Introduction
The capacity of estrogen to facilitate various learning and memory processes in females has been demonstrated in various species, including humans (see Daniel, 2006; van Haaren et al., 1990; Korol, 2004; Dohanich, 2002). Although estrogen can modulate several neurotransmitter systems centrally (see McEwen, 2002), the effects of estrogen on learning and memory are considered to be mediated largely through potentiation of cholinergic neurotransmission in the septo-hippocampal pathway and other related brain areas (e.g. Gibbs et al., 1994, 1997; Luine, 1985; Daniel and Dohanich, 2001; Gibbs and Pfaff, 1992; Singh et al., 1994). Somewhat surprisingly, the capacity of androgens to facilitate learning and memory in males has not been studied to the same extent as estradiol even though testosterone can affect spatial and working memory by altering cholinergic activity (van Haaren et al., 1990). For example, gonadectomized (GX) adult male rats had reduced levels of choline acetyltransferase (ChAT) immunoreactivity in the medial septum and hippocampus as compared to gonadally intact males, and exogenous testosterone replacement partially restored these depleted levels (Nakamura et al., 2002). Likewise, gonadectomy and the consequent removal of testosterone reduced acetylcholinesterase (AChE) activity levels in the cerebral hemispheres and preoptic suprachiasmatic area of male rats (Libertun et al., 1973; James and Kanungo, 1978), and in the medial preoptic area-anterior hypothalamus of adult male gerbils (Commins and Yahr, 1984); exogenous testosterone replacement dose-dependently restored AChE activity levels in these GX males (James and Kanungo, 1978; Commins and Yahr, 1984). In the absence of a direct effect of androgens on this enzyme, these findings pose the possibility that testosterone is increasing cholinergic function by enhancing ACh release and thereby producing a compensatory increase in AChE activity. Increased ACh release in areas of the brain such as the hippocampus and prefrontal cortex is generally associated with enhanced responding under various learning and memory tasks (e.g., Fadda et al., 2000; Stancampiano et al., 1999; Orsetti et al., 1996; McIntyre et al., 2002; Hironaka et al., 2001; Arnold et al., 2002), whereas decreased ACh release in these areas is associated with reduced responding under the same types of tasks (e.g., Leanza et al., 1996; McDonald et al., 1997; Shen et al., 1996; Vnek et al., 1996; Lehmann et al., 2002). For instance, low synaptic levels of ACh due to decreased cholinergic innervation in the hippocampal formation and cortex has been hypothesized to be the source of the cognitive decline associated with Alzheimer's disease (for review see Kasa et al., 1997), a finding that is also the basis of the ‘cholinergic hypothesis’ (Bartus et al., 1982; Bartus et al., 1985).
Understanding the interaction between testosterone and the cholinergic system as it relates to learning and memory in males has also been complicated by findings from this laboratory and others indicating that testosterone replacement in gonadectomized males can either improve (e.g., Frye and Seliga, 2001; Kritzer et al., 2001; Aubele et al., 2008) or impair (Leonard et al., 2007; Gibbs and Johnson, 2008) responding on learning and memory tasks, depending upon the particular type of task and the associated stimuli. In one study from this laboratory, for example, Daniel et al. (2003) found that gonadectomy in male rats enhanced the error-increasing effects, but not the rate-decreasing effects, of scopolamine and mecamylamine on working memory, compared to gonadally intact rats, as measured in an eight-arm radial maze. This study, which clearly involved explicit memory of spatial orientation and spatial stimuli, suggested that the presence of testosterone tonically increases cholinergic function, as its loss through gonadectomy potentiated the disruptions of two cholinergic antagonists. However, these data directly contrast with the interactive effects of testosterone and scopolamine obtained under a non-spatial operant learning procedure involving a repeated-acquisition technique (Leonard et al., 2007), which suggested that testosterone tonically decreases cholinergic function. In this study involving a non-spatial task where male rats learned different response sequences each session, gonadectomy attenuated the disruptive effects of scopolamine on both response rate and the percentage of errors when compared to the effects in gonadally intact males and gonadectomized males with testosterone replacement (GX + T males).
The present study was conducted to help clarify these issues by examining the interaction between testosterone and a cholinesterase inhibitor (i.e., donepezil), in male rats responding on a non-spatial operant task. Another purpose of the present study was to determine if gonadectomy in male rats can reduce AChE activity levels in areas of the brain that mediate learning and memory processes, such as the hippocampus and striatum. Donepezil, a centrally-acting, second-generation AChE inhibitor frequently prescribed for humans with mild to moderate dementia associated with Alzheimer's disease (Shigeta and Homma, 2001; Sugimoto, 2001), enhances cholinergic function by blocking the synaptic degradation of ACh through inhibition of AChE. Because synaptic levels of ACh are increased by donepezil in many areas of the brain including the hippocampus (Rogers et al., 2009; Kawashima et al., 1994; Wilkinson et al., 2004), the effects of donepezil should be enhanced in an intact male compared to a gonadectomized male if testosterone increases cholinergic function, or attenuated in an intact male compared to a gonadectomized male if testosterone decreases cholinergic function. This particular drug interaction may also have important ramifications for elderly males with reduced testosterone levels that have been prescribed donepezil. The interactive effects of donepezil and estradiol were also investigated in males because testosterone can mediate learning and memory processes directly through the androgen receptor or indirectly through the estrogen receptor via aromatization to estradiol (e.g., Kritzer et al., 2007; Gibbs, 2005; Luine and Rodriguez, 1994; Leonard et al., 2008).
The non-spatial baseline of operant behavior was a multiple-schedule procedure that has been used previously to examine drug-hormone interactions (Leonard et al., 2007, 2008; Daniel et al., 2002), and one that was comprised of both repeated-acquisition and performance components (Moerschbaecher et al., 1979). During the repeated-acquisition components, animals acquired a different predetermined sequence of responses (of fixed length) each session. During the performance components, animals were reinforced for responding on an invariant sequence of responses, and responding in this component served as a behavioral control for the nonspecific effects of either drug or hormone administration. Both responding to acquire a sequence and responding on the invariant sequence were maintained by food presentation. The advantage of using this type of multiple schedule for testing the effects of drugs and hormones on learning was that learning was demonstrated each session by the within-session pattern of responding in which fewer incorrect responses and an increasing number of consecutive correct responses were made as the session progressed. Across sessions, this “learning” curve was repeatedly demonstrated as the predetermined sequence changed each session.
Methods
Subjects
Twenty-seven Long-Evans hooded rats (Harlan, Indianapolis, IN) comprised the behavioral portion of the study, and of these, 12 were used for assaying AChE activity. All rats were housed singly in a colony room maintained at 21±2°C with 50±10% relative humidity on a 14-h light/10-h dark cycle (lights on at 0600 h CST). Each rat earned 45-mg food pellets (TestDiets, Richmond, IN) during the experimental sessions, and when necessary, were provided with standard rodent chow (Rodent Diet 5001, PMI Inc., Brentwood, MO) in the home cage after the test sessions in order to maintain them at 90% of their free-feeding weight. Water was freely available in their home cage. This study was carried out in accordance with the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center, and in compliance with the recommendations of the National Research Council in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Apparatus
Twelve identical modular test chambers (Coulbourn Instruments, Allentown, PA, Model E10-10TC) configured specifically for rodents were used. Located on the front wall of each chamber were a houselight, speaker, auditory feedback relay, pellet trough (5.5 cm above the floor and centered), and three response keys aligned horizontally (8 cm apart, center to center, and 14.5 cm above the floor). Each response key could be transilluminated by three Sylvania 28ESB indicator lamps, one with a red plastic cap, one with a yellow cap, and one with a green cap. Response keys required a minimum force of 0.15 N for activation and correct responses produced an audible click of the feedback relay. Each chamber was enclosed within a sound-attenuating cubicle equipped with a fan for ventilation and white noise to mask extraneous sounds. All test chambers were connected to a computer programmed in MED-PC for Windows, Version IV (MED Associates, Inc., St. Albans, VT), and to cumulative recorders (Gerbrands, Arlington, MA) located within the same room.
Behavioral procedure
Preliminary training for the repeated-acquisition task has been described previously (Winsauer et al., 1995) and included magazine training, shaping of the response (nose press), and reinforcing responses on individually illuminated keys after shaping. To train repeated acquisition, all three response keys were illuminated with yellow light, but only one of the three response keys was correct for a particular session and each response emitted on that key resulted in the delivery of a food pellet. Responding on either of the other two illuminated keys was considered an incorrect response or error and resulted in a 5-s timeout during which the key lights were extinguished and responding had no programmed consequence. For each additional session during this stage of training, the position for the correct response was varied in a mixed order across sessions. After rats reliably acquired this task, regardless of the key position, a second response was added to create a sequence or chain of responding such that two correct responses were necessary to obtain a food pellet. This type of sequential responding is procedurally defined as a “chain” because each response except the last produces a discriminative stimulus controlling the response that follows (Kelleher, 1966). The key positions for the correct responses varied both within the two-response sequence and across sessions; the color of the key lights changed after each correct response. A third response was added to the sequence or response chain when stable responding was obtained under the two-response sequence. Training continued until response rates and the percentage of errors did not vary by more than ± 20% of the mean for 10 consecutive sessions. A second component was then added to the schedule so that the animals responded under a multiple schedule of repeated acquisition and performance of response chains.
During the acquisition components, the three response keys were illuminated at the same time with one of three colors: red, yellow, or green. As described above, responding on the correct key in the presence of one color changed the color of the key lights as well as the position for the next correct response (e.g., keys green, center correct; keys red, left correct; keys yellow, right correct). When the response sequence was completed by emitting three correct responses (i.e., one correct response in the presence of each color), the key lights were extinguished and the stimulus light in the pellet trough was illuminated for 0.4 s. Subsequently, the response keys were illuminated with the first stimulus (i.e., green) and the sequence was reset. Within a given session, the correct response that was associated with a particular color did not change, and the same sequence (in this case, center–left–right or C–L–R) was repeated during all acquisition components of a given session. Responding on this sequence was maintained by food presentation under a second-order fixed-ratio (FR) 3 schedule, such that every three completions of the sequence resulted in the presentation of a 45-mg food pellet. When rats responded on an incorrect key (in the example, the left or right key when the green lights were illuminated), the error was followed by a 5-s timeout. An incorrect response did not reset the three response sequence (i.e., the stimuli and the position of the correct response were the same before and after a timeout).
To establish a steady state of repeated acquisition, the sequence was changed from session to session. An example of sequences for five consecutive sessions was: C–L–R, L–R–C, C–R–L, R–L–C, and L–C–R. The sequences were carefully selected to be equivalent in several ways, and there were restrictions on their ordering across sessions. Briefly, each sequence was scheduled with equal frequency and consecutive correct responses within a sequence were scheduled on different keys. Occasionally, a correct sequence position for a given color was the same for two consecutive sessions (as in the list of sequences above, L–R–C and C–R–L).
During performance components, the houselight and the response keys were illuminated. The houselight served as a discriminative stimulus for responding during this component, and unlike the acquisition component, the sequence in this component remained the same from session to session (i.e., R-C-L). In all other aspects (colored stimuli for each response in the sequence, second-order FR 3 schedule of food presentation, 5-s timeout, etc.), the performance components were identical to the acquisition components. Experimental sessions always began with an acquisition component, which then alternated with a performance component after 40 reinforcers or 20 min, whichever occurred first. Each session terminated after 200 reinforcers or 80 min, whichever occurred first. Throughout testing, sessions were generally conducted 6 days per week, Monday through Saturday.
When training was complete and responding stabilized under the multiple schedule, 19 of the 27 rats were gonadectomized and assigned to one of three hormone-treated groups: gonadectomized with no hormone replacement (GX), gonadectomized with testosterone replacement (GX + T), and gonadectomized with estradiol replacement (GX + E). The remaining 8 rats did not undergo hormonal manipulation and thus comprised the gonadally intact group (Intact). Gonadectomy involved removing the testes through small bilateral incisions in the scrotum while the animals were under isoflurane anesthesia. Concurrent with gonadectomy, 6 of the 19 rats were implanted subcutaneously in the interscapular region of the neck with an empty 36-mm Silastic® capsule (GX; 1.57 mm i.d. and 3.18 mm o.d.; Dow Corning, Midland, MI), 6 rats were implanted with a 36-mm Silastic® capsule containing 30 mm active length of testosterone propionate (GX + T; 4-androsten-17β-ol-3-one propionate; Steraloids, Inc., Newport, RI), and 7 rats were implanted with a 10-mm Silastic® capsule (1.47 mm i.d. and 1.95 mm o.d.) containing 5 mm active length of 17β-estradiol (GX + E; 1,3,5[10]-estratrien-3,17β-diol; Steraloids). The ends (2-3 mm) of each capsule were sealed with silicone rubber cement, and incubated separately in sterile 0.9% saline for 24 hours prior to implantation. Capsules of these dimensions have been shown to maintain physiological levels of circulating testosterone (Butler et al., 2001; Woodson & Balleine, 2002) and estradiol (Pinilla et al., 1999) in male rats. In order to allow for the clearance of residual hormones and the reinstatement of circulating hormone titers, a total of ten days elapsed (i.e., 3 days in which the animals were allowed to recover from the surgery and 7 days in which they were tested behaviorally, but data were not included in the statistical analysis) before determination of the donepezil dose-effect curve (0.56–5.6 mg/kg). To maintain consistent hormone levels, hormone capsules were replaced with a new, identical capsule every 30 days.
Doses of donepezil hydrochloride (Sequoia Research Products, Pangbourne, UK) were dissolved in 0.9% saline and administered intraperitoneally (i.p.) in a mixed order on Tuesdays and Fridays whenever dose-effects curves were established. Saline (control) injections were administered on Thursdays, and baseline sessions (no injections) occurred on the remaining days. Higher doses of donepezil were administered only once a week. The volume of injection for both drug and saline was 0.1 ml/100 g of body weight, and all injections were administered 15 min prior to the start of the testing session.
Hormone Assays
Blood samples for evaluation of hormone titers were collected by saphenous venepuncture from a total of 24 rats (this study and Leonard et al., 2008). Serum was isolated and stored at −80°C until analyzed via enzymatic immunoassay (EIA) using testosterone (EIA-1559) and estradiol (EIA-4399) assay kits from DRG International, Inc. (Mountainside, NJ, USA). The analytical sensitivity of the ELISA assay kits is reported by the manufacturer to be 0.083 ng/ml for testosterone and < 1.4 pg/ml for estradiol. Also, as reported by the manufacturer, the intra- and interassay coefficients of variation (CV) for testosterone are 3.3-4.2% and 4.7-9.9% respectively, and the intra- and interassay CV for estradiol are reported to be 3.9-11.4 % and 5.5-7.9 % respectively.
Acetylcholinesterase Activity Assay
Six brain regions from 6 of the gonadally intact and 6 of the GX males were used to assess acetylcholinesterase (AChE) activity. The specific regions were the hippocampus, striatum, prefrontal cortex, midbrain, cerebral cortex, and hypothalamus. The former three areas were chosen because they are integral to learning and memory (e.g.,McDonald and White, 1993; White and McDonald, 2002; Packard and McGaugh, 1996), whereas the latter three served as controls. Immediately following decapitation, brains were rapidly removed and placed in cold saline for 10 minutes. Dissections were performed on an ice-cooled glass plate according to the methods of Glowinski & Iversen (1966). After separation, each region was immediately flash-frozen in liquid nitrogen and stored at -80° C. Prior to analyses, each brain region was weighed to the nearest 0.1 mg and homogenized individually in reaction buffer (Cell Technology, Inc. Mountain View, CA) in the ratio of 7 μl buffer:1 mg tissue. The homogenization buffer contained the protease inhibitors leupetin, benzamidine and phenylmethylsulfonyl fluoride (Sigma) at final concentrations of 1 μg/ml, 3 mM, and 1 mM respectively. Tissue was homogenized in a Dounce homogenizer, the homogenate was clarified by centrifugation at 20,000 × g for 15 min. at 4°C, and the supernatant was used to measure AChE activity. Since AChE activity varied greatly between brain regions, and did not change linearly with supernatant dilution (data not shown), an appropriate amount of supernatant protein providing an activity measurement within the linear range of the assay was chosen for each brain region. Subsequent experiments used identical amounts of supernatant protein to enable comparison of the same brain region between experiments. Protein concentration was measured with the Pierce BCA Protein Assay Kit (ThermoScientific, Rockford, IL) using bovine serum albumin as a standard. Acetycholinesterase activity was then assessed with a commercially available fluorescence assay kit (Fluoro AChE 100-2; Cell Technology) in accordance with the manufacturer's protocol. Acetylcholine hydrochloride (Sigma) was used as a substrate for AChE in a series of coupled enzyme reactions. After incubation in the dark at room temperature for 10 minutes, fluorescence generated by these reactions was measured at Ex: 530nm Em: 590 nm using a fluorescent 96-well microplate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA). After duplicate determinations were made, mean AChE activity (mU/ml) was calculated for each brain area and expressed as a percent control of the gonadally intact group. Background signal of the quenched detection reagent in each sample was subtracted prior to statistical analysis.
Data Analysis
The data collected for both components of the multiple schedule were analyzed in terms of: (1) the overall response rate (total responses/min, excluding timeouts) and (2) the overall accuracy, expressed as the percentage of errors [(incorrect responses/incorrect + correct responses) × 100]. However, when the response rate was less than 5 responses/min, data were excluded from the analyses for percent errors because of the small number of responses involved. The mean data for each subject were grouped and analyzed statistically for an effect of donepezil on responding in each component with a two-way analysis of variance (ANOVA; SigmaStat Statistical Software, SYSTAT Software, Inc. Point Richmond, CA), with donepezil dose and hormone status as main factors. Holm-Sidak post-hoc tests were used to compare drug sessions with control sessions where appropriate. In addition to these measures based on session totals, within-session changes in responding were monitored by the cumulative recorder and computer. For example, acquisition of a response sequence was indicated by within-session error reduction; that is, a decrease in the number of errors between food presentations as the session progressed.
With respect to the biochemical data, mean AChE activity data were analyzed with a two-way repeated-measures ANOVA (hormonal condition × brain region). Following the two-way ANOVA, differences in AChE activity between the two groups of males (intact and GX) for each brain region were determined with paired t-tests. Significance was accepted at α level ≤ 0.05 for all statistical tests. Differences in the sensitivity of each of the four groups to the effects of donepezil were also quantified by comparing the ED50 values of the dose-effect curves for both response rate and accuracy. These ED50 values were determined by linear regression using two or more data points reflecting the downward slopes of the descending curve for response rate or the upward slopes of the ascending curve for the percentage of errors.
Results
Stable responding was evident for all animals in both components of the multiple schedule during the baseline and control (saline) sessions that preceded and followed administration of donepezil. In addition, the daily pattern of responding of each subject during the acquisition components was characterized by a steady state in terms of within-session error reduction, which was indicated by a distinct decrease in the number of errors and a concomitant increase in consecutive correct completions of the response sequence. Responding of each subject during the performance component under baseline and control conditions was also characterized by consistent overall response rates and percentages of error from component to component and session to session.
Gonadectomy reduced testosterone titers to approximately 19% of the titers for intact males, and estradiol titers to approximately 35% of intact male titers (Table 1). Behaviorally, response rates and error levels under control conditions were comparable between GX and intact males as shown in Figure 1. However, the disruptive effects of donepezil on response rate differed between these two groups as indicated by a significant interaction of dose and hormone status in both the acquisition (F5,83 = 3.07, p < 0.02) and performance (F5,83 = 2.46, p < 0.05) components. Further analysis of the interaction indicated that donepezil produced rate-decreasing effects in both groups of males in the acquisition [intact: (F5,47 = 4.26, p < 0.005); GX: (F5,35 = 20.23, p < 0.001)] and performance [intact: (F5,47 = 3.34, p < 0.02); GX: (F5,35 = 13.54, p < 0.001)] components, and that the GX males were more sensitive to the rate-decreasing effects in the acquisition component after 3.2 mg/kg donepezil (F1,13 = 12.21, p < 0.005) and after 3.2 mg/kg and 5.6 mg/kg donepezil (F1,13 = 9.10, p < 0.02 and F1,13 = 6.30, p < 0.05, respectively) in the performance component compared to the intact males. This difference in sensitivity between GX and intact males was also evident in the ED50values for both groups; that is, the ED50 value for response rate in the acquisition and performance components was 2.9 and 3 mg/kg for GX males, respectively, and 4.2 mg/kg in both components for intact males.
Table 1.
Serum titers of testosterone and estradiol from gonadally intact, gonadectomized, testosterone-replaced, and estradiol-replaced male rats.
| Group | T (ng/ml) mean ± SEM |
n | Estradiol (pg/ml) mean ± SEM |
n |
|---|---|---|---|---|
| Intact | 1.249 ± 0.196 | 8 | 4.79 ± 1.54 | 10 |
| GX | 0.232 ± 0.019 | 3 | 1.69 ± 0.25 | 9 |
| GX + T | 20.297 ± 0.649 | 6 | ----- | |
| GX +E | ----- | 137.74 ± 13.57 | 5 |
Serum titers expressed as mean ± standard error of the mean, T = testosterone; Intact = gonadally intact, GX = gonadectomized, GX + T = testosterone-replaced, and GX + E = estradiol-replaced.
Fig. 1.
Effects of donepezil in gonadally intact and gonadectomized male rats responding under a multiple schedule of repeated acquisition and performance of response sequences. Circles represent intact males (Intact) and squares represent males after gonadectomy (GX). The mean data from the acquisition components are represented by the unfilled symbols, whereas the mean data from the performance components are represented by the filled symbols. Error bars represent ± standard error of the mean (S.E.M). Any points without vertical lines indicate instances in which the S.E.M. is encompassed by the data point. Numbers in parentheses adjacent to the data points indicate instances when the number of rats were less than eight (Intact) or nine (GX). Asterisks indicate doses of donepezil that were significantly different from control (S = saline) and pound signs indicate significant differences between hormonally manