2005) and/or out-competition for transport by Ca-permeable channels (Perfus-Barbeoch et al., 2002).
Role of Cd2+-transporters in accumulation
Molecular approaches indicate that transporters for essential metals such as Zn2+ and Fe2+ can mediate Cd2+ uptake by root cells (Korshunova et al., 1999; Nakanishi et al., 2006). Xylem loading of Cd has been shown to be mediated by the P-type ATPase transporter AtHMA4 in Arabidopsis thaliana (Verret et al., 2004) and its homologues in the Cd-hyperaccumulator plants Thlaspi caerulescens and Arabidopsis halleri (Papoyan and Kochian, 2004; Hanikenne et al., 2008). Specifically, the higher expression of AtHMA4 found in A. halleri is attributable to a combination of modified cis-regulatory sequences and an increase in copy number, in comparison to the non-accumulating sister species A. thaliana (Hanikenne et al., 2008). Physiological experiments also demonstrated the existence of phloem-mediated Cd transport into seeds of various plants (Popelka et al., 1996; Herren and Feller, 1997; Harris and Taylor, 2001; Tanaka et al., 2003).
Cation transporters with broad substrate specificity are the likely entry point of Cd2+ into cells (Clemens, 2001). It is surprising therefore that the site of accumulation of Zn and Cd, while similar in the roots of T. caerulescens, differs in the shoot (Küpper et al., 1999; Vázquez et al., 1992, 1994). This possibly results from the preferential complexing with PCs prior to transport of Cd, but not Zn, or differences in leaf cell-type expression or specificities of respective transporters (Küpper et al., 1999, 2000). Indeed in T. caerulescens fed high levels of Zn, with leaves studied by Synchrotron XAS, Zn (but not Cd) was again found in the epidermis, but not bound to any strong ligands (Küpper et al., 2000, 2004). Adding to the complexity, A. halleri, previously known as Cardaminopsis halleri L. Hayek, is one species known to hyperaccumulate Cd and Zn within the same leaf cell types (Küpper et al., 2000; Bert et al., 2003).
The majority of proteins identified with affinity for Cd2+ and Zn2+ transport belong the ZIP (ZR, IRT-like protein), IRT (Korshunova et al., 1999; Clemens, 2001), NRAMP (natural resistance-associated macrophage protein) (Cailliatte et al., 2009; Thomine et al., 2000), MTP HMA (P-type ATPase) families (Guerinot, 2000; Ramesh et al., 2003; Puig and Peñarrubia, 2009). By complementation of a yeast Zn-transport-defective mutant with a T. caerulescens cDNA library, Lasat et al. (2000) cloned the ZNT1 cDNA, which encodes a high-affinity Zn transporter. However, ZNT1 can also mediate low-affinity Cd transport (Lasat et al., 2000). Based on the study of two T. caerulescens ecotypes, Lombi et al. (2000) suggested that Cd may be transported in the low Cd-accumulation ecotype via ZNT1 but, conversely, that Cd may be mediated in the high-accumulation ecotype via a high-affinity Cd transporter. This latter point was further strengthened given that the expression of ZNT1 in high Cd-accumulating T. caerulescens by in situ hybridization was lower in the non-accumulating cell types in the leaf (Kupper et al., 2007b).
Other proteins found to transport Cd2+ include the calcium exchanger (CAX) family in arabidopsis. AtCAX1 and AtCAX2, tonoplast localized calcium/proton antiporters, were first identified in a screen for rescuing a Ca2+ transport-deficient Saccharomyces cerevisiae line (Hirschi et al., 1996). Over-expression of full-length and N-terminally truncated (constitutively active) versions of AtCAX1 (Hirschi, 1999); AtCAX2 (Hirschi et al., 2000); AtCAX2, AtCAX4 and AtCAX11 (now called CCX5) in tobacco have been shown to increase the rate of Cd2+ (and Ca2+) uptake by root tonoplast vesicles (Korenkov et al., 2007). The expression levels of AtCAX2, AtCAX4 and AtCAX11, but not AtCAX1 or AtCAX3, are higher in the root than the shoot, and within the root the cell-specific expression levels mimic the Cd accumulation profile (EFP browser; data from Yang et al., 2008). This evidence suggests that these members of the so-called CAX2-like clade may be responsible for mediating vacuolar sequestration of Cd2+ (and other heavy metals), despite only being induced poorly by Cd2+ (Shigaki et al., 2006; Edmond et al., 2009). Another two pieces of evidence support the distinction between clades of the CAX family: (1) co-expression of AtCAX2 and AtCAX4 in tobacco using two root-selective promoters resulted in increased Cd accumulation in the root and 15–25 % reduced leaf Cd (Korenkov et al., 2009); (2) expression of AtCAX2 (Hirschi et al., 2000), but not AtCAX1 (Hirschi, 1999) under the 35S promoter leads to a significant increase in root, but not shoot [Cd]. It would be interesting to measure cell-specific [Cd] and [Zn] in the 35S::AtCAX2 over-expressing line in the root and shoot, to see if this gene and its homologues in T. caerulescens contribute to the differential accumulation of these ions, or to see if they are post-translationally regulated.
A study in barley identifying changes in the tonoplast proteome after exposure to varying levels of Cd has affirmed theories regarding the aforementioned transporter families (Schneider et al., 2009). Schneider et al. (2009) found Cax1a (implicated in Cd2+ transport), a member of the MRP family of ABC transporters (implicated in transport of Cd–PC complexes) and an NRAMP member to be up-regulated in the presence of 20 µm Cd2+. Each of these transporters plays a different putative role in Cd detoxification, with their simultaneous up-regulation suggesting the importance of multiple strategies to enhance cellular tolerance.
Finally, a wheat gene, LCT1, was cloned by its ability to rescue a S. cerevisiae mutant deficient in K+ uptake (Schachtman et al., 1997). It was later shown that LCT1 is able to transport not only K+ and Na+, but also Ca2+ and Cd2+, in yeast (Clemens et al., 1998). Expression of this gene in tobacco resulted in lower shoot and root [Cd2+], higher shoot [Ca2+] and an increased Cd tolerance, but only when plants were grown in the presence of 1 mm Ca (Antosiewicz and Hennig, 2004). Heterologous expression of LCT1 in the methylotrophic yeast, Pichia pastoris, also resulted in reduced endogenous Cd transport activity (Diatloff et al., 2006). Together, LCT1 seems to be linked with Cd tolerance, via a Ca2+-dependent pathway, but, with no known homologues in rice or arabidopsis, transcriptional profiling and protein interaction work will need to be performed in the future to determine the mechanism of reducing endogenous Cd uptake.
FUTURE RESEARCH AND CONCLUDING REMARKS
Within the limited scope of this review, we have found that cell-specific accumulation of ions (Na, Cd, Ca and K) is typically robust within an individual plant. However, the cell type in which ions accumulate may differ between plant species, the contrast between leaf Ca accumulation in the Poales and eudicots being a case in point (Table 2 and Fig. 1). Furthermore, Ca and K (and P) are never localized, when soluble, at high levels within the same leaf cell types, while Cd accumulation within the leaf differs between hyperaccumulators and non-accumulators. Na and Cd predominantly accumulate within roots, but Cd within the apoplast and Na with specific cell types, to prevent translocation to the shoot. When in the shoot, accumulation mechanisms are needed to exclude the toxic ions from the mesophyll cytoplasm.
Means of enhancing the tolerance of plants to heavy metals and toxic cations was outside the scope of this review, yet it is clear that the same protein can transport various ions. As a result, knocking out these genes may reduce accumulation of not just the toxic ion. Therefore, protein engineering strategies may be required to change the selectivity of certain ion transport proteins to alter toxic ion accumulation without perturbing overall critical ion homeostasis.
It is clear that a large body of work exists describing the cell-specific ion accumulation within a variety of plants using a number of different techniques for their quantification. Contemporary efforts to combine knowledge have emerged for the cell-specific transcriptome (EFP browser; Winter et al., 2007), the proteome (Weckwerth et al., 2008), the interactome (Geisler-Lee et al., 2007) and the ‘ionome’ of various plants (PiiMS database; Baxter et al., 2007). Integration of these ‘omes’ on a cell-specific basis would permit greater understanding of the control of the ion profile. Furthermore, among this expanse of knowledge may reside the links to enhancing the success of bio-fortification and phytoremediation strategies.
ACKNOWLEDGEMENTS
This work is an output from an Australian Research Council Discovery award to Roger Leigh (R.A.L.), Brent Kaiser and Stephen Tyerman. The authors also acknowledge funding from the Faculty of Sciences, University of Adelaide to R.A.L. and M.G. We thank R.A.L. for advice on the manuscripts content and critical evaluation by Dr Vanessa Conn and two anonymous referees.
LITERATURE CITED
- Ager FJ, Ynsa MD, Dominguez-Solis JR, Gotor C, Respaldiza MA, Romero LC. Cadmium localization and quantification in the plant Arabidopsis thaliana using micro-PIXE. Nuclear Instruments & Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2002;189:494–498. [Google Scholar]
- Ager FJ, Ynsa MD, Dominguez-Solis JR, Lopez-Martin MC, Gotor C, Romero LC. Nuclear micro-probe analysis of Arabidopsis thaliana leaves. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2003;210:401–406. [Google Scholar]
- Akesson A, Persson S, Love J, Boss WF, Widell S, Sommarin M. Overexpression of the Ca2+-binding protein calreticulin in the endoplasmic reticulum improves growth of tobacco cell suspensions (Nicotiana tabacum) in high-Ca2+ medium. Physiologia Plantarum. 2005;123:92–99. [Google Scholar]
- Ali R, Ma W, Lemtiri-Chlieh F, et al. Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. The Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann A, Blatt MR. Regulation of macronutrient transport. New Phytologist. 2009;181:35–52. doi: 10.1111/j.1469-8137.2008.02666.x. [DOI] [PubMed] [Google Scholar]
- Amtmann A, Wang B, Fricke W, Volkov V. Comparative analysis of ion transport in Arabidopsis related species differing in salt tolerance. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2005;141:S339–S339. [Google Scholar]
- Andreev IM. Functions of the vacuole in higher plant cells. Russian Journal of Plant Physiology. 2001;48:672–680. [Google Scholar]
- Anil VS, Krishnamurthy H, Mathew MK. Limiting cytosolic Na+ confers salt tolerance to rice cells in culture: a two-photon microscopy study of SBFI-loaded cells. Physiologia Plantarum. 2007;129:607–621. [Google Scholar]
- Anil VS, Rajkumar P, Kumar P, Mathew MK. A plant Ca2+ pump, ACA2, relieves salt hypersensitivity in yeast: modulation of cytosolic calcium signature and activation of adaptive Na+ homeostasis. Journal of Biological Chemistry. 2008;283:3497–3506. doi: 10.1074/jbc.M700766200. [DOI] [PubMed] [Google Scholar]
- Antosiewicz D, Hennig J. Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environmental Pollution. 2004;129:237–245. doi: 10.1016/j.envpol.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Apse MP, Sottosanto JB, Blumwald E. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. The Plant Journal. 2003;36:229–239. doi: 10.1046/j.1365-313x.2003.01871.x. [DOI] [PubMed] [Google Scholar]
- Bangerth F. Calcium-related physiological disorders in plants. Annual Reviews of Phytopathology. 1979;17:97–122. [Google Scholar]
- Barta DJ, Tibbitts TW. Calcium localization and tipburn development in lettuce leaves during early enlargement. Journal of the American Society for Horticultural Science. 2000;125:294–298. [PubMed] [Google Scholar]
- Baxter I, Tchieu J, Sussman MR, et al. Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiology. 2003;132:618–628. doi: 10.1104/pp.103.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter I, Ouzzani M, Orcun S, Kennedy B, Jandhyala SS, Salt DE. Purdue ionomics information management system; an integrated functional genomics platform. Plant Physiology. 2007;143:600–611. doi: 10.1104/pp.106.092528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter I, Prashant S, Hosmani S, et al. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genetics. 2009;5:1–12. doi: 10.1371/journal.pgen.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter IR, Vitek O, Lahner B, et al. The leaf ionome as a multivariable system to detect a plant's physiological status. Proceedings of the National Academy of Sciences of the USA. 2008;105:12081–12086. doi: 10.1073/pnas.0804175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L, Urias U, Milstein D, et al. A candidate gene survey of quantitative trait loci affecting chemical composition in tomato fruit. Journal of Experimental Botany. 2008;59:2875–2890. doi: 10.1093/jxb/ern146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert V, Meerts P, Saumitou-Laprade P, Salis P, Gruber W, Verbruggen N. Genetic basis of Cd tolerance and hyperaccumulation in Arabidopsis halleri. Plant and Soil. 2003;249:9–18. [Google Scholar]
- Bidwell SD, Crawford SA, Woodrow IE, Sommer-Knudsen J, Marshall AT. Sub-cellular localization of Ni in the hyperaccumulator, Hybanthus floribundus (Lindley) F. Muell. Plant, Cell & Environment. 2004;27:705–716. [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, et al. A gene expression map of the arabidopsis root. Science. 2003;302:1956–1960. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Romanowsky S, Harper JF. The mutation of two Ca2+ pumps ACA4 and ACA11 provides evidence for communication between the tonoplast and plasma membrane in the regulation of Ca2+ signals leading to a programmed cell death. 18th New Phytologist Symposium Calcium-based Signalling Systems in Plants. 2007 5–7 December 2007, Dublin, Ireland. [Google Scholar]
- Boursier P, Läuchli A. Mechanisms of chloride partitioning in the leaves of salt-stressed Sorghum bicolor L. Physiologia Plantarum. 1989;77:537–544. [Google Scholar]
- Brandt SP. Microgenomics: gene expression analysis at the tissue-specific and single-cell levels. Journal of Experimental Botany. 2005;56:495–505. doi: 10.1093/jxb/eri066. [DOI] [PubMed] [Google Scholar]
- Brinch-Pedersen H, Sorensen LD, Holm PB. Engineering crop plants: getting a handle on phosphate. Trends in Plant Science. 2002;7:118–125. doi: 10.1016/s1360-1385(01)02222-1. [DOI] [PubMed] [Google Scholar]
- Britto DT, Ruth TJ, Lapi S, Kronzucker HJ. Cellular and whole-plant chloride dynamics in barley: insights into chloride-nitrogen interactions and salinity responses. Planta. 2004;218:615–622. doi: 10.1007/s00425-003-1137-x. [DOI] [PubMed] [Google Scholar]
- Britto DT, Szczerba MW, Kronzucker HJ. A new, non-perturbing, sampling procedure in tracer exchange measurements. Journal of Experimental Botany. 2006;57:1309–1314. doi: 10.1093/jxb/erj105. [DOI] [PubMed] [Google Scholar]
- Broadley MR, Willey NJ, Wilkins JC, Baker AJM, Mead A, White PJ. Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytologist. 2001;152:9–27. doi: 10.1046/j.0028-646x.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- Broadley MR, Bowen HC, et al. Variation in the shoot calcium content of angiosperms. Journal of Experimental Botany. 2003;54:1431–1446. doi: 10.1093/jxb/erg143. [DOI] [PubMed] [Google Scholar]
- Brown SL, Chaney RL, Lloyd CA, Angle JS, Ryan JA. Relative uptake of cadmium by garden vegetables and fruits grown on long term biosolid-amended soils. Environmental Science & Technology. 1996;30:3508–3511. [Google Scholar]
- Brownell PF, Crossland CJ. The requirement for sodium as a micronutrient by species having the C4 dicarboxylic photosynthetic pathway. Plant Physiology. 1972;49:794–797. doi: 10.1104/pp.49.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell PF, Crossland CJ. Growth responses to sodium by Bryophyllum tubiflorum under conditions inducing crassulacean acid metabolism. Plant Physiology. 1974;54:416–417. doi: 10.1104/pp.54.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune A, Urbach W, Dietz K-J. Differential toxicity of heavy metals is partly related to a loss of preferential extraplasmic compartmentation: a comparison of Cd-, Mo-, Ni- and Zn-stress. New Phytologist. 1995;129:403–409. [Google Scholar]
- Byrt C, Platten JD, Spielmeyer W, et al. HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiology. 2007;143:1918–1928. doi: 10.1104/pp.106.093476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailliatte R, Lapeyre B, Briat J-F, Mari S, Curie C. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochemical Journal. 2009;422:217–228. doi: 10.1042/BJ20090655. [DOI] [PubMed] [Google Scholar]
- Canut H, Carrasco A, Rossignol M, Ranjeva R. Is vacuole the richest store of IP3-mobilizable calcium in plant cells? Plant Science. 1993;90:135–143. [Google Scholar]
- Carden DEE. Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiology. 2003;131:676. doi: 10.1104/pp.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Pan SQ, Jan ZH, Avila EL, Girke T, Raikhel NV. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. The Plant Cell. 2004;16:3285–3303. doi: 10.1105/tpc.104.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CW, Schorrak LM, Smith RK, Jr, Bent AF, Sussman MR. A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiology. 2003;132:728–731. doi: 10.1104/pp.102.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CWM, Wohlbach DJ, Rodesch MJ, Sussman MR. Transcriptional changes in response to growth of Arabidopsis in high external calcium. FEBS Letters. 2008;582:967–976. doi: 10.1016/j.febslet.2008.02.043. [DOI] [PubMed] [Google Scholar]
- Chaney RL, Ryan JA, Li YM, Angle JS. Transfer of cadmium through plants to the food chain. In: Syers JK, Gochfeld M, editors. Environmental cadmium in the food chain: sources, pathways, and risks. Brussels: Belgian Academy of Sciences; 2000. [Google Scholar]
- Chen ZH, Pottosin II, Cuin TA, et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology. 2007;145:1714–1725. doi: 10.1104/pp.107.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. The Plant Cell. 2003;15:347–364. doi: 10.1105/tpc.007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, et al. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiology. 2005;138:2048–2060. doi: 10.1104/pp.105.061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, Moeder W, Yoshioka K. Biological roles of cyclic-nucleotide-gated ion channels in plants: what we know and don't know about this 20 member ion channel family. Botany – Botanique. 2009;87:668–677. [Google Scholar]
- Chino M. Species differences in calcium and potassium distributions within plant roots. Soil Science and Plant Nutrition. 1981;27:487–503. [Google Scholar]
- Cho D, Kim SA, Murata Y, et al. De-regulated expression of the plant glutamate receptor homolog AtGLR3·1 impairs long-term Ca2+-programmed stomatal closure. The Plant Journal. 2009;58:437–449. doi: 10.1111/j.1365-313X.2009.03789.x. [DOI] [PubMed] [Google Scholar]
- Cho M, Chardonnens AN, Dietz KJ. Differential heavy metal tolerance of Arabidopsis halleri and Arabidopsis thaliana: a leaf slice test. New Phytologist. 2003;158:287–293. [Google Scholar]
- Choi HI, Park HJ, Park JH, et al. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiology. 2005;139:1750–1761. doi: 10.1104/pp.105.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YE, Harada E, Wada M, et al. Detoxification of cadmium in tobacco plants: formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta. 2001;213:45–50. doi: 10.1007/s004250000487. [DOI] [PubMed] [Google Scholar]
- Choi YE, Harada E. Roles of calcium and cadmium on Cd-containing intra- and extracellular formation of Ca crystals in tobacco. Journal of Plant Biology. 2005;48:113–119. [Google Scholar]
- Cholewa E, Peterson CA. Evidence for symplastic involvement in the radial movement of calcium in onion roots. Plant Physiology. 2004;134:1793–1802. doi: 10.1104/pp.103.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT. Calcium transport between tissues and its distribution in the plant. Plant, Cell & Environment. 1984;7:449–456. [Google Scholar]
- Clarkson DT. Root structure and sites of ion uptake. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. New York, NY: Marcel Dekker; 1991. [Google Scholar]
- Clemens S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 2001;212:475–486. doi: 10.1007/s004250000458. [DOI] [PubMed] [Google Scholar]
- Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006;88:1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proceedings of the National Academy of Sciences of the USA. 1998;95:1243–1248. doi: 10.1073/pnas.95.20.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocozza C, Minnocci A, Tognetti R, Iori V, Zacchini M, Scarascia Mugnozza G. Distribution and concentration of cadmium in root tissue of Populus alba determined by scanning electron microscopy and energy-dispersive x-ray microanalysis. iForest – Biogeosciences and Forestry. 2008;1:96–103. [Google Scholar]
- Cuin TA, Miller AJ, Laurie SA, Leigh RA. Potassium activities in cell compartments of salt-grown barley leaves. Journal of Experimental Botany. 2003;54:657–661. doi: 10.1093/jxb/erg072. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Betts SA, Chalmandrier R, Shabala S. A root's ability to retain K+ correlates with salt tolerance in wheat. Journal of Experimental Botany. 2008;59:2697–2706. doi: 10.1093/jxb/ern128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angeli A, Monachello D, Ephritikhine G, et al. CLC-mediated anion transport in plant cells. Philosophical Transactions of the Royal Society B–Biological Sciences. 2009;364:195–201. doi: 10.1098/rstb.2008.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJM. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist. 2007;175:387–404. doi: 10.1111/j.1469-8137.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- Diatloff E, Forde BG, Roberts SK. Expression and transport characterisation of the wheat low-affinity cation transporter (LCT1) in the methylotrophic yeast Pichia pastoris. Biochemical and Biophysical Research Communications. 2006;344:807–813. doi: 10.1016/j.bbrc.2006.03.212. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Schramm M, Betz M, Busch H, Durr C, Martinoia E. Characterization of the epidermis from barley primary leaves. 1. Isolation of epidermal protoplasts. Planta. 1992a;187:425–430. doi: 10.1007/BF00199959. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Schramm M, Lang B, Lanzlschramm A, Durr C, Martinoia E. Characterization of the epidermis from barley primary leaves. 2. The role of the epidermis in ion compartmentation. Planta. 1992b;187:431–437. doi: 10.1007/BF00199960. [DOI] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, et al. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science. 2008;320:942–945. doi: 10.1126/science.1153795. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Gardner MJ, Hotta CT, et al. The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science. 2007;318:1789–1792. doi: 10.1126/science.1146757. [DOI] [PubMed] [Google Scholar]
- Echlin P. Low temperature microscopy and analysis. New York, NY: Plenum Press; 1992. [Google Scholar]
- Edmond C, Shigaki T, Ewert S, et al. Comparative analysis of CAX2-like cation transporters indicates functional and regulatory diversity. Biochemical Journal. 2009;418:145–154. doi: 10.1042/BJ20081814. [DOI] [PubMed] [Google Scholar]
- Elenany AE. Alleviation of cadmium toxicity on maize seedlings by calcium. Biologia Plantarum. 1995;37:93–99. [Google Scholar]
- Epimashko S, Meckel T, Fischer-Schliebs E, Luttge U, Thiel G. Two functionally different vacuoles for static and dynamic purposes in one plant mesophyll leaf cell. The Plant Journal. 2004;37:294–300. doi: 10.1046/j.1365-313x.2003.01958.x. [DOI] [PubMed] [Google Scholar]
- Ernst WHO, Verkleij JAC, Schat H. Metal tolerance in plants. Acta Botanica Neerlandica. 1992;41:229–248. [Google Scholar]
- Fernando DR, Bakkaus EJ, Perrier N, et al. Manganese accumulation in the leaf mesophyll of four tree species: a PIXE/EDAX localization study. New Phytologist. 2006;171:751–758. doi: 10.1111/j.1469-8137.2006.01783.x. [DOI] [PubMed] [Google Scholar]
- Fernie AR, Geigenberger P, Stitt M. Flux an important, but neglected, component of functional genomics. Current Opinion in Plant Biology. 2005;8:174–182. doi: 10.1016/j.pbi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Dalmond D. Protein-synthesis in halophytes: the influence of potassium, sodium and magnesium in vitro. Plant and Soil. 1992;146:153–161. [Google Scholar]
- Flowers TJ, Hajibagheri MA. Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant and Soil. 2001;231:1–9. [Google Scholar]
- Flowers TJ, Troke PF, Yeo AR. The mechanism of salt tolerance in halophytes. Annual Review of Plant Physiology. 1977;28:89–121. [Google Scholar]
- Flowers TJ, Hajibagheri MA, Yeo AR. Ion accumulation in the cell walls of rice plants growing under saline conditions: evidence for the Oertli hypothesis. Plant, Cell & Environment. 1991;14:319–325. [Google Scholar]
- Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annual Review of Plant Biology. 2005;56:41–71. doi: 10.1146/annurev.arplant.56.032604.144106. [DOI] [PubMed] [Google Scholar]
- Fricke W. Solute sorting in grass leaves: the transpiration stream. Planta. 2004;219:507–514. doi: 10.1007/s00425-004-1262-1. [DOI] [PubMed] [Google Scholar]
- Fricke W, Leigh RA, Tomes AD. Concentrations of inorganic and organic solutes in extracts from individual epidermal, mesophyll and bundle-sheath cells of barley leaves. Planta. 1994a;192:310–316. [Google Scholar]
- Fricke W, Leigh RA, Tomos AD. Epidermal solute concentrations and osmolality in barley leaves studied at the single cell level: changes along the leaf blade, during leaf aging and NaCl stress. Planta. 1994b;192:317–323. [Google Scholar]
- Fricke W, Pritchard E, Leigh RA, Tomes AD. Cells of the upper and lower epidermis of barley (Hordeum vulgare L.) leaves exhibit distinct patterns of vacuolar solutes. Plant Physiology. 1994c;104:1201–1208. doi: 10.1104/pp.104.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W, Hinde PS, Leigh RA, Tomos AD. Vacuolar solutes in the upper epidermis of barley leaves: intercellular differences follow patterns. Planta. 1995;196:40–49. [Google Scholar]
- Fricke W, Leigh RA, Tomos AD. The intercellular distribution of vacuolar solutes in the epidermis and mesophyll of barley leaves changes in response to NaCl. Journal of Experimental Botany. 1996;47:1413–1426. [Google Scholar]
- Fricker M, Runions J, Moore I. Quantitative fluorescence microscopy: from art to science. Annual Review of Plant Biology. 2006;57:79–107. doi: 10.1146/annurev.arplant.57.032905.105239. [DOI] [PubMed] [Google Scholar]
- Fricker MD, Plieth C, Knight H, et al. Fluorescence and luminescence techniques to probe ion activities in living plant cells. In: Mason B, editor. Fluorescent and luminescent probes for biological activity. San Diego, CA: Academic Press; 1999. [Google Scholar]
- Galbraith DW, Birnbaum K. Global studies of cell type-specific gene expression in plants. Annual Review of Plant Biology. 2006;57:451–475. doi: 10.1146/annurev.arplant.57.032905.105302. [DOI] [PubMed] [Google Scholar]
- Geisler M, Axelsen KB, Harper JF, Palmgren MG. Molecular aspects of higher plant P-type Ca2+-ATPases. Biochimica et Biophysica Acta: Biomembranes. 2000;1465:52–78. doi: 10.1016/s0005-2736(00)00131-0. [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J, O'Toole N, Ammar R. A predicted interactome for Arabidopsis. Plant Physiology. 2007;145:317–329. doi: 10.1104/pp.107.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliham M. Membrane structure and methods for studying membrane transport. In: Yeo AR, Flowers TJ, editors. Plant solute transport. Oxford: Blackwell Publishing; 2007. [Google Scholar]
- Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJM. Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. Journal of Experimental Botany. 2006;57:791–800. doi: 10.1093/jxb/erj064. [DOI] [PubMed] [Google Scholar]
- Grusak MA, DellaPenna D. Improving the nutrient composition of plants to enhance human nutrition and health. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:133–161. doi: 10.1146/annurev.arplant.50.1.133. [DOI] [PubMed] [Google Scholar]
- Guerinot ML. The ZIP family of metal transporters. Biochimica et Biophysica Acta: Biomembranes. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Guo KM, Babourina O, Christopher DA, Borsics T, Rengel Z. The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiologia Plantarum. 2008;134:499–507. doi: 10.1111/j.1399-3054.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- Guo KM, Babourina O, Rengel Z. Na+/H+ antiporter activity of the SOS1 gene: lifetime imaging analysis and electrophysiological studies on Arabidopsis seedlings. Physiologia Plantarum. 2009;137:155–165. doi: 10.1111/j.1399-3054.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Hajibagheri MA, Flowers TJ, Collins JC, Yeo AR. A comparison of the methods of X-ray microanalysis, compartmental analysis and longitudinal ion profiles to estimate cytoplasmic ion concentrations in 2 maize varieties. Journal of Experimental Botany. 1988;39:279–290. [Google Scholar]
- Hall J. Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany. 2002;53:1–11. [PubMed] [Google Scholar]
- Halperin SJ, Lynch JP. Effects of salinity on cytosolic Na+ and K+ in root hairs of Arabidopsis thaliana: in vivo measurements using the fluorescent dyes SBFI and PBFI. Journal of Experimental Botany. 2003;54:2035–2043. doi: 10.1093/jxb/erg219. [DOI] [PubMed] [Google Scholar]
- Hampton CR, Broadley MR, White PJ. Short review: the mechanisms of radiocaesium uptake by arabidopsis roots. Nukleonika. 2005;50:S3–S8. [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, et al. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- Hansen T, Laursen K, Persson D, Pedas P, Husted S, Schjoerring J. Micro-scaled high-throughput digestion of plant tissue samples for multi-elemental analysis. Plant Methods. 2009;5:12. doi: 10.1186/1746-4811-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NS, Taylor GJ. Remobilization of cadmium in maturing shoots of near isogenic lines of durum wheat that differ in grain cadmium accumulation. Journal of Experimental Botany. 2001;52:1473–1481. doi: 10.1093/jexbot/52.360.1473. [DOI] [PubMed] [Google Scholar]
- Hayter ML, Peterson CA. Can Ca2+ fluxes to the root xylem be sustained by Ca2+-ATPases in exodermal and endodermal plasma membranes? Plant Physiology. 2004;136:4318–4325. doi: 10.1104/pp.104.041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Yan J, Shen G, et al. Expression of an Arabidopsis vacuolar sodium/proton antiporter gene in cotton improves photosynthetic performance under salt conditions and increases fiber yield in the field. Plant Cell Physiology. 2005a;46:1848–1854. doi: 10.1093/pcp/pci201. [DOI] [PubMed] [Google Scholar]
- He ZY, Li JC, Zhang HY, Ma M. Different effects of calcium and lanthanum on the expression of phytochelatin synthase gene and cadmium absorption in Lactuca sativa. Plant Science. 2005b;168:309–318. [Google Scholar]
- Hepler PK, Wayne RO. Calcium and plant development. Annual Review of Plant Physiology and Plant Molecular Biology. 1985;36:397–439. [Google Scholar]
- Herren T, Feller U. Transport of cadmium via xylem and phloem in maturing wheat shoots: comparison with the translocation of zinc, strontium and rubidium. Annals of Botany. 1997;80:623–628. [Google Scholar]
- Hetherington AM, Brownlee C. The generation of Ca2+ signals in plants. Annual Review of Plant Biology. 2004;55:401–427. doi: 10.1146/annurev.arplant.55.031903.141624. [DOI] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Hirschi KD. Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. The Plant Cell. 1999;11:2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD. The calcium conundrum: both versatile nutrient and specific signal. Plant Physiology. 2004;136:2438–2442. doi: 10.1104/pp.104.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR. CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 1996;93:8782–8786. doi: 10.1073/pnas.93.16.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ. Expression of Arabidopsis CAX2 in tobacco: altered metal accumulation and increased manganese tolerance. Plant Physiology. 2000;124:125–133. doi: 10.1104/pp.124.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LC, White PJ. A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Annals of Botany. 2005;95:571–581. doi: 10.1093/aob/mci065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson MJ, Sangster AG. Subcellular-localization of mineral deposits in the roots of wheat (Triticum aestivum L.) Protoplasma. 1989;151:19–32. [Google Scholar]
- Hodson MJ, Sangster AG. Mineral deposition in the needles of white spruce Picea glauca (Moench.) Voss. Annals of Botany. 1998;82:375–385. [Google Scholar]
- Hokura A, Onuma R, Kitajima H, et al. 2-D X-ray fluorescence imaging of cadmium hyperaccumulating plants by using high-energy synchrotron radiation X-ray microbeam. Chemistry Letters. 2006;35:1246–1249. [Google Scholar]
- Hu P-J, Qiu R-L, Senthilkumar P, et al. Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environmental and Experimental Botany. 2009;66:317–325. [Google Scholar]
- Huang CX, Van Steveninck RFM. Effect of moderate salinity on patterns of potassium, sodium and chloride accumulation in cells near the root tip of barley: role of differentiating metaxylem vessels. Physiologia Plantarum. 1988;73:525–533. [Google Scholar]
- Hwang I, Harper JF, Liang F, Sze H. Calmodulin activation of an endoplasmic reticulum-located calcium pump involves an interaction with the N-terminal autoinhibitory domain. Plant Physiology. 2000a;122:157–167. doi: 10.1104/pp.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sze H, Harper JF. A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 2000b;97:6224–6229. doi: 10.1073/pnas.97.11.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Ae N, Murakami M, Wagatsuma T. Is Brassica juncea a suitable plant for phytoremediation of cadmium in soils with moderately low cadmium contamination? Possibility of using other plant species for Cd-phytoextraction. Soil Science and Plant Nutrition. 2006;52:32–42. [Google Scholar]
- James RA, Munns R, Von Caemmerer S, Trejo C, Miller C, Condon T. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl− in salt-affected barley and durum wheat. Plant, Cell & Environment. 2006;29:2185–2197. doi: 10.1111/j.1365-3040.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Kachenko AG, Bhatia NP, Siegele R, Walsh KB, Singh B. Nickel, Zn and Cd localisation in seeds of metal hyperaccumulators using μ-PIXE spectroscopy. Nuclear Instruments & Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2009;267:2176–2180. [Google Scholar]
- Kader MA, Lindberg S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. determined by the fluorescent dye SBFI. Journal of Experimental Botany. 2005;56:3149–3158. doi: 10.1093/jxb/eri312. [DOI] [PubMed] [Google Scholar]
- Kang JM, Turano FJ. The putative glutamate receptor 1·1 (AtGLR1·1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the USA. 2003;100:6872–6877. doi: 10.1073/pnas.1030961100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karley AJ, White PJ. Moving cationic minerals to edible tissues: potassium, magnesium, calcium. Current Opinion in Plant Biology. 2009;12:291–298. doi: 10.1016/j.pbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Karley AJ, Leigh RA, Sanders D. Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends in Plant Science. 2000a;5:465–470. doi: 10.1016/s1360-1385(00)01758-1. [DOI] [PubMed] [Google Scholar]
- Karley AJ, Leigh RA, Sanders D. Differential ion accumulation and ion fluxes in the mesophyll and epidermis of barley. Plant Physiology. 2000b;122:835–844. doi: 10.1104/pp.122.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J. Single cell technology. Current Opinion in Plant Biology. 2003;6:617–621. doi: 10.1016/j.pbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Kerton M, Newbury HJ, Hand D, Pritchard J. Accumulation of calcium in the centre of leaves of coriander (Coriandrum sativum L.) is due to an uncoupling of water and ion transport. Journal of Experimental Biology. 2009;60:227–235. doi: 10.1093/jxb/ern279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR. Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. The Plant Journal. 2000;23:267–278. doi: 10.1046/j.1365-313x.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- Kim BG, Waadt R, Cheong YH, et al. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. The Plant Journal. 2007;52:473–484. doi: 10.1111/j.1365-313X.2007.03249.x. [DOI] [PubMed] [Google Scholar]
- Kim SA, Kwak JM, Jae S-K, Wang M-H, Nam HG. Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiology. 2001;42:74–84. doi: 10.1093/pcp/pce008. [DOI] [PubMed] [Google Scholar]
- Kiser MR, Reid CD, Crowell AS, Phillips RP, Howell CR. Exploring the transport of plant metabolites using positron emitting radiotracers. HFSP Journal. 2008;2:189–204. doi: 10.2976/1.2921207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klich MG. Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity. Environmental and Experimental Botany. 2000;44:171–183. doi: 10.1016/s0098-8472(00)00056-3. [DOI] [PubMed] [Google Scholar]
- Knight H. Calcium signaling during abiotic stress in plants. International Reviews of Cytology. 2000;192:269–324. doi: 10.1016/s0074-7696(08)62707-2. [DOI] [PubMed] [Google Scholar]
- Korenkov V, Park S, Cheng NH, et al. Enhanced Cd2+-selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta. 2007;225:403–411. doi: 10.1007/s00425-006-0352-7. [DOI] [PubMed] [Google Scholar]
- Korenkov V, King B, Hirschi K, Wagner GJ. Root-selective expression of AtCAX4 and AtCAX2 results in reduced lamina cadmium in field-grown Nicotiana tabacum L. Plant Biotechnology Journal. 2009;7:219–226. doi: 10.1111/j.1467-7652.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- Koroleva OA, Tomos AD, Farrar J, Roberts P, Pollock CJ. Tissue distribution of primary metabolism between epidermal, mesophyll and parenchymatous bundle sheath cells in barley leaves. Australian Journal of Plant Physiology. 2000;27:747–755. [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Molecular Biology. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Kralj J, Player A, Sedrick H, et al. T7-based linear amplification of low concentration mRNA samples using beads and microfluidics for global gene expression measurements. Lab on a Chip. 2009;9:917–924. doi: 10.1039/b811714d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Moazami-Goudarzi M, Britto DT. The cytosolic Na+:K+ ratio does not explain salinity-induced growth impairment in barley: a dual-tracer study using K42+ and Na24+ Plant, Cell & Environment. 2006;29:2228–2237. doi: 10.1111/j.1365-3040.2006.01597.x. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Schulze LM, Britto DT. Non-reciprocal interactions between K+ and Na+ ions in barley (Hordeum vulgare L.) Journal of Experimental Botany. 2008;59:2793–2801. doi: 10.1093/jxb/ern139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Zhao FJ, McGrath SP. Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiology. 1999;119:305–311. doi: 10.1104/pp.119.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Lombi E, Zhao FJ, McGrath SP. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta. 2000;212:75–84. doi: 10.1007/s004250000366. [DOI] [PubMed] [Google Scholar]
- Küpper H, Lombi E, Zhao FJ, Wieshammer G, McGrath SP. Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. Journal of Experimental Botany. 2001;52:2291–2300. doi: 10.1093/jexbot/52.365.2291. [DOI] [PubMed] [Google Scholar]
- Küpper H, Mijovilovich A, Meyer-Klaucke W, Kroneck P. Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiology. 2004;134:748–757. doi: 10.1104/pp.103.032953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Kroneck PMH. Heavy metal uptake by plants and cyanobacteria. In: Sigel A, Sigel H, Sigel R, editors. Metal ions in biological systems. Vol. 44. London: Taylor & Francis; 2005. pp. 97–142. Biogeochemistry, availability, and transport of metals in the environment. [PubMed] [Google Scholar]
- Küpper H, Parameswaran A, Leitenmaier B, Trtílek M, Šetlík I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytologist. 2007a;175:655–674. doi: 10.1111/j.1469-8137.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- Kupper H, Seib LO, Sivaguru M, Hoekenga OA, Kochian LV. A method for cellular localization of gene expression via quantitative in situ hybridization in plants. The Plant Journal. 2007b;50:159–175. doi: 10.1111/j.1365-313X.2007.03031.x. [DOI] [PubMed] [Google Scholar]
- Lahner B, Gong J, Mahmoudian M, et al. Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nature Biotechnology. 2003;21:1215–1221. doi: 10.1038/nbt865. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Ehrhardt DW, Frommer WB. Shining light on signaling and metabolic networks by genetically encoded biosensors. Current Opinion in Plant Biology. 2005;8:574–581. doi: 10.1016/j.pbi.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasat MM, Pence NS, Garvin DF, Ebbs SD, Kochian LV. Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens. Journal of Experimental Botany. 2000;51:71–79. [PubMed] [Google Scholar]
- Läuchli A, Boursier PJ. Compartmentation in root cells and tissues: X-ray-microanalysis of specific ions. Methods in Enzymology. 1989;174:267–277. [Google Scholar]
- Läuchli A, James RA, Huang C, McCully M, Munns R. Cell-specific localization of Na+ in roots of durum wheat and possible control points for sodium exclusion. Plant, Cell & Environment. 2008;31:1565–1574. doi: 10.1111/j.1365-3040.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA. A role for HKT1 in sodium uptake by wheat roots. The Plant Journal. 2002;32:139–149. doi: 10.1046/j.1365-313x.2002.01410.x. [DOI] [PubMed] [Google Scholar]
- Lazof D, Läuchli A. Complementary analysis of freeze-dried and frozen-hydrated plant tissue by electron-probe X-ray microanalysis: spectral resolution and analysis of calcium. Planta. 1991;184:327–333. doi: 10.1007/BF00195333. [DOI] [PubMed] [Google Scholar]
- Lee S, Petros D, Moon JS, Ko T-S, Goldsbrough PB, Korban SS. Higher levels of ectopic expression of Arabidopsis phytochelatin synthase do not lead to increased cadmium tolerance and accumulation. Plant Physiology and Biochemistry. 2003;41:903–910. [Google Scholar]
- Lee SM, Kim HS, Han HJ, et al. Identification of a calmodulin-regulated autoinhibited Ca2 + -ATPase (ACA11) that is localized to vacuole membranes in Arabidopsis. FEBS Letters. 2007a;581:3943–3949. doi: 10.1016/j.febslet.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Leigh RA. Solute composition of vacuoles. In: Leigh RA, Sanders D, editors. The plant vacuole: advances in botanical research incorporating advances in plant pathology. Vol. 25. New York, NY: Academic Press; 1997. pp. 298–337. [Google Scholar]
- Leigh RA, Storey R. Intercellular compartmentation of ions in barley leaves in relation to potassium nutrition and salinity. Journal of Experimental Botany. 1993;44:755–762. [Google Scholar]
- Leigh RA, Tomos AD. Ion distribution in cereal leaves: pathways and mechanisms. Philosophical Transactions of the Royal Society of London Series B–Biological Sciences. 1993;341:75–86. [Google Scholar]
- Leigh RA, Chater M, Storey R, Johnston AE. Accumulation and subcellular-distribution of cations in relation to the growth of potassium-deficient barley. Plant, Cell & Environment. 1986;9:595–604. [Google Scholar]
- Li H-T, Liu H, Gao X-S, Zhang H. Knockout of Arabidopsis AtNHX4 enhances tolerance to salt stress. Biochemical and Biophysical Research Communications. 2009;382:637–641. doi: 10.1016/j.bbrc.2009.03.091. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang QQ, Yu MM, Zhang YY, Wu YB, Zhang HX. Transgenic salt-tolerant sugar beet (Beta vulgaris L.) constitutively expressing an Arabidopsis thaliana vacuolar Na+/H+ antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots. Plant, Cell & Environment. 2008;31:1325–1334. doi: 10.1111/j.1365-3040.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- Lobinski R, Moulin C, Ortega R. Imaging and speciation of trace elements in biological environment. Biochimie. 2006;88:1591–1604. doi: 10.1016/j.biochi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Lombi E, Zhao FJ, Dunham SJ, McGrath SP. Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytologist. 2000;145:11–20. [Google Scholar]
- Loveland WD, Morrissey DJ, Seaborg GT. Radiotracers. In: Loveland WD, Morrissey DJ, Seaborg GT, editors. Modern nuclear chemistry. New York, NY: Wiley; 2005. pp. 91–128. [Google Scholar]
- Lozano-Rodríguez E, Hernández LE, Bonay P, Cárpena-Ruiz RO. Distribution of Cd in shoot and root tissues of maize and pea plants: physiological distribution. Journal of Experimental Botany. 1997;48:123–128. [Google Scholar]
- Luan S, Lan W, Lee SC. Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL–CIPK network. Current Opinion in Plant Biology. 2009;12:339–346. doi: 10.1016/j.pbi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK. Shaping the calcium signature. New Phytologist. 2009;181:275–294. doi: 10.1111/j.1469-8137.2008.02682.x. [DOI] [PubMed] [Google Scholar]
- McDowell LR. Calcium and phosphorous. In: McDowell LR, editor. Minerals in animal and human nutrition. Oxford: Elsevier Health Sciences; 2003. [Google Scholar]
- McLaughlin MJ, Parker DR, Clarke JM. Metals and micronutrients: food safety issues. Field Crops Research. 1998a;60:143–163. [Google Scholar]
- McLaughlin MJ, Andrew SJ, Smart MK, Smolders E. Effects of sulfate on cadmium uptake by Swiss chard. I. Effects of complexation and calcium competition in nutrient solutions. Plant and Soil. 1998b;202:211–216. [Google Scholar]
- Ma W, Ali R, Berkowitz GA. Characterization of plant phenotypes associated with loss-of-function of AtCNGC1, a plant cyclic nucleotide gated cation channel. Plant Physiology and Biochemistry. 2006;44:494–505. doi: 10.1016/j.plaphy.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM. The role of monovalent cation transporters in plant responses to salinity. Journal of Experimental Botany. 2006;57:1137–1147. doi: 10.1093/jxb/erj001. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Filatov V, Herzyk P, et al. Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. The Plant Journal. 2003;35:675–692. doi: 10.1046/j.1365-313x.2003.01839.x. [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC. Fluxes and compartmentation in plant cells. Annual Review of Plant Physiology. 1971;22:75–96. [Google Scholar]
- Maeshima M. Tonoplast transporters: organization and function. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:469–497. doi: 10.1146/annurev.arplant.52.1.469. [DOI] [PubMed] [Google Scholar]
- Malone M, Leigh RA, Tomos AD. Extraction and analysis of sap from individual wheat leaf cells: the effect of sampling speed on the osmotic pressure of extracted sap. Plant, Cell & Environment. 1989;12:919–926. [Google Scholar]
- Malone M, Leigh RA, Tomos AD. Concentrations of vacuolar inorganic-ions in individual cells of intact wheat leaf epidermis. Journal of Experimental Botany. 1991;42:305–309. [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. London: Academic Press; 1995. [Google Scholar]
- Martin C, Bhatt K, Baumann K. Shaping in plant cells. Current Opinion in Plant Biology. 2001;4:540–549. doi: 10.1016/s1369-5266(00)00213-2. [DOI] [PubMed] [Google Scholar]
- Maser P, Thomine S, Schroeder JI, et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiology. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel A, Leshem Y, Tiwari BS, Levine A. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e) Plant Physiology. 2004;134:118–128. doi: 10.1104/pp.103.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesjasz-Przybylowicz J, Balkwill K, Przybylowicz WJ, Annegarn HJ. Proton microprobe and X-ray fluorescence investigations of nickel distribution in serpentine flora from South Africa. Nuclear Instruments and Methods in Physics Research. 1994;89:208–212. [Google Scholar]
- Mesjasz-Przybylowicz J, Przybylowicz WJ, Pineda CA. Nuclear microprobe studies of elemental distribution in apical leaves of the Ni hyperaccumulator Berkheya coddii. South African Journal of Science. 2001a;97:591–593. [Google Scholar]
- Mesjasz-Przybylowicz J, Przybylowicz WJ, Rama DBK, Pineda CA. Elemental distribution in Senecio anomalochrous, a Ni hyperaccumulator from South Africa. South African Journal of Science. 2001b;97:593–595. [Google Scholar]
- Metzner R, Schneider HU, Breuer U, Schroeder WH. Imaging nutrient distributions in plant tissue using time-of-flight secondary ion mass spectrometry and scanning electron microscopy. Plant Physiology. 2008;147:1774–1787. doi: 10.1104/pp.107.109215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff O, Muller K, Roelfsema MR, et al. AtGLR3·4, a glutamate receptor channel-like gene is sensitive to touch and cold. Planta. 2005;222:418–427. doi: 10.1007/s00425-005-1551-3. [DOI] [PubMed] [Google Scholar]
- Meyers BC, Galbraith DW, Nelson T, Agrawal V. Methods for transcriptional profiling in plants: be fruitful and replicate. Plant Physiology. 2004;135:637–652. doi: 10.1104/pp.104.040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Milla MAR, Uno Y, Chang IF, et al. A novel yeast two-hybrid approach to identify CDPK substrates: characterization of the interaction between AtCPK11 and AtDi19, a nuclear zinc finger protein. FEBS Letters. 2006;580:904–911. doi: 10.1016/j.febslet.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Smith SJ. Cytosolic nitrate ion homeostasis: could it have a role in sensing nitrogen status? Annals of Applied Biology. 2008;101:485–489. doi: 10.1093/aob/mcm313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Wells DM. Electrochemical methods and measuring trans-membrane ion gradients. In: Volkov AJ, editor. Plant electrophysiology: theory and methods. Berlin: Springer-Verlag; 2006. [Google Scholar]
- Miller AJ, Cookson SJ, Smith SJ, Wells DM. The use of microelectrodes to investigate compartmentation and the transport of metabolized inorganic ions in plants. Journal of Experimental Botany. 2001;52:541–549. [PubMed] [Google Scholar]
- Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proceedings of the National Academy of Sciences of the USA. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I, Gilliham M, Jha D, et al. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. The Plant Cell. 2009;21:2163–2178. doi: 10.1105/tpc.108.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J, Hawthorne KM, Hotze T, Abrams SA, Hirschi KD. Nutritional impact of elevated calcium transport activity in carrots. Proceedings of the National Academy of Sciences of the USA. 2008;105:1431–1435. doi: 10.1073/pnas.0709005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühling K, Läuchli A. Effect of salt stress on growth and cation compartmentation in leaves of two plant species differing in salt tolerance. Journal of Plant Physiology. 2002a;159:137–146. [Google Scholar]
- Mühling KH, Läuchli A. Determination of apoplastic Na+ in intact leaves of cotton by in vivo fluorescence ratio-imaging. Functional Plant Biology. 2002b;29:1491–1499. doi: 10.1071/FP02013. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nagasaki N, Tomioka R, Maeshima M. A hydrophilic cation-binding protein of Arabidopsis thaliana, AtPCaP1, is localized to plasma membrane via N-myristoylation and interacts with calmodulin and the phosphatidylinositol phosphates PtdIns(3,4,5)P-3 and PtdIns(3,5)P-2. FEBS Journal. 2008;275:2267–2282. doi: 10.1111/j.1742-4658.2008.06379.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Science and Plant Nutrition. 2006;52:464–469. [Google Scholar]
- Nawy T, Lee JY, Colinas J, et al. Transcriptional profile of the Arabidopsis root quiescent center. The Plant Cell. 2005;17:1908–1925. doi: 10.1105/tpc.105.031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DH, Leidi E, Zhang Q. Loss of halophytism by interference with SOS1 expression. Plant Physiology. 2009;151:210–222. doi: 10.1104/pp.109.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega R. Chemical elements distribution in cells. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2005;231:218–223. [Google Scholar]
- Outlaw WH, Tarczynski MC, Miller WI. Histological compartmentation of phosphate in Vicia faba L. leaflet: possible significance to stomatal functioning. Plant Physiology. 1984;74:430–433. doi: 10.1104/pp.74.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkutlu F, Ozturk L, Erdem H, McLaughlin M, Cakmak I. Leaf-applied sodium chloride promotes cadmium accumulation in durum wheat grain. Plant and Soil. 2007;290:323–331. [Google Scholar]
- Papoyan A, Kochian LV. Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance: characterization of a novel heavy metal transporting ATPase. Plant Physiology. 2004;136:3814–3823. doi: 10.1104/pp.104.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Cheng NH, Pittman JK, et al. Increased calcium levels and prolonged shelf life in tomatoes expressing Arabidopsis H+/Ca2+ transporters. Plant Physiology. 2005;139:1194–1206. doi: 10.1104/pp.105.066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. International Journal of Biochemistry & Cell Biology. 2009;41:1665–1677. doi: 10.1016/j.biocel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. The Plant Journal. 2002;32:539–548. doi: 10.1046/j.1365-313x.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- Plieth C. Plant calcium signaling and monitoring: pros and cons and recent experimental approaches. Protoplasma. 2001;218:1–23. doi: 10.1007/BF01288356. [DOI] [PubMed] [Google Scholar]
- Plieth C. Aequorin as a reporter gene. Methods in Molecular Biology. 2006;323:307–327. doi: 10.1385/1-59745-003-0:307. [DOI] [PubMed] [Google Scholar]
- Popelka JC, Schubert S, Schulz R, Hansen AP. Cadmium uptake and translocation during reproductive development of peanut (Arachis hypogaea L) Journal of Applied Botany: Angewandte Botanik. 1996;70:140–143. [Google Scholar]
- Pottosin II, Schönknecht G. Vacuolar calcium channels. Journal of Experimental Botany. 2007;58:1559–1569. doi: 10.1093/jxb/erm035. [DOI] [PubMed] [Google Scholar]
- Puig S, Peñarrubia L. Placing metal micronutrients in context: transport and distribution in plants. Current Opinion in Plant Biology. 2009;12:299–306. doi: 10.1016/j.pbi.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Punshon T, Guerinot ML, Lanzirotti A. Using synchrotron X-ray fluorescence microprobes in the study of metal homeostasis in plants. Annals of Botany. 2009;103:665–672. doi: 10.1093/aob/mcn264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Marrison JL, Leech RM. Temporal and spatial development of the cells of the expanding 1st leaf of Arabidopsis thaliana (L) Heynh. Journal of Experimental Botany. 1991;42:1407–1416. [Google Scholar]
- Ramesh SA, Shin R, Eide DJ, Schachtman DP. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiology. 2003;133:126–134. doi: 10.1104/pp.103.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O'Shea EK. Control of stochasticity in eukaryotic gene expression. Science. 2004;304:1811–1814. doi: 10.1126/science.1098641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE. Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin, and metallothioneins. Cell Biochemistry and Biophysics. 1999;31:19–48. doi: 10.1007/BF02738153. [DOI] [PubMed] [Google Scholar]
- Reid RJ, Smith FA. The limits of sodium/calcium interactions in plant growth. Australian Journal of Plant Physiology. 2000;27:709–715. [Google Scholar]
- Richardson A, Boscari A, Schreiber L, et al. Cloning and expression analysis of candidate genes involved in wax deposition along the growing barley (Hordeum vulgare) leaf. Planta. 2007;226:1459–1473. doi: 10.1007/s00425-007-0585-0. [DOI] [PubMed] [Google Scholar]
- Roy SJ, Cuin TA, Leigh RA. Nanolitre-scale assays to determine the activities of enzymes in individual plant cells. The Plant Journal. 2003;34:555–564. doi: 10.1046/j.1365-313x.2003.01744.x. [DOI] [PubMed] [Google Scholar]
- Roy SJ, Gilliham M, Berger B, et al. Investigating glutamate-like receptor gene co-expression in Arabidopsis thaliana. Plant, Cell & Environment. 2008;31:861–871. doi: 10.1111/j.1365-3040.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Burnett P, Rizzuto R, et al. Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: significance for the regulation of pyruvate dehydrogenase activity. Proceedings of the National Academy of Sciences of the USA. 1996;93:5489–5494. doi: 10.1073/pnas.93.11.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Baxter I, Lahner B. Ionomics and the study of the plant ionome. Annual Review of Plant Biology. 2008;59:709–733. doi: 10.1146/annurev.arplant.59.032607.092942. [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. The Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqib M, Zorb C, Rengel Z, Schubert S. The expression of the endogenous vacuolar Na+/H+ antiporters in roots and shoots correlates positively with the salt resistance of wheat (Triticum aestivum L.) Plant Science. 2005;169:959–965. [Google Scholar]
- Sarret G, Saumitou-Laprade P, Bert V, et al. Forms of zinc accumulated in the hyperaccumulator Arabidopsis halleri. Plant Physiology. 2002;130:1815–1826. doi: 10.1104/pp.007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Kumar R, Schroeder JI, Marsh EL. Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants. Proceedings of the National Academy of Sciences of the USA. 1997;94:11079–11084. doi: 10.1073/pnas.94.20.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheckel KG, Hamon R, Jassogne L, Rivers M, Lombi E. Synchrotron X-ray absorption-edge computed microtomography imaging of thallium compartmentalization in Iberis intermedia. Plant and Soil. 2007;290:51–60. [Google Scholar]
- Schneider T, Schellenberg M, Meyer S, et al. Quantitative detection of changes in the leaf-mesophyll tonoplast proteome in dependency of a cadmium exposure of barley (Hordeum vulgare L.) plants. Proteomics. 2009;9:2668–2677. doi: 10.1002/pmic.200800806. [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. Potassium transport and plant salt tolerance. Physiologia Plantarum. 2007;133:651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- Shabala S, Demidchik V, Shabala L, et al. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiology. 2006;141:1653–1665. doi: 10.1104/pp.106.082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Cuin TA, Pottosin I. Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Letters. 2007;581:1993–1999. doi: 10.1016/j.febslet.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Shen ZG, Zhao FJ, McGrath SP. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the non-hyperaccumulator Thlaspi ochroleucum. Plant, Cell & Environment. 1997;20:898–906. [Google Scholar]
- Sherma J, Van Lenten FJ. Ion-exchange paper chromatography of metal ions with mixed aqueous-organic solvents containing mineral acid and a selective extractant. Separation Science and Technology. 1971;6:199–206. [Google Scholar]
- Shi HZ, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. The Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Wu SJ, Zhu JK. Overexpression of a plasma membrane Na+/H+ antiporter improves salt tolerance in Arabidopsis. Nature Biotechnology. 2003;21:81–85. doi: 10.1038/nbt766. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi KD. Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biology. 2006;8:419–429. doi: 10.1055/s-2006-923950. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Pittman JK, Hirschi KD. Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. Journal of Biological Chemistry. 2003;278:6610–6617. doi: 10.1074/jbc.M209952200. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Rees I, Nakhleh L, Hirschi KD. Identification of three distinct phylogenetic groups of CAX cation/proton antiporters. Journal of Molecular Evolution. 2006;63:815–825. doi: 10.1007/s00239-006-0048-4. [DOI] [PubMed] [Google Scholar]
- de Silva DLR, Honour SJ, Mansfield TA. Estimations of apoplastic concentrations of K+ and Ca2+ in the vicinity of stomatal guard cells. New Phytologist. 1996;134:463–469. [Google Scholar]
- Smith J. Ion-transport and the transpiration stream. Botanica Acta. 1991;104:416. [Google Scholar]
- Sottosanto JB, Saranga Y, Blumwald E. Impact of AtNHX1, a vacuolar Na+/H+ antiporter, upon gene expression during short-term and long-term salt stress in Arabidopsis thaliana. BMC Plant Biology. 2007;7 doi: 10.1186/1471-2229-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck Z, Siwiec A, Chotuj D. Distribution of calcium in tomato plants in response to heat stress and plant growth regulators. Plant and Soil. 1994;167:143–148. [Google Scholar]
- Stephens NR, Qi Z, Spalding EP. Glutamate receptor subtypes evidenced by differences in desensitization and dependence on the GLR3·3 and GLR3·4 genes. Plant Physiology. 2008;146:529–538. doi: 10.1104/pp.107.108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey R, Leigh RA. Processes modulating calcium distribution in citrus leaves: an investigation using x-ray microanalysis with strontium as a tracer. Plant Physiology. 2004;136:3838–3848. doi: 10.1104/pp.104.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey R, Schachtman DP, Thomas MR. Root structure and cellular chloride, sodium and potassium distribution in salinized grapevines. Plant, Cell & Environment. 2003a;26:789–800. doi: 10.1046/j.1365-3040.2003.01005.x. [DOI] [PubMed] [Google Scholar]
- Storey R, Wyn Jones RG, Schachtman DP, Treeby MT. Calcium-accumulating cells in the meristematic region of grapevine root apices. Functional Plant Biology. 2003b;30:719–727. doi: 10.1071/FP02212. [DOI] [PubMed] [Google Scholar]
- Sunarpi, Horie T, Motoda J, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. The Plant Journal. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Fujimaki S, Fujiwara T, Yoneyama T, Hayashi H. Cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.) treated with a nutrient solution containing cadmium. Soil Science and Plant Nutrition. 2003;49:311–313. [Google Scholar]
- Tauris B, Borg S, Gregersen PL, Holm PB. A roadmap for zinc trafficking in the developing barley grain based on laser capture microdissection and gene expression profiling. Journal of Experimental Botany. 2009;60:1333–1347. doi: 10.1093/jxb/erp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Leigh RA. Partitioning of nutrient transport processes in roots. Journal of Experimental Biology. 2001;52:445–457. doi: 10.1093/jexbot/52.suppl_1.445. [DOI] [PubMed] [Google Scholar]
- Thomine S, Wang RC, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proceedings of the National Academy of Sciences of the USA. 2000;97:4991–4996. doi: 10.1073/pnas.97.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomos AD, Sharrock RA. Cell sampling and analysis (SiCSA): metabolites measured at single cell resolution. Journal of Experimental Botany. 2001;52:623–630. [PubMed] [Google Scholar]
- Treeby MT, Vansteveninck RFM, Devries HM. Quantitative estimates of phosphorus concentrations within Lupinus luteus leaflets by means of electron probe X-ray microanalysis. Plant Physiology. 1987;85:331–334. doi: 10.1104/pp.85.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano FJ, Muhitch MJ, Felker FC, McMahon MB. The putative glutamate receptor 3·2 from Arabidopsis thaliana (AtGLR3·2) is an integral membrane peptide that accumulates in rapidly growing tissues and persists in vascular-associated tissues. Plant Science. 2002;163:43–51. [Google Scholar]
- Ueno D, Iwashita T, Zhao F-J, Ma JF. Characterization of Cd translocation and identification of the Cd form in xylem sap of the Cd-hyperaccumulator Arabidopsis halleri. Plant and Cell Physiology. 2008;49:540–548. doi: 10.1093/pcp/pcn026. [DOI] [PubMed] [Google Scholar]
- Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. Journal of Experimental Botany. 2009;60:2677–2688. doi: 10.1093/jxb/erp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez MD, Barcelo J, Poschenrieder C, et al. Localization of zinc and cadmium in Thlaspi caerulescens (Brassicaceae), a metalophyte that can hyperaccumulate both metals. Journal of Plant Physiology. 1992;140:350–355. [Google Scholar]
- Vázquez MD, Poschenrieder C, Barcelo J, Baker AJM, Hatton P, Cope GH. Compartmentation of zinc in roots and leaves of the zinc hyperaccumulator Thlaspi caerulescens. Botanica Acta. 1994;1087:243–250. [Google Scholar]
- Verbruggen N, Hermans C, Schat H. Mechanisms to cope with arsenic or cadmium excess in plants. Current Opinion in Plant Biology. 2009;12:364–372. doi: 10.1016/j.pbi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Verkleij JAC, Koevoets P, Vantriet J, Bank R, Nijdam Y, Ernst WHO. Poly(gamma-glutamylcysteinyl)glycines or phytochelatins and their role in cadmium tolerance of Silene-vulgaris. Plant, Cell & Environment. 1990;13:913–921. [Google Scholar]
- Verret F, Gravot A, Auroy P, et al. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Letters. 2004;576:306–312. doi: 10.1016/j.febslet.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Vogel-Mikuš K, Regvar M, Mesjasz-Przybylowicz J, et al. Spatial distribution of cadmium in leaves of metal hyperaccumulating Thlaspi praecox using micro-PIXE. New Phytologist. 2008a;179:712–721. doi: 10.1111/j.1469-8137.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- Vogel-Mikuš K, Simcic J, Pelicon P, et al. Comparison of essential and non-essential element distribution in leaves of the Cd/Zn hyperaccumulator Thlaspi praecox as revealed by micro-PIXE. Plant, Cell & Environment. 2008b;31:1484–1496. doi: 10.1111/j.1365-3040.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- Volkov V, Amtmann A. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+/Na+ homeostasis under salinity stress. The Plant Journal. 2006;48:342–353. doi: 10.1111/j.1365-313X.2006.02876.x. [DOI] [PubMed] [Google Scholar]
- Wagner GJ. Compartmentation in plant cells: the role of the vacuole. Recent Advances in Phytochemistry. 1982;16:1–45. [Google Scholar]
- Wagner GJ, Yeargan R. Variation in cadmium accumulation potential and tissue distribution of cadmium in tobacco. Plant Physiology. 1986;82:274–279. doi: 10.1104/pp.82.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NA, Pitman MG. Measurement of fluxes across membranes. In: Luttge U, Pitman MG, editors. Encyclopedia of plant physiology. Berlin: Springer-Verlag; 1976. [Google Scholar]
- Wang C, Song H. Calcium protects Trifolium repens L. seedlings against cadmium stress. Plant Cell Reports. 2009;28:1341–1349. doi: 10.1007/s00299-009-0734-y. [DOI] [PubMed] [Google Scholar]
- Weckwerth W, Baginsky S, van Wijk K. The multinational Arabidopsis steering subcommittee for proteomics assembles the largest proteome database resource for plant systems biology. Journal of Proteome Research. 2008;7:4209–4210. doi: 10.1021/pr800480u. [DOI] [PubMed] [Google Scholar]
- Wegner LH, Raschke K. Ion channels in the xylem parenchyma of barley roots: a procedure to isolate protoplasts from this tissue and a patch-clamp exploration of salt passageways into xylem vessels. Plant Physiology. 1994;105:799–813. doi: 10.1104/pp.105.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RM. The impact of mineral nutrients in food crops on global human health. Plant and Soil. 2002;247:83–90. [Google Scholar]
- White PJ. The pathways of calcium movement to the xylem. Journal of Experimental Botany. 2001;52:891–899. doi: 10.1093/jexbot/52.358.891. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Calcium in plants. Annals of Botany. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets: iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- Williams ML, Thomas BJ, Farrar JF, Pollock CJ. Visualizing the distribution of elements within barley leaves by energy-dispersive X-ray image maps (EDX maps) New Phytologist. 1993;125:367–372. doi: 10.1111/j.1469-8137.1993.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An ‘electronic fluorescent pictograph’ browser for exploring and analyzing large-scale biological data sets. PLoS ONE. 2007;2:e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcik M, Vangronsveld J, 'Haen J, Tukiendorf A. Cadmium tolerance in Thlaspi caerulescens. II. Localization of cadmium in Thlaspi caerulescens. Environmental and Experimental Botany. 2005;53:163–171. [Google Scholar]
- Wu QY, Lim W, Park SH. Physiological characterization of co-expressing of a CAX1 calcium transporter and a CRT calcium-binding protein in planta. In vitro Cellular & Developmental Biology – Plant. 2009;45:511. [Google Scholar]
- Wu YY, Chen QJ, Chen M, Chen J, Wang XC. Salt-tolerant transgenic perennial ryegrass (Lolium perenne L.) obtained by Agrobacterium tumefaciens-mediated transformation of the vacuolar Na+/H+ antiporter gene. Plant Science. 2005;169:65–73. [Google Scholar]
- Xue D, Chen M, Zhang G. Mapping of QTLs associated with cadmium tolerance and accumulation during seedling stage in rice (Oryza sativa L.) Euphytica. 2009;165:587–596. [Google Scholar]
- Yadav R, Flowers TJ, Yeo AR. The involvement of the transpirational bypass flow in sodium uptake by high- and low-sodium-transporting lines of rice developed through intravarietal selection. Plant, Cell & Environment. 1996;19:329–336. [Google Scholar]
- Yang X, Baligar VC, Martens DC, Clark RB. Cadmium effects on influx and transport of mineral nutrients in plant species. Journal of Plant Nutrition. 1996;19:643–656. [Google Scholar]
- Yang YZ, Costa A, Leonhardt N. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;4:6. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, Quintero FJ, Cubero B, et al. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. The Plant Journal. 2002;30:529–539. doi: 10.1046/j.1365-313x.2002.01309.x. [DOI] [PubMed] [Google Scholar]
- Yu XC, Zhu SY, Gao GF, et al. Expression of a grape calcium-dependent protein kinase ACPK1 in Arabidopsis thaliana promotes plant growth and confers abscisic acid-hypersensitivity in germination, postgermination growth, and stomatal movement. Plant Molecular Biology. 2007;64:531–538. doi: 10.1007/s11103-007-9172-9. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Maeshima M. Organ specificity of a vacuolar Ca2+-binding protein RVCaB in radish and its expression under Ca2+-deficient conditions. Plant Molecular Biology. 2001;47:633–640. doi: 10.1023/a:1012355205991. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nature Biotechnology. 2001;19:765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Hodson JN, William JP, Blumwald E. Engineering salt tolerant brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proceedings of the National Academy of Sciences of the USA. 2001;98:12832–12836. doi: 10.1073/pnas.231476498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Song FB. Cadmium uptake and distribution by different maize genotypes in maturing stage. Communications in Soil Science and Plant Analysis. 2008;39:1517–1531. [Google Scholar]
- Zhang WH, Zhou YC, Dibley KE, Tyerman SD, Furbank RT, Patrick JW. Nutrient loading of developing seeds. Functional Plant Biology. 2007;34:314–331. doi: 10.1071/FP06271. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Lombi E, Breedon T, McGrath SP. Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant, Cell & Environment. 2000;23:507–514. [Google Scholar]
- Zhao J, Barkla B, Marshall J, Pittman J, Hirschi K. The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta. 2008;227:659–669. doi: 10.1007/s00425-007-0648-2. [DOI] [PubMed] [Google Scholar]