Abstract
Background
Function, patient global assessment and pain are routinely measured in rheumatoid arthritis (RA) clinical trials. However, other patient-reported outcomes identified as important to patients in qualitative studies, such as fatigue and quality of life, are commonly not included, and modern treatment regimens may have changed patients’ expectations of treatment outcomes.
Objective
To elicit patient priority treatment outcomes for pharmacological interventions since the common use of anti-TNF therapy, which will form the basis of a core set of patient priorities to complement existing professional core sets.
Methods
In-depth interviews were conducted with 23 RA patients, purposively sampled for age, gender, medication (anti-TNF or other DMARDs), disease severity and work status. Grounded Theory guided iterative data collection and analysis. Coding of the data was peer reviewed. A patient research partner collaborated in the research design and analysis.
Results
63 different outcomes important to patients were generated from the interviews. Four major categories of patient outcomes from pharmacological treatments were developed: ‘RA under control’, ‘Doing things’, ‘Emotional health’, and ‘Coping with illness’. The core category (or overall theme) was ‘Minimising the personal impact of RA’.
Conclusion
Although the routine outcomes of pain, function and overall well-being were raised by patients, they also generated a further 60 important outcomes that they look for from treatment. This difference in perspective may potentially influence treatment decisions. The next step is therefore to use these data to develop a patient core set.
Background
Measurement of disease activity in rheumatoid arthritis (RA) clinical trials has been standardised by the development of core sets of variables and composite indexes that use a mixture of objective measures and patient reported outcomes (PROs). The American College of Rheumatology (ACR) core set was developed by the OMERACT (Outcome Measures for Rheumatology Clinical Trials) group, who used a literature review and nominal groups with experts in clinical trials, health services research, and biostatistics (1). The Disease Activity Score (DAS) was constructed after a prospective study of RA patients where disease activity markers described in the literature and chosen by rheumatologists were tested for their relative contribution to assessing changes in inflammation (2). The International Classification of Functioning, Disability and Health (ICF) core set for RA was developed using the Delphi technique with 17 experts, a systematic review and empirical data collection (3). Thus all these methods contain outcomes deemed important by health professionals for assessing disease activity.
However, there is evidence that assessment and valuation of outcomes for pharmacological treatment of RA differ between health professionals and patients. Differences have been shown between physician and patient assessments of pain, overall health, physical and mental function (4-6). In addition, only difficulty with outcomes of personal importance to patients results in a high personal impact, independent of the level of actual severity (7). Through the Patient Perspective Workshop at OMERACT conferences and the increasing presence of patients at other conferences, the importance of the patient voice has grown since these professional core sets were developed (8). In the past decade, focus group studies with RA patients on first-line Disease Modifying Anti-Rheumatic Drugs (DMARDs) have provided evidence of treatment outcomes important to patients that are frequently not being measured in clinical trials, such as fatigue, sleep, social roles, coping, and life enjoyment (9-11). Therefore, not all indicators of impact important to patients are being assessed in clinical trials, or as part of clinical practice and review as recommended by the National Institute for Clinical Excellence (NICE) (12). The development of a patient core set would enable the standardisation of PROs important to patients.
No studies have determined what outcomes are important to patients on anti-TNF therapy or for those who have discontinued. Two qualitative studies that have examined the patient experience of anti-TNF therapy reported participants feeling like a ‘normal’ person, being socially more dynamic, feeling well in themselves for the first time in years, and that the improvement was ‘life-changing’ (13,14). Such outcomes have not been reported in earlier studies of outcomes important to patients (9-11). The magnitude and speed of change on anti-TNF therapy compared to traditional DMARDs (14) indicate that different outcomes may be important. These studies did not specifically address the valuation of outcomes, did not include participants who had discontinued therapy in focus groups (13) or had experience of adalimumab (13,14), included patients who had planned cessation of infliximab after six months (14), and interviewed small numbers (14). The need to understand the meaning of well-being and normality in RA is also indicated by qualitative studies (10,13,14), and by the OMERACT patient perspective workshop (15). Since these concepts have not been defined in detail, they are currently difficult to measure (16).
