Abstract
Background
The essentiality of boron (B) for plant growth was established >85 years ago. In the last decade, it has been revealed that one of the physiological roles of B is cross-linking the pectic polysaccharide rhamnogalacturonan II in primary cell walls. Borate cross-linking of pectic networks serves both for physical strength of cell walls and for cell adhesion. On the other hand, high concentrations of B are toxic to plant growth. To avoid deficiency and toxicity problems, it is important for plants to maintain their tissue B concentrations within an optimum range by regulating transport processes. Boron transport was long believed to be a passive, unregulated process, but the identification of B transporters has suggested that plants sense and respond to the B conditions and regulate transporters to maintain B homeostasis.
Scope
Transporters responsible for efficient B uptake by roots, xylem loading and B distribution among leaves have been described. These transporters are required under B limitation for efficient acquisition and utilization of B. Transporters important for tolerating high B levels in the environment have also been identified, and these transporters export B from roots back to the soil. Two types of transporters are involved in these processes: NIPs (nodulin-26-like intrinsic proteins), boric acid channels, and BORs, B exporters. It is demonstrated that the expression of genes encoding these transporters is finely regulated in response to B availability in the environment to ensure tissue B homeostasis. Furthermore, plants tolerant to stress produced by low B or high B in the environment can be generated through altered expression of these transporters.
Conclusions
The identification of the first B transporter led to the discovery that B transport was a process mediated not only by passive diffusion but also by transporters whose activity was regulated in response to B conditions. Now it is evident that plants sense internal and external B conditions and regulate B transport by modulating the expression and/or accumulation of these transporters. Results obtained in model plants are applicable to other plant species, and such knowledge may be useful in designing plants or crops tolerant to soils containing low or high B.
Keywords: Boron, rhamnogalacturonan II, transporter, channel, xylem loading, phloem, over-expression, Arabidopsis thaliana, Oryza sativa, Hordeum vulgare, Triticum aestivum
INTRODUCTION
Boron (B) is an essential micronutrient for plants, and its deficiency generally causes growth defects mainly in young and growing portions of plants. Boron deficiency is one of the most widespread deficiencies among plant micronutrients in agriculture (Loomis and Durst, 1992). Boron deficiency causes a wide range of symptoms including the cessation of root elongation, reduced leaf expansion and the loss of fertility, depending on the plant species and developmental stage (Gupta, 1979; Dell and Huang, 1997; Goldbach et al., 2001). Boron deficiency reduces not only yield but also the quality of crops (Shorrocks, 1997; Brown et al., 2002). Boron-deficient soils are mostly found in regions with high rainfall such as southeast Asia and southeast China. Boron in soil is relatively soluble and easily leached by rainfall.
High concentrations of B are toxic to living organisms including plants (Nable et al., 1997). Boron toxicity reduces growth, particularly of shoots, and causes chlorosis starting at the leaf tip and margins of mature leaves (Nable et al., 1997; Reid et al., 2004; Reid and Fitzpatrick, 2009). This presumably results from the accumulation of B transported through the transpiration stream. As retranslocation of B is negligible in many plant species, B tends to accumulate in old leaves (Marschner, 1995). Boron toxicity is a significant problem in semi-arid areas in the world including South Australia, Turkey, Mediterranean countries, California and Chile.
Boron belongs to Group 13 in the periodic table and its properties lie in between those of metals and non-metals. Although it belongs to Group 13, its chemical properties are very different from those of the other elements in this Group, such as Al or Ga, and B is referred to as an orphan of the periodic table (Tanaka and Fujiwara, 2007). Its unique properties make B an important element in organic chemistry. A wide range of B-containing chemicals including boronic acids and borohydrides are commonly used in the synthesis of organic compounds.
Boron in soil solution is mainly present as boric acid or borate. The pKa of boric acid is 9·25 and, at neutral pH, the equilibrium is shifted greatly toward boric acid. Boric acid, a charge-neutral molecule, is the major chemical form of B taken up by plants (Marschner, 1995). Among the elements required by plants that are taken up from the soil, B is the only element that is taken up by plants not as an ion, but as an uncharged molecule. When the Casparian strip, a hydrophobic layer in the cell wall between endodermal cells, develops, it blocks apoplastic flow of solutes into the stele. In the root tip where the Casparian strip is not matured, solutes are accessible to the xylem through apoplastic flow, and apoplastic flux is shown to be an important pathway for transport to shoots in the case of Ca2+ (White, 2001). For the majority of the root length, for most mineral nutrients to be transported across the root from the soil to the xylem, they need to move across plasma membranes at least twice because of the Casparian strip: first uptake into cells and subsequently export out of the cells into the xylem. For solutes that do not permeate lipid membranes readily, two types of transport protein must be present in the plasma membrane of root cells, an influx transporter and an efflux transporter for symplastic flux. In the case of B, however, only passive diffusion (i.e. transport that is not catalysed by proteins) across the plasma membrane was thought to occur and, until the 1990s, this was considered capable of fulfilling the B requirements of plants. This hypothesis was based on the high permeability of lipid bilayers to boric acid (Raven, 1980). Furthermore, the idea of B transport by passive diffusion appeared to coincide well with the patterns of symptoms of B deficiency and toxicity in young growing and mature tissues, respectively (Marschner, 1995). This hypothesis is now known not to be true. The first B transporter in biological systems was identified in Arabidopsis thaliana and this protein is required for the transport of boric acid against its concentration gradients under low B conditions. The identification of this transport protein revealed the physiological functions of transporters for B homeostasis and expanded our views of B in biology.
PHYSIOLOGICAL FUNCTION OF B: BORATE CROSS-LINKING OF PECTIC POLYSACCHARIDE RHAMNOGALACTURONAN II
Although the essential nature of B was established >85 years ago, the molecular basis of the B requirement of plants has been understood for <10 years. Boric acid forms esters with alcohols in a pH-dependent manner (Power and Woods, 1997). The most stable borate esters are formed with cis-diols on a furanoid ring, such as ribose and apiose.
Biochemical and genetic studies have uncovered that one of the physiological functions of B in plants is dimerization of the pectic polysaccharide rhammnogaracturonan II (RG II) by borate cross-linking (Ishii and Matsunaga, 1996; Kobayashi et al., 1996; O'Neill et al., 2001). This function of B in cell walls was consistent with the observations that B is predominantly distributed to the water-insoluble fractions of cells under conditions of limited B supply (Matoh et al., 1992; Hu and Brown, 1994; Dannel et al., 1999; Noguchi et al., 2000). Borate-dimerized RG II complex is present not only in angiosperms and gymnosperms but also in pteridophytes and bryophytes (Matsunaga et al., 2004; O'Neill et al., 2004). Due to the high complexity of the glycosyl compositions and linkages of RG II, whole RG II synthesis processes are not completely understood although several enzymes for RG II synthesis have beeen identified (Iwai et al., 2002; Bonin et al., 2003; Ahn et al., 2006; Egelund et al., 2006, 2008; Delmas et al., 2008). Further, it remains unknown when and where dimerization of the RG II monomer by borate occurs in plant cells.
Other than cross-linking of pectins in plants, possible functions of B in membrane function and metabolic activities have also been suggested (Bolanos et al., 2004). Bassil et al. (2004) proposed that B performs a structural function in the cytoskeleton from the observation that boronic acids, which compete with boric acid for binding to cis-diols, caused the disruption of cytoplasmic strands and cell–cell wall detachment in cultured tobacco cells. Wimmer et al. (2009) reported isolation of possible B-binding proteins from microsomes by phenylboronate affinity chromatography. Molecular mechanisms underlying these functions of B remain to be uncovered.
Of the 17 elements essential for plant growth and development, B has long been considered to be required only for plants but not for animals (Marschner, 1995). A specific requirement for B in plant cell walls supports this idea. However, increasing evidence suggests essential roles for B in other organisms including animals and bacteria. The functions of B are obviously not cell wall related in these organisms. Boron-containing compounds have been identified in micro-organisms. Borophycin and boromycin are B-containing antibiotics produced by the cyanobacterium Nostoc spongiaeforme var. tenue and by Streptmyces griseus, respectively (Dembitsky et al., 2002). Autoinducer-2, a molecule for quorum sensing in the marine bacterium Vibrio harveyi, contains B (Chen et al. 2002), and vibrioferrin, a B-containing siderophore produced by particular marine bacteria, is required for the growth of the toxic, bloom-forming dinoflagellate Gymnodinium catenatum (Amin et al., 2007).
ROOT UPTAKE AND XYLEM LOADING OF B UNDER LIMITED B AVAILABILITY
Boron occurs in the soil solution predominantly as undissociated, electroneutral boric acid, B(OH)3. The uptake of B by plant roots from the soil solution was long considered to be a passive process that was not facilitated by transport proteins. However, recent physiological studies have revealed the presence of channel-mediated facilitated diffusion and energy-dependent active transport against concentration gradients in B transport systems (Dannel et al., 2000, 2001; Stangoulis et al., 2001). The molecular identity of the transport systems then became a major focus. Subsequently, molecular genetic studies of A. thaliana successfully identified two types of B transporters, NIP5;1 and BOR1, both of which are important for efficient transport of B across the plasma membrane under B limitation.
In the initial uptake process, NIP5;1, a boric acid channel, facilitates B influx to root cells. NIP5;1 was identified from microarray experiments as a gene upregulated by low B supply in roots of A. thaliana. NIP5;1 mRNA accumulation in roots increased by about 15 fold 24 h after the initiation of a low B treatment (Takano et al., 2006). Expression of NIP5;1 facilitated the influx of boric acid to Xenopus laevis oocytes. T-DNA insertion mutants of A. thaliana exhibited reduced uptake of boric acid by roots and growth defects only under limited B supply. These results revealed that NIP5;1 is a boric acid channel required for efficient B uptake in A. thaliana roots (Takano et al., 2006).
NIP5;1 is a member of the major intrinsic protein (MIP) superfamily, which includes the aquaporins. The MIP superfamily can be divided into four sub-families including the small basic intrinsic proteins (SIPs), tonoplast intrinsic proteins (TIPs), nodulin-26-like intrinsic proteins (NIPs) and the plasma membrane intrinsic proteins (PIPs). Involvement of other members of the MIP superfamily in B transport is also suggested. In addition to NIP6;1, as discussed below, it has been demonstrated that expresssion of maize Zm-PIP1 in Xenopus laevis oocytes resulted in a 30 % increase in B permeability (Dordas and Brown, 2000, 2001) and expression of Hv-PIP1;3 and Hv-PIP1;4 from Hordeum vulgare increased the sensitivity of yeast cells to B (Fitzpatrick and Reid, 2009). However, the functions of these transporters in plant cells remain to be clarified.
BOR1, a B exporter, plays a key role in xylem loading. The A. thaliana bor1-1 mutant was isolated as being more susceptible to B deficiency than the wild type. In bor1-1, leaf expansion in upper leaves was severely inhibited under low B conditions (Noguchi et al., 1997). When B concentrations of root cell saps and xylem saps were determined in plants exposed to various concentration of B, the B concentrations in root cell saps were almost the same as the B concentrations in the media, both in wild-type plants and in the bor1-1 mutant (Takano et al., 2002). In contrast, B concentrations in xylem saps were always higher in the wild type than in the mutant. In the wild type, B concentrations in xylem saps were higher than those in the media with low B supply, but those of the bor1-1 mutant were not (Takano et al., 2002), suggesting that BOR1 was required for B transport to the xylem against its concentration gradient. BOR1 was identified by positional cloning. BOR1 showed a high similarity to anion exchanger proteins, including the well-characterized Band3 protein of animal erythrocytes (Takano et al., 2002). BOR1 is expressed predominantly in the root pericycle cells surrounding the xylem. The BOR1–green fluorescent protein (GFP) fusion protein was localized to the plasma membrane. When A. thaliana BOR1 is expressed in a Saccharomyces cerevisiae strain that lacks the yeast BOR1 gene, B concentrations in yeast cells were reduced by 3-fold, suggesting that BOR1 is an exporter of B. BOR1 was the first B transporter identified in biological systems. It has been demonstrated that Oryza sativa BOR1, an orthologue of A. thaliana BOR1, plays a critical role in B acquisition by roots and translocation of B into shoots (Nakagawa et al., 2007).
Expression of A. thaliana BOR1 is regulated post-transcriptionally (Takano et al., 2005). Accumulation of BOR1 protein decreases in response to a high B concentration in the medium. Under low B supply, BOR1 is accumulated mainly in the plasma membrane. Upon exposure to high B, BOR1 is incorporated into endosomes and transported to the vacuole for degradation (Takano et al., 2005). This B-dependent degradation of BOR1 might be beneficial for plants as it allows them to avoid overaccumulation of B in shoots when B concentrations in soils are high.
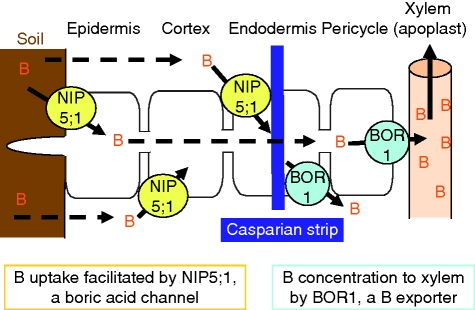
Under B-limited conditions, the combination of NIP5;1 for uptake and BOR1 for efflux into xylem is likely to be key to the efficient transcellular transport of B from the soil solution to the xylem (Fig. 1). BOR1 is likely to generate a concentration gradient between root cells and the medum. This concentration gradient is probably essential for NIP5;1 to facilitate B uptake into root cells because NIP5;1 is likely to be a boric acid channel.
Fig. 1.
A schematic model of B transport in A. thaliana roots under B limitation. Cell layers of a cross-section of A. thaliana roots where the Casparian strip is developed in the endodermis (State I; White, 2001) are illustrated. It is likely that endodermal cells subsequently become suberized and unable to take up solutes directly from the root apoplast (Moore et al., 2002). Under B limitation, NIP5;1 increases the permeability of boric acid to cell membranes, and facilitates influx of B into root cells from the soil. BOR1 exports B out of the cells toward the xylem against the concentration gradient. It is likely that the co-ordinated expression patterns of BOR1 and NIP5;1 are essential for efficient transcellular transport of B as NIP5;1 possibly facilitates B influx, following the B concentration gradient that BOR1 generates. Under high levels of B supply, expression of both NIP5;1 and BOR1 is decreased by transcriptional and post-transcriptional regulation, respectively. The downregulation of NIP5;1 and BOR1 might be beneficial for avoidance of overloading of high concentrations of B to shoots.
B DISTRIBUTION IN SHOOTS OF PLANTS WITH LIMITED B SUPPLY
BOR1 is involved not only in B transport into the xylem but also in B distribution within shoots. Takano et al. (2001) demonstrated that the wild-type A. thaliana distributed B preferentially to young leaves, while such preferential transport was not evident in the bor1-1 mutant. BOR1 is expressed in shoots, but it is not known how BOR1, an efflux transporter of B, regulates B distribution among leaves. It is possible that BOR1 may be involved in directing B from the xylem to phloem for preferential delivery to young leaves.
Another transporter has also been shown to be involved in B distribution in shoots. NIP6;1 is the gene most similar to NIP5;1 among the nine NIP genes present in the A. thaliana genome. NIP6;1 is also localized to the plasma membrane and facilitates boric acid uptake into oocytes, but does not show water channel activity (Tanaka et al., 2008). This is in contrast to the case of NIP5;1, which is permeable to both boric acid and water. T-DNA insertion mutant lines of NIP6;1 exhibited defects in the expansion of young leaves. In the mutant, B concentrations in young leaves under B limitation were lower than in wild-type plants. Strong NIP6;1 promoter activity was observed in the phloem region at nodes of the stem. It is proposed that NIP6;1 functions in xylem–phloem transfer for preferential distribution of B into young growing tissues (Tanaka et al., 2008).
B EFFLUX AT TOXIC LEVELS OF B IN ROOTS AND SHOOTS
One of the physiological functions of the A. thaliana BOR1 homologue in a single cell was shown to be the efflux of B back to the environment to reduce the B concentration in cells. YNL275w (Sc-BOR1) is the sole At-BOR1 homologue in S. cerevisiae. It was previously characterized as an anion transporter localized to plasma membrane, and potential substrates include HCO3−, I−, Br−, NO3− and Cl− (Zhao and Reithmeier, 2001). Bor1p, the product of Sc-BOR1, is a B exporter (Takano et al., 2002). Disruption of Sc-BOR1 increased sensitivity to toxic levels of B, presumably resulting from elevated concentrations of B in cells (Takano et al., 2002, 2007). These findings suggested that one physiological function of Bor1p was to export B from the cell. Nozawa et al. (2007) demonstrated the possible involvement of FPS1 (a glycerol channel) and DUR3 (a urea transporter) in tolerance of high B in yeast. Kaya et al. (2009) recently found that ATR1 is responsible for most of the tolerance of high B in S. cerevisiae. ATR1 encodes a multidrug resistance transport protein of the major facilitator superfamily. Disruption of ATR1 increased sensitivity to high B in the environment, and expression of ATR1 conferred a greater tolerance. ATR1–GFP was localized to the cell membrane and it was suggested that ATR1 was a B exporter. Genes similar to ATR1 are widely present in bacteria and fungi, but not in plants.