In summary, although treatment decisions may be based on core sets of key outcomes, these have been generated by professionals and there is evidence of discordant ratings between professionals and patients. In addition, modern treatment regimens may offer a wider range of outcome possibilities. Yet there has been no detailed exploration of outcomes important to patients that might contribute to treatment decisions. The aims of this study were therefore to establish the treatment outcomes important to patients since the use of anti-TNF therapy became common, and to develop a thematic framework of treatment outcomes to guide the future development of a core set of patient priority outcomes to complement existing professional core sets and standardise the use of PROs important to patients.
Patients and Methods
Patients diagnosed with RA according to ACR criteria (17) were recruited from the Bristol Royal Infirmary outpatients department for in-depth interviews. Participants were purposively sampled for a range of medication, DAS patient opinion (general health Numerical Rating Scale 0-10), disease duration, gender and age, as shown in Table 1.Recruitment initially focused on patients with experience of anti-TNF therapy, to determine whether additional important outcomes could be identified from those on other DMARDs, both in comparison to previous literature from focus groups and in-depth interviews in this study. Interview questions (Box 1) were guided by a conceptual framework developed from a literature review on treatment outcomes in RA, well-being and normality. Question six was included to explore benefit-finding in relation to understanding well-being. The interviews were recorded and transcribed verbatim. Data collection and analysis were guided by an iterative Grounded Theory approach (18), allowing data analysis of early interviews to enrich the data collection of later interviews. Data saturation was achieved when no new substantive codes were being elicited.
Table 1. Participant characteristics.
| Patient code |
Age (years) |
Gender | Disease duration* (years) |
Current medication** (discontinued) |
HAQ† | DAS global†† |
|---|---|---|---|---|---|---|
| AA | 53 | Male | 10 | Et | 0.625 | 5.1 |
| AB | 69 | Female | 9 | (In) | 1.375 | 1.3 |
| AC | 50 | Female | 17 | Et | 1.750 | 2.6 |
| AE | 69 | Female | 40 | In | 1.875 | 1.2 |
| AF | 65 | Male | 40 | In | 0.000 | 2.6 |
| AG | 42 | Female | 19 | (In, Et, Ad) | 1.500 | 0.7 |
| AH | 27 | Female | 18 | Et | 1.250 | 2.3 |
| AI | 74 | Male | 27 | In | 0.000 | 1.3 |
| AK | 54 | Male | 9 | Et | 0.000 | 1.6 |
| AL | 72 | Female | 19 | Et (In) | 1.625 | 6.3 |
| AM | 35 | Female | 4 | (In, Et, Ad) | 2.375 | 7.4 |
| AN | 48 | Female | 19 | Ad (In, Et) | 2.125 | 8.2 |
| AO | 59 | Female | 9 | In | 1.625 | 9.7 |
| AP | 60 | Female | 5 | Ad | 1.125 | 3.6 |
| AQ | 52 | Female | 15 | (Et) | 1.000 | 2.2 |
| AR | 70 | Female | 32 | Ad(Et) | 2.625 | 7.5 |
| AS | 67 | Female | 27 | In | 1.375 | 4.9 |
| AT | 65 | Female | 6 | Methotrexate | 1.250 | 1.2 |
| AU | 70 | Female | 6 | Methotrexate | 0.000 | 2.4 |
| AV | 74 | Female | 39 | Sulphasalazine | 1.875 | 4.8 |
| AX | 79 | Male | 31 | Sulphasalazine | 0.000 | 1.3 |
| AY | 52 | Female | 3 | Methotrexate | 0.875 | 3.3 |
| AZ | 53 | Female | 3 | Sulphasalazine | 2.500 | 3.5 |
Onset of symptoms was reported as up to 4 years before diagnosis in some cases
Et=Etanercept; In=Infliximab; Ad=Adalimumab (anti-TNF therapy drugs)
HAQ score 0 – 3 (3 is most disabled)
DAS patient global VAS 0 – 10 cm (0 = doing very well, 10 = doing very badly)
Box 1: Interview schedule.
Tell me about your health.....
What is important in terms of your health?
How do you know when a treatment is working?
Other patients have talked about ‘returning to normal’. What does that mean to you?
What makes you feel well (or better)?
Other patients have described finding benefits from having rheumatoid arthritis. What benefits have you experienced (if any)?