It has also been demonstrated that homologues of A. thaliana BOR1 are involved in tolerance of high B in other plant species. Hayes and Reid (2004) observed that the high B-tolerant barley cultivar, Sahara, maintained low B concentrations in roots by active B efflux, and this resulted in decreased accumulation of B in shoots and elevated tolerance of high B. Sutton et al. (2007) performed a quantitative trait locus (QTL) analysis of two barley cultivars differing in their tolerance of high B conditions, and found that the mapped region contained a BOR1 homologue. They named this gene Bot1. The same gene was cloned by Reid (2007) and was named Hv-BOR2, as it is similar to rice BOR2. The terminology for Bot1 may be correct in terms of the effect of the gene, but obviously it potentially causes confusion as similar transporters are now named differently. Sutton et al. (2007) demonstrated that expression of Bot1 conferred tolerance of high B in S. cerevisiae, as was reported for At-BOR1. mRNA from Bot1 has been detected in both roots and shoots, but more is present in roots. The tolerant cultivar possesses multiple copies of Bot1 and has greater amounts of Bot1 mRNA than the susceptible cultivar. Sutton et al. (2007) suggested that increased expression of the B exporter in roots leads to reduction in B concentrations in roots. At the same time, Reid (2007) cloned Hv-BOR2 and Ta-BOR2 from barley and wheat, respectively. Both are similar to At-BOR1. Positive correlations between mRNA levels of BOR2 genes and tolerance of high B were described among different cultivars in both barley and wheat, supporting roles for BOR2 in tolerance of high B. Furthermore, Reid and Fitzpatrick (2009) noticed that necrosis in leaves was observed at higher leaf B concentrations in the tolerant cultivars in barley and wheat. It was shown that B concentrations in leaf protoplasts were lower in the tolerant cultivar, suggesting different partitioning of B within the shoot. It is likely that B transporters export toxic B out of cells into the apoplast and alleviate B toxicity symptoms in the cytoplasm of leaf cells. Boron exporters encoded by BOR1 homologues not only reduce B uptake by roots as a primary B tolerance mechanism, but also redistribute toxic B in leaves to confer further tolerance of high B.
In addition to B exclusion, the presence of another mechanism affecting tolerance of high B in plants has been suggested. Ochiai et al. (2008) found that ‘japonica’ rice cultivars were more tolerant than ‘indica’ cultivars to high B supply. However, B concentrations in roots and shoots were not significantly different. The molecular basis of this phenomenon has not been reported yet, but a major QTL has been identified that accounts for the phenotypic variation.
PLANTS TOLERANT TO B DEFICIENCY AND B TOXICITY GENERATED BY ENHANCED EXPRESSION OF TRANSPORTERS
When A. thaliana BOR1 was over-expressed under the control of the cauliflower mosaic virus 35S RNA promoter in A. thaliana plants, shoot growth and fertility were increased compared with wild-type plants when grown under low B conditions (Miwa and Takano et al., 2006). Elevated levels of BOR1 presumably increased translocation of B from roots to shoots. This elevated B transport to shoots is likely to be beneficial for plants with low B supply. This study represented the first example of altering the expression of genes encoding transport proteins for essential nutrients to generate plants tolerant to a low availability of the essential nutrient. The advantage of this method is the lack of negative effects of over-expression of BOR1 on growth under normal or high levels of B supply. This is probably due to the degradation of BOR1 in plants grown in high B environments.
Over-expression of BOR1 enhanced shoot growth under limited B supply, but not root growth. Kato et al. (2009) demonstrated that enhanced expression of NIP5;1 improved root elongation in low B environments. The combination of enhanced expression of BOR1 and NIP5;1 enabled A. thaliana plants to become remarkably tolerant to B limitation in terms of the growth of both shoots and roots. From these experiments, it could be speculated that a limiting process for root and shoot growth under low B conditions is B uptake by NIP5;1 and xylem loading by BOR1 in A. thaliana wild-type plants.
To increase tolerance of high B, over-expresssion of BOR4, a BOR1 paralogue, in A. thaliana was very effective (Miwa et al., 2007). The transgenic plants tolerated 10 mm boric acid in the medium, while the untransformed wild-type plants barely extended roots. In contrast to BOR1, BOR4 is not degraded even under high levels of B supply. In one BOR4-expressing A. thaliana line, B concentrations in shoots and roots were decreased by about 3-fold, and plant growth was greatly improved under high levels of B supply. This result further supports the idea that endogenous Hv-BOR2/Bot1 and Ta-BOR2 function in tolerance of high B in barely and wheat.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Molecular, genetic and physiological studies have revealed that B transport in plants not only occurs by passive diffusion across membranes but is also catalysed by regulated transport proteins. These transporters function to support normal growth under both low and high B conditions. Our understanding of B acquisition from soils by roots has greatly advanced; however, the processes of B distribution within the aerial portions of plants are still poorly understood. In particular, more emphasis should be placed on the molecular study of B transport to reproductive organs during the reproductive stages under limited B conditions, because B limitation causes sterility, which is the major cause of yield reduction of crops under low B conditions. At the cellular level, mechanisms for preferential partitioning of B into cell walls remain to be uncovered.
It is established that the expression of some B transporters is regulated in response to B conditions; NIP5;1 at the mRNA level and BOR1 at the protein level. This observation supports the idea that plants sense their B status and control B transport activities to maintain B homeostasis. It is very important to know where plants sense (local or systemic), what plants sense (boric acid or metabolites) and how plants sense (binding protein) their B status.
Distinct roles and transport properties of the gene products have been found among the BOR genes present in plants, although all of them so far tested encode efflux-type B transporters. At-BOR1 is required for B export into xylem for efficient translocation of B under limited B supply, and is degraded under high B supply. In contrast, Hv-BOR2/Bot1, Ta-BOR2 and At-BOR4 are likely to remove B from plant cells to confer tolerance to high B. Since a high requirement for B and a requirement to load B into the xylem probably emerged during the evolution of vascular plants, it is of great interest to elucidate how BOR genes have evolved from the ancestral gene common to the anion exchangers in humans, which do not seem to transport boric acid, to fulfil their distinct roles in B transport.
From the view of application to agriculture, modulation of the expression of genes encoding B transporters has been shown to have great potential to improve tolerance of B stresses in A. thaliana plants. In addition to the transgenic approaches, these genes can be useful as genetic makers for crop breeding since genes homologous to BOR1 and NIP5;1 are widely present in plant species. It would be satisfying if the identification of the key genes and processes involved in tolerating B stresses in model plants led to the improvement of crop plants.
ACKNOWLEDGEMENTS
We appreciate the support of the JSPS Research Fellowships for young scientists and the Hokkaido University leader development system in basic interdisciplinary research to K. M. This work was supported in part by a grant (Kiban S) from the Japanese Society for the Promotion of Science to T. F., from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation Grant IPG-0005 to T.F.), and a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to T. F.
LITERATURE CITED
- Ahn JW, Verma R, Kim M, et al. Depletion of UDP-d-apiose/UDP-d-xylose synthases results in rhamnogalacturonan-II deficiency, cell wall thickening, and cell death in higher plants. Journal of Biological Chemistry. 2006;281:13708–13716. doi: 10.1074/jbc.M512403200. [DOI] [PubMed] [Google Scholar]
- Amin SA, Kupper FC, Green DH, Harris WR, Carrano CJ. Boron binding by a siderophore isolated from marine bacteria associated with the toxic dinoflagellate Gymnodinium catenatum. Journal of the American Chemical Society. 2007;129:478–479. doi: 10.1021/ja067369u. [DOI] [PubMed] [Google Scholar]
- Bassil E, Hu H, Brown PH. Use of phenylboronic acids to investigate boron function in plants. Possible role of boron in transvacuolar cytoplasmic strands and cell-to-wall adhesion. Plant Physiology. 2004;136:3383–3395. doi: 10.1104/pp.104.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos L, Lukaszewski K, Bonilla I, Blevins D. Why boron? Plant Physiology and Biochemistry. 2004;42:907–912. doi: 10.1016/j.plaphy.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Bonin CP, Freshour G, Hahn MG, Vanzin GF, Reiter WD. The GMD1 and GMD2 genes of Arabidopsis encode isoforms of GDP-d-mannose 4,6-dehydratase with cell type-specific expression patterns. Plant Physiology. 2003;132:883–892. doi: 10.1104/pp.103.022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Bellaloui N, Wimmer MA, et al. Boron in plant biology. Plant Biology. 2002;4:205–223. [Google Scholar]
- Chen X, Schauder S, Potier N, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- Dannel F, Pfeffer H, Römheld V. Distribution within the plant or compartmentation does not contribute substantially to the detoxification of excess boron in sunflower Helianthus annuus. Australian Journal of Plant Physiology. 1999;26:95–99. [Google Scholar]
- Dannel F, Pfeffer H, Römheld V. Characterization of root boron pools, boron uptake and boron translocation in sunflower using the stable isotope 10B and 11B. Australian Journal of Plant Physiology. 2000;156:756–761. [Google Scholar]
- Dannel F, Pfeffer H, Walch-Liu P, Romheld V. Plant nutrition – food security and sustainability of agro-ecosystems. Dordrecht: Kluwer; 2001. pp. 162–163. [Google Scholar]
- Dell B, Huang LB. Physiological response of plants to low boron. Plant and Soil. 1997;193:103–120. [Google Scholar]
- Delmas F, Seveno M, Northey JGB, et al. The synthesis of the rhamnogalacturonan II component 3-deoxy-d-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation. Journal of Experimental Botany. 2008;59:2639–2647. doi: 10.1093/jxb/ern118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembitsky VM, Smoum R, Al-Quntar A, Ali HA, Pergament I, Srebnik M. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Science. 2002;163:931–942. [Google Scholar]
- Dordas C, Brown PH. Permeability of boric acid across lipid bilayers and factors affecting it. Journal of Membrane Biology. 2000;175:95–105. doi: 10.1007/s002320001058. [DOI] [PubMed] [Google Scholar]
- Dordas C, Brown PH. Evidence for channel mediated transport of boric acid in squash (Cucurbita pepo) Plant and Soil. 2001;235:95–103. [Google Scholar]
- Egelund J, Petersen BL, Motawia MS, et al. Arabidopsis thaliana RGXT1 and RGXT2 encode Golgi-localized (1,3)-alpha-d-xylosyltransferases involved in the synthesis of pectic rhamnogalacturonan-II. The Plant Cell. 2006;18:2593–2607. doi: 10.1105/tpc.105.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelund J, Damager I, Faber K, et al. Functional characterisation of a putative rhamnogalacturonan II specific xylosyltransferase. FEBS Letters. 2008;582:3217–3222. doi: 10.1016/j.febslet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KL, Reid R. The involvement of aquaglyceroporins in transport of boron in barley root. Plant, Cell and Environment. 2009;32:1357–1365. doi: 10.1111/j.1365-3040.2009.02003.x. [DOI] [PubMed] [Google Scholar]
- Gupta UC. Boron nutrition of crops. Advances in Agronomy. 1979;31:273–307. [Google Scholar]
- Goldbach HE, Yu Q, Wingender R, et al. Rapid response reactions of roots to boron deprivation. Journal of Plant Nutrition and Soil Science. 2001;164:173–181. [Google Scholar]
- Hayes JE, Reid RJ. Boron tolerance in barley is mediated by efflux of boron from the roots. Plant Physiology. 2004;136:3376–3382. doi: 10.1104/pp.103.037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Brown PH. Localization of boron in cell walls of squash and tobacco and its association with pectin. Evidence for a structural role of boron in the cell wall. Plant Physiology. 1994;105:681–689. doi: 10.1104/pp.105.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T. Isolation and characterization of a boron–rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydrate Research. 1996;284:1–9. [Google Scholar]
- Iwai H, Masaoka N, Ishii T, Satoh S. A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proceedings of the National Academy of Sciences, USA. 2002;99:16319–16324. doi: 10.1073/pnas.252530499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Miwa K, Takano J, Wada M, Fujiwara T. Highly boron deficiency-tolerant plants generated by enhanced expression of NIP5;1, a boric acid channel. Plant and Cell Physiology. 2009;50:58–66. doi: 10.1093/pcp/pcn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya A, Karakaya HC, Fomenko DE, Gladyshev VN, Koc A. Identification of a novel system for boron transport: Atr1 is a main boron exporter in yeast. Molecular and Cellular Biology. 2009;29:3665–3674. doi: 10.1128/MCB.01646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Matoh T, Azuma JI. Two chains of rhamnogalacturonan II are cross-linked by borate–diol ester bonds in higher plant cell walls. Plant Physiology. 1996;110:1017–1020. doi: 10.1104/pp.110.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WD, Durst RW. Chemistry and biology of boron. Biofactors. 1992;3:229–239. [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Matoh T, Ishigaki K, Mizutani M, Matsunaga W, Takabe K. Boron nutrition of cultured tobacco BY-2 cells. 1. Requirement for and intracellular localization of boron and selection of cells that tolerate low levels of boron. Plant and Cell Physiology. 1992;33:1135–1141. [Google Scholar]
- Matsunaga T, Ishii T, Matsumoto S, et al. Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiology. 2004;134:339–351. doi: 10.1104/pp.103.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Takano J, Fujiwara T. Improvement of seed yields under boron-limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. The Plant Journal. 2006;46:1084–1091. doi: 10.1111/j.1365-313X.2006.02763.x. [DOI] [PubMed] [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. Plants tolerant of high boron levels. Science. 2007;318:1417. doi: 10.1126/science.1146634. [DOI] [PubMed] [Google Scholar]
- Moore CA, Bowen HC, Scrase-Field S, Knight M, White PJ. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. The Plant Journal. 2002;30:457–465. doi: 10.1046/j.1365-313x.2002.01306.x. [DOI] [PubMed] [Google Scholar]
- Nable RO, Bañuelos GS, Paull JG. Boron toxicity. Plant and Soil. 1997;193:181–198. [Google Scholar]
- Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T. Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. The Plant Cell. 2007;19:2624–2635. doi: 10.1105/tpc.106.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Yasumori M, Imai T, et al. bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiology. 1997;115:901–906. doi: 10.1104/pp.115.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Dannel F, Heidrun P, Römheld V, Hayashi H, Fujiwara T. Defect in root–shoot translocation of boron in Arabidopsis thaliana mutant bor1-1. Journal of Plant Physiology. 2000;156:756–761. [Google Scholar]
- Nozawa A, Takano J, Kobayashi M, von Wirén N, Fujiwara T. Roles of BOR1, DUR3, and FPS1 in boron transport and tolerance in Saccharomyces cerevisiae. FEMS Microbiology Letters. 2006;262:216–222. doi: 10.1111/j.1574-6968.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- Ochiai K, Uemura S, Shimizu A, Okumoto Y, Matoh T. Boron toxicity in rice (Oryza sativa L.). I. Quantitative trait locus (QTL) analysis of tolerance to boron toxicity. Theoretical and Applied Genetics. 2008;117:125–133. doi: 10.1007/s00122-008-0758-7. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annual Review of Plant Biology. 2004;55:109–139. doi: 10.1146/annurev.arplant.55.031903.141750. [DOI] [PubMed] [Google Scholar]
- Power PP, Woods WG. The chemistry of boron and its speciation in plants. Plant and Soil. 1997;193:1–13. [Google Scholar]
- Raven JA. Short- and long-distance transport of boric acid in plants. New Phytologist. 1980;84:231–249. [Google Scholar]
- Reid R. Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant and Cell Physiology. 2007;48:1673–1678. doi: 10.1093/pcp/pcm159. [DOI] [PubMed] [Google Scholar]
- Reid R, Fitzpatrick K. Influence of leaf tolerance mechanisms and rain on boron toxicity in barley and wheat. Plant Physiology. 2009;151:413–420. doi: 10.1104/pp.109.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RJ, Hayes JE, Post A, Stangoulis JC, Graham RD. A critical analysis of the causes of boron toxicity in plants. Plant, Cell and Environment. 2004;27:1405–1414. [Google Scholar]
- Shorrocks VM. The occurrence and correction of boron deficiency. Plant and Soil. 1997;193:121–148. [Google Scholar]
- Stangoulis JC, Reid RJ, Brown PH, Graham RD. Kinetic analysis of boron transport in Chara. Planta. 2001;213:142–146. doi: 10.1007/s004250000484. [DOI] [PubMed] [Google Scholar]
- Sutton T, Baumann U, Hayes J, et al. Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science. 2007;318:1446–1449. doi: 10.1126/science.1146853. [DOI] [PubMed] [Google Scholar]
- Takano J, Yamagami M, Noguchi K, Hayashi H, Fujiwara T. Preferential translocation of boron to young leaves in Arabidopsis thaliana regulated by the BOR1 gene. Soil Science and Plant Nutrition. 2001;47:345–357. [Google Scholar]
- Takano J, Noguchi K, Yasumori M, et al. Arabidopsis boron transporter for xylem loading. Nature. 2002;420:337–340. doi: 10.1038/nature01139. [DOI] [PubMed] [Google Scholar]
- Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proceedings of the National Academy of Sciences, USA. 2005;102:12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. The Plant Cell. 2006;18:1498–1509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Kobayashi M, Noda Y, Fujiwara T. Saccharomyces cerevisiae Bor1p is a boron exporter and a key determinant of boron tolerance. FEMS Microbiology Letters. 2007;267:230–235. doi: 10.1111/j.1574-6968.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fujiwara T. Physiological roles and transport mechanisms of boron: perspectives from plants. Pflugers Archives-European Journal of Physiology. 2007;456:671–677. doi: 10.1007/s00424-007-0370-8. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. The Plant Cell. 2008;20:2860–2875. doi: 10.1105/tpc.108.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. The pathways of calcium movement to the xylem. Journal of Experimental Botany. 2001;52:891–899. doi: 10.1093/jexbot/52.358.891. [DOI] [PubMed] [Google Scholar]
- Wimmer MA, Lochnit G, Bassil E, Mühling KH, Goldbach HE. Membrane-associated, boron-interacting proteins isolated by boronate affinity chromatography. Plant and Cell Physiology. 2009;50:1292–1304. doi: 10.1093/pcp/pcp073. [DOI] [PubMed] [Google Scholar]
- Zhao RM, Reithmeier RA. Expression and characterization of the anion transporter homologue YNL275w in Saccharomyces cerevisiae. American Journal of Physiology. Cell Physiology. 2001;281:C33–C45. doi: 10.1152/ajpcell.2001.281.1.C33. [DOI] [PubMed] [Google Scholar]