The data were analysed using both a systematic approach of coding (18), managed in NVivo2, and an analytical tool called Framework to aid contextual understanding (19). Three levels of coding were used: open coding (level 1) to identify relevant data, axial coding (level 2) to group similar codes into categories through constant comparison, and theoretical coding (level 3) to develop conceptual analysis (18). The categories were then used to create the Framework, with category headings forming the themes (columns) and the context of each participant easily accessible (rows) (19). Peer and patient partner checking enhanced analytical rigour. The wording of outcomes was grounded in the interview data and the labelling of categories was decided in collaboration with two patient research partners. For example, ‘e.g. empowered’ was suggested as a clarification to the ‘feeling mentally stronger’ outcome. Ethics approval was granted by Bath Local Research Ethics Committee (LREC) (ref. 07/Q2001/30) and written consent was obtained from participants prior to interviews. A patient research partner (P.R.) was part of the study steering group.
Results
Elicitation of patients’ important treatment outcomes
Interviews with 23 patients were conducted: 13 patients on anti-TNF therapy (four on etanercept, five on infliximab and four on adalimumab), four who had discontinued anti-TNF therapy and six on other types of DMARD (Table 1).
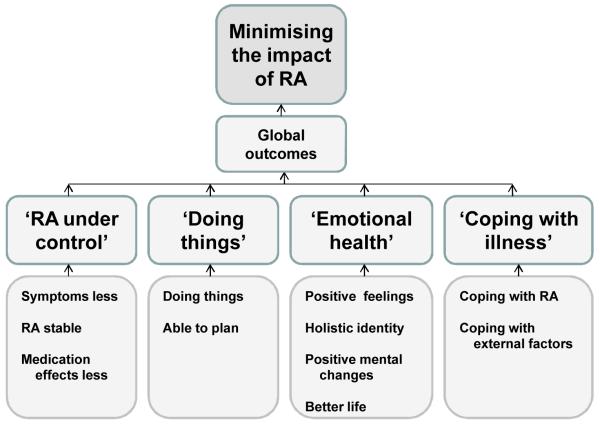
88 codes (Level 1) relating to important treatment outcomes were initially identified from three areas covered within the interviews: impact of treatment (actual treatment outcomes); patients’ important outcomes; and what patients suggested should be measured. Through constant comparison, and in collaboration with two patient research partners, very similar codes (of the 88) were merged to form 63 different codes (Table 2). For example, ‘Less tired from RA’ merged with ‘Less fatigue’. There were both specific codes, such as ‘Able to drive’, and more general ones such as ‘Able to do everyday things’. Eleven categories emerged from the data (Level 2 coding): ‘Less symptoms’, ‘RA stable’, ‘Medication effects’, ‘Doing things’, ‘Able to plan’, ‘Positive feelings’, ‘Holistic identity’, ‘Positive mental changes’, ‘Better life’, ‘Coping with RA’ and ‘Coping with health system’ (Table 2). These were grouped through constant comparison into four major categories: ‘RA under control’, ‘Doing things’, ‘Emotional health’, and ‘Coping with illness’ (Figure 1). A brief description of each major category is given below, with supporting quotations.
Table 2. Major categories, categories and codes of treatment outcome data.
| Major category | Category | Code |
|---|---|---|
| RA under control | Symptoms less | Less Pain |
| Less stiffness | ||
| Less swelling | ||
| Quicker recovery | ||
| Less fatigue | ||
| More energy | ||
| Sleeping better | ||
| Feet better | ||
| Feeling better | ||
| Visible signs less | ||
| RA stable | More ‘good’ days | |
| Less (bad) flares | ||
| More predictable disease | ||
| Avoid surgery | ||
| No more joint damage | ||
| Medication effects | Effect on fertility | |
| Less side effects | ||
| Reducing medication | ||
| Doing things | Doing things | More mobility |
| More independent (physically) | ||
| Able to drive | ||
| Can exercise | ||
| Able to work | ||
| Doing things you want to do | ||
| Doing everyday things | ||
| Better personal circumstances | ||
| Tiredness is earned | ||
| Others aware of improvement | ||
| Having physical relationship (sex) | ||
| Able to plan | Confident in planning | |
| Emotional health | Positive feelings | Better mood |
| Less depressed | ||
| Less embarrassed by appearance | ||
| Less stressed | ||
| Less frustrated | ||
| Feeling useful | ||
| Feel more settled | ||
| Close others happier | ||
| Holistic identity | Brings you out of yourself | |
| Feel younger /equivalent to peers | ||
| Maintain / Regain sense of own identity | ||
| Positive mental changes |
More confident (trying things, self-worth) | |
| Feeling mentally stronger e.g. empowered | ||
| More possibilities / choices | ||
| New interest in things / people / life | ||
| More motivation | ||
| New relationships / Socialise more | ||
| Focus not on RA | ||
| Better life | ||
| Life more manageable | ||
| New lease on life / freedom | ||
| Life back on track / give life back | ||
| Enjoy life | ||
| Coping with illness | Coping with RA | Coping (better) emotionally |
| Coping (better) physically | ||
| Feeling in control of aspects of RA | ||
| Coping with external pressures | ||
| Coping with health system |
Improved health system | |
| Improved access to treatment | ||
| Better relationship with RA Dr | ||
| Global outcomes | Feeling well | |
| Quality of life improved | ||
| Return to /maintain a normal life | ||
| Feeling (more) normal |
Figure 1. Thematic framework of patient-generated treatment outcomes.
‘RA under control’
This major category was most often described in terms of a reduction in symptoms, commonly including a reduction in the magnitude or quality of pain, swelling, and fatigue:
“The swellings in my hands have gone down. I haven’t got so much pain in the hands.” (AE-Infliximab)
“Apart from the discomfort and pain, there always seemed to be a feeling of tiredness and lethargy.” (AL-Etanercept)
“Before I had this anti-TNF drug, everything that I did was an effort, there’s no doubt about it. I say that I’m tired now at night but it’s a different sort of tiredness. It’s more of a feeling of achievement tiredness.” (AF-Infliximab)
Interviewees described the avoidance of joint damage as an important treatment outcome, which they judged by visible change, that resulted in surgery and consequent disruption:
“During all this time things were deteriorating. I could actually see joints starting to bend and swirl, and I was getting very upset about it.” (AK-Etanercept)
“I’ve got quite weak wrists because once the joints are damaged, the joints are damaged.” (AI-Infliximab)
“A lot of the surgery that I had, particularly the sorts of things I had done to my ankle, I’d have three months in plaster afterwards. So you’re completely stuck. Life just falls apart.” (AY-Methotrexate)
There were descriptions of RA being under control because effective treatment resulted in the disease being more stable or reduction of medication:
“Now I’ve not had a flare up really bad for months and months and it’s brilliant.” (AC-Etanercept)
“I am cutting down now on the steroids I was taking. I’m just down to 5 mg a day.” (AF-Infliximab)
In contrast, ineffective treatment meant experiencing systemic physical unwellness:
“You feel tired, it’s just your body feels heavy, it feels like it weighs a ton. That will normally come at the beginning of a bad flare up and you just feel awful.” (AM-Discont.all anti-TNF therapies)
“When there’s that fluey feeling, I’m not bothered with things, want to sleep, be left alone.” (AN-Adalimumab)
‘Doing things’
The outcomes in both this major category and ‘RA under control’, result from the physical impact of the condition, which patients have experienced as a result of effective medication or hope to experience when a medication is ‘working’. In this major category, the important outcomes relate to the inability to carry out everyday activities and to plan them:
“I’m able to grip things and open tops and things with the right hand, whereas previously I’d hold it somehow with my knees and do it with my left hand.” (AX-Sulphasalazine)
“We’ve been sat at the weekend planning to go out on Wednesday, which I would never have done before.” (AC-Etanercept)
However, participants also highlighted how important it was to be able to do activities that were not essential, but provided enjoyment:
“When I was in a flare and I was really suffering from fatigue, I was quite cross with the fact that I couldn’t do all the things I wanted to do.” (AB-Discont. infliximab)
“I want to be full of life, not tired, not hurting. I want to go away at weekends, to do what I want to do.” (AT-Methotrexate)
Mobility, in the sense of getting around, was another important outcome and commonly feet were the focus:
“When I was in work, I could hardly hobble round the office in flat shoes. I was in agony. And it hurt just to walk from my desk to the photocopy [sic].” (AP-Adalimumab)
“It’s a second floor flat and the stairs are really beginning to get to me.” (AB-Discont. infliximab)
“I’m using a stick because if it’s over uneven ground I find I need to have perhaps the confidence support of the stick.” (AX-Sulphasalazine)
The outcome ‘Better personal circumstances’ was often related to an improvement of other treatment outcomes within the category ‘Doing things’. For example, being able to work would improve financial circumstances, and being able to fulfil social roles would improve relationships:
“The issues that it created for me at home in terms of the family… you know… the problems that developed with my husband.” (AY-Methotrexate)
“Had I been as I was before infusions I would have been in a totally different position because she [his wife] is absolutely reliant on me. So we’d both be in a home.” (AI-Infliximab)
‘Emotional health’
This major category contained outcomes that provide the psychological signs of whether the RA is under control (the term ‘emotional’ was preferred by the patient partners as being less stigmatising than ‘mental’ or ‘psychological’). Examples of the psychological impact of ineffective treatment include:
“You can feel really down and miserable and snappy. I feel as though I’m moaning all the time.” (AO-Infliximab)
“It’s just like something’s taken over you and that you’re losing control of what you want to do.” (AG-Discont. all)
In contrast, the impact of effective treatment is illustrated in this quotation:
“I just can’t wait for each day to come along [since anti-TNF]. You know, I go to bed and I can’t wait to get up in the morning. Whereas before I didn’t want to get up.” (AE-Infliximab)
Participants often acknowledged that everyone experiences mood fluctuations and that it was important to judge the psychological impact more broadly. For example, whether it was possible to enjoy life in general and have a sense of overall well-being:
“I feel better when I’ve done something enjoyable. Feeling well is being able to enjoy life and being able to do everything.” (AM-Discont.all)
Effective treatment enabled a broader positive psychological functioning, including interest in things, people or life, opportunities for personal growth, and hope for the future:
“My sister remarked on, you know, on the fact that I sort of seemed to be much better on it [anti-TNF therapy].... because I was more interested in doing things and managing to do them as well.” (AL-Etanercept)
“I feel more confident now [on anti-TNF]. […] You just feel more positive about yourself and about life.” (AQ-Discont. etanercept)
Although outcomes such as ‘Better mood’ or ‘Focus not on arthritis’ may be indicators that disease activity has reduced, patients also wanted these to be targeted with interventions and measured as important outcomes in their own right:
“They only look at the physical aspect of that [the RA], nobody looks at the emotional side of that, or any kind of emotional effect that it has on you.” (AZ-Sulphasalazine)
“I certainly, certainly think that one of the biggest bits of this disease is the emotional part. I think it should be far more part of the core treatment for the disease and not just when someone presents with a problem.” (AH-Etanercept)
‘Coping with illness’
This major category represents patients’ individual ability to physically and mentally cope with RA, which was an outcome that was frequently raised:
“I try to do one physical thing a day, and not more than one, in the sense of gardening or housework, or walking down to the doctor’s, or down to the Post Office.” (AU-Methotrexate)
“I’ve come in here today on the bus and I didn’t think I’d be able to do that but I have. I make myself do things but I think you’ve got to do it.” (AR-Adalimumab)
The codes in this category linked to personal control or effort, rather than the codes in the ‘RA under control’ major category, which was formed from the reduction of physical symptoms of RA through medication or intervention of the multidisciplinary team. A few participants also described having to cope with the health care system into which the disease forces them:
“I should be having regular um blood tests, once a month and that, but I don’t bother now. Because there’s a lack of joined up thinking, I don’t get the results.” (AA-Etanercept)
“I was angry at times because I knew that this treatment [anti-TNF therapy] was available but I wasn’t eligible for it.” (AK-Etanercept)
Such events may undermine personal coping strategies and have a negative emotional impact.
Interrelationship of categories
Overall, the data show the interrelatedness between the different treatment outcome categories and illustrated the importance of considering the individual’s context and ability to adapt to the condition:
“It’s very dependent on your physical side and that in turn gives drive to how you feel emotionally. It’s very interlinked.” (AB-Discont. infliximab)
“At first you try to go through that barrier and that’s the worst thing you can do because the next day you are in agony. […] I’m more sensible now. Whereas years ago I would have carried on, but then you aggravate things.” (AR-Adalimumab)
The outcomes ‘Feeling well’, ‘Return to/ maintain a normal life’ and ‘Feeling (more) normal’ generated rich and complex data, and were shown to be multi-dimensional concepts that incorporated many of the more specific outcomes. These global outcomes will be described in detail elsewhere (publications submitted).
From the majority of interviewees’ descriptions, their physical symptoms (‘RA under control’) and functional abilities (‘Doing things’) most strongly influenced their perceptions of overall well-being:
“There is no way that I could be at my absolute illest … my hips and knees needing replacing, and just because I’m in love and having a nice time at home everything feels alright.” (AH-Etanercept)
“When it’s really, really bad you just can’t get away from the pain because it’s not like its one bit of me is painful, everything’s painful.” (AG-Discont.all)
However, self-management strategies and adaptation (‘Coping with RA’) appeared to modify this relationship in some cases:
“The first three years I used to do nothing, I used to go home, the pain was so bad I just used to just sit and now I manage to override the pain and I lead quite a normal life.” (AM-Discont. all)
For this reason, the core category, the prime issue emerging from the data, was ‘Minimising the personal impact of RA’.
Discussion
63 treatment outcomes were elicited from the in-depth interviews, which could be grouped into four major outcome categories: ‘RA under control’ (outcomes resulting from impact of disease activity), ‘Doing things’ (activity/participation), ‘Emotional health’ (psychological impact) and ‘Coping with illness’ (adaptation and context). These are underpinned by the core category of ‘Minimising the personal impact of RA’, which reveals that patients’ concerns focus on the consequences of disease activity, rather than disease activity per se. In addition, several outcomes were identified as highly complex global outcomes (‘Feeling well’, ‘Feeling normal’ and ‘Return to/maintain normal life’) and are reported in depth elsewhere (publications submitted).
Many of the 63 outcomes (Table 2) described by interviewees as important are not currently included in the commonly used professional core sets (ACR and DAS). Pain and swelling are captured in the ACR core set and the DAS, and OMERACT has formed a consensus that fatigue should now be measured alongside the ACR core set (20). However, other patient important outcomes raised here in the ‘RA under control’ category (disease activity) such as ‘No more joint damage’ and ‘Sleeping better’ are currently not covered in RA trials. In relation to the ‘Doing things’ category (activity/participation), disability in activities of daily living (ADL) is commonly measured by the Health Assessment Questionnaire (21) for the ACR patient’s assessment of function variable. Mobility (including driving), independence and ability to work could be captured in the AIMS2 (22), which is recommended for use by ACR. The Valued Life Activities (VLA) scale (23) includes discretionary (optional) activities as well as obligatory (essential) activities and committed (regular, necessary) ones. However, the use of AIMS2 and the VLA scale are not standard in RA clinical trials (24). In the ‘emotional health’ category (psychological impact), ‘Better mood’, ‘Less depressed’, and ‘Less stressed’ would also be measured if the AIMS2 was used. However, non-emotional impact (or signs of positive psychological functioning) would not be covered, including important outcomes such as ‘Maintain sense of own identity’, ‘New interest in things/ people/ life’ and ‘Feeling normal’. Last, in the ‘coping with illness’ category (adaptation), it appears that none of the important outcomes generated by patients are measured by the ACR recommended instruments.
Similarly, although the ICF core set for RA has comprehensive categories for body functions and structures, activities and participation, and environmental factors (3), the psychological outcomes are not well represented, despite it having later been validated with RA patients (25). The ICF categories ‘Experience of self and time functions’ and ‘Emotional functions’ do not convey all the different aspects of psychological health evident in the outcomes presented in this paper, particularly in relation to coping with the illness. The DAS patient opinion scale (disease activity or general health) (2) may capture aspects of the different categories globally depending on what is most salient for the patient at the time of completing the scale. However, a patient’s global perception of disease activity or health may be more resistant to change than when separate domains are measured, as was illustrated by Cummins in relation to QOL instruments, comparing a global QoL VAS to subscales for different domains of QoL (26).
Some treatment outcomes were generated from the interviews that had not previously been reported as important for RA patients in other qualitative studies (9-11). For example, ‘Confident in planning’ was an outcome that was described as improving many aspects of life, including socialising, family relationships, holiday and ability to work. This important outcome may reflect a reduction in disease activity, which means that disease is more stable and predictable. Other outcomes connected to psychological well-being that have not previously been identified were ‘New interest in things, people or life’ and ‘More possibilities/choices’, and were important in relation to expectations for the future. However, other outcomes were similar conceptually to those identified in other studies, but used different words to describe them. For example, ‘Coping (better) with RA’ could be linked to ‘Not feel helpless about my arthritis’ from Hewlett et al.’s study (27).
This study has strengths and weaknesses. A limitation is that all interviews were conducted with RA patients from one UK hospital, which may provide a distinctive type of care. In addition, the numbers on each type of medication, and with experience of discontinuing or switching anti-TNF therapies remain small. However, the important outcomes were common across medications and no substantive outcome codes were emerging by the final interview. Future work using a multi-centred postal survey will address the issue of generalisability of these important patient outcomes. Another limitation is that recruitment of patients from ethnic minority groups was difficult, with only one interviewee being from a non-white population. Further research is required to determine whether the outcomes and thematic framework generated here are applicable to a non-Western culture. A strength of the study is that the in-depth interviews enabled a detailed understanding of the way in which the treatment outcome categories are related and the importance of considering patients’ adaptation and contextual factors. The rich qualitative data will ensure that the subsequent set of patient priority outcomes that will be developed will be grounded in the patient perspective.
Conclusion
RA patients generated 63 treatment outcomes through in-depth interviews. Whilst some of these were in common with professional core sets and indices, there were important differences in treatment outcome priorities. All 63 outcomes will be taken forward to the next study where RA patients will prioritise the most important outcomes to improve through pharmacological intervention, to develop an RA patient core set, which will complement the existing professional core sets. Capturing the patient perspective of important changes from pharmacological therapies has the potential to enhance decision-making in clinical practice and influence the research agenda.
Acknowledgements
The authors thank the patients and staff at the BRI, and the Arthritis Research Campaign for funding the PhD studentship.
References
- 1.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary cores set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36(6):729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 2.van der Heijde D, Hof M, Piet L, Putte L. Development of a disease activity score based on judgement in clinical practice by rheumatologists. J Rheumatol. 1993;20(3):579–81. [PubMed] [Google Scholar]
- 3.Stucki G, Cieza A, Geyh S, Battistella L, Lloyd J, Symmons D, et al. ICF core sets for rheumatoid arthritis. J Rehabil Med. 2004 Jul;(Supp(44)):87–93. doi: 10.1080/16501960410015470. [DOI] [PubMed] [Google Scholar]
- 4.Kwoh CK, O’Connor GT, Regan-Smith MG, et al. Concordance between clinical and patient assessment of physical and mental health status. J Rheumatol. 1992;19:1031–7. [PubMed] [Google Scholar]
- 5.Berkanovic E, Hurwicz ML, Lachenbruch PA. Concordant and discrepant views of patients’ physical functioning. Arthritis Care Res. 1995;8:94–101. doi: 10.1002/art.1790080207. [DOI] [PubMed] [Google Scholar]
- 6.Suarez-Almazor ME, Conner-Spady B, Kendall CJ, Russel AS, Skeith K. Lack of congruence in the ratings of patients’ health status by patients and their physicians. Med Dec Making. 2001;21:113–21. doi: 10.1177/0272989X0102100204. [DOI] [PubMed] [Google Scholar]
- 7.Hewlett S. Patients and clinicians have different perspectives on outcomes in arthritis. J Rheumatol. 2003;30(4):877–9. [PubMed] [Google Scholar]
- 8.Kirwan J, Heiberg T, Hewlett S, Hughes R, Kvien T, Ahlmen M, et al. Outcomes from the patient perspective workshop at OMERACT 6. J Rheumatol. 2003 Apr 01;30(4):868–72. 2003. [PubMed] [Google Scholar]
- 9.McPherson KM, Brander P, Taylor WJ, McNaughton HK. Living with arthritis: What is important? Disabil Rehabil. 2001 Nov 10;23(16):706–21. doi: 10.1080/09638280110049919. [DOI] [PubMed] [Google Scholar]
- 10.Carr A, Hewlett S, Hughes R, Mitchell H, Ryan S, Carr M, et al. Rheumatology outcomes: The patient’s perspective. J Rheumatol. 2003 Apr 01;30(4):880–3. 2003. [PubMed] [Google Scholar]
- 11.Ahlmen M, Nordenskiold U, Archenholtz B, Thyberg I, Ronnqvist R, Linden L, et al. Rheumatology outcomes: The patient’s perspective. A multicentre focus group interview study of Swedish rheumatoid arthritis patients. Rheumatology. 2005 Jan;44(1):105–10. doi: 10.1093/rheumatology/keh412. [DOI] [PubMed] [Google Scholar]
- 12.NICE . NICE clinical guideline 79. NICE; London: 2009. Rheumatoid arthritis: The management of rheumatoid arthritis in adults. [Google Scholar]
- 13.Marshall NJ, Wilson G, Lapworth K, Kay LJ. Patients’ perceptions of treatment with anti-TNF therapy for rheumatoid arthritis: A qualitative study. Rheumatology. 2004;43:1034–8. doi: 10.1093/rheumatology/keh237. [DOI] [PubMed] [Google Scholar]
- 14.Edwards J. An exploration of patients experiences of anti-TNF therapy. Musculoskeletal Care. 2004;2(1):40–50. doi: 10.1002/msc.55. [DOI] [PubMed] [Google Scholar]
- 15.Kirwan JR, Hewlett S, Heiberg T, Hughes R, Carr M, Hehir M, et al. Incorporating the patient perspective into outcome assessment in rheumatoid arthritis - progress at OMERACT 7. J Rheumatol. 2005;32(11):2250–6. [PubMed] [Google Scholar]
- 16.Heller JE, Shadick NA. Outcomes in rheumatoid arthritis: Incorporating the patient perspective. Curr Opin Rheumatol. 2007 Mar;19(2):101–5. doi: 10.1097/BOR.0b013e32802bf79d. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, McShane DJF, Cooper NS, Healey LA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Strauss A, Corbin J. Grounded theory methodology: An overview. In: Denzin N, Lincoln Y, editors. Strategies of qualitative inquiry. Sage; London: 1998. [Google Scholar]
- 19.Ritchie J, Lewis J, editors. Qualitative research practice: A guide for social science students and researchers. SAGE; London: 2003. [Google Scholar]
- 20.Kirwan J, Minnock P, Adebajo A, Bresnihan B, Choy E, de Wit M, et al. Patient perspective: Fatigue as a recommended patient centered outcome measure in rheumatoid arthritis. J Rheumatol. 2007;34(5):1174–7. [PubMed] [Google Scholar]
- 21.Fries JF, Spitz PW, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 22.Guillermin F, Coste J, Pouchot J, Ghezail M, Bregeon C, Sany J, et al. The AIMS2-SF: A short form of the arthritis impact measurement scales 2. Arthritis Rheum. 1997;40(7):1267–74. doi: 10.1002/1529-0131(199707)40:7<1267::AID-ART11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Katz PP, Morris A, Yelin EH. Prevalence and predictors of disability in valued life activities among individuals with rheumatoid arthritis. Ann Rheum Dis. 2006 Jun;65(6):763–9. doi: 10.1136/ard.2005.044677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalyoncu U, Dougados M, Daures J, Gossec L. Reporting of patient-reported outcomes in recent trials in rheumatoid arthritis: A systematic literature review. Ann Rheum Dis. 2009;68:183–90. doi: 10.1136/ard.2007.084848. [DOI] [PubMed] [Google Scholar]
- 25.Coenen M, Stamm T, Cieza A, Amann E, Kollerits B, Stucki G. Validation of the comprehensive ICF core set for rheumatoid arthritis from the patients’ perspective using focus groups. Arthritis Res Ther. 2006;8(4):R84. doi: 10.1186/ar1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummins RA. On the train of the gold standard for subjective well-being. Social Indic Res. 1995;35:179–200. [Google Scholar]
- 27.Hewlett S, Carr M, Ryan S, Kirwan J, Richards P, Carr A, et al. Outcomes generated by patients with rheumatoid arthritis: How important are they? Musculoskeletal Care. 2005;3(3):131–42. doi: 10.1002/msc.3. [DOI] [PubMed] [Google Scholar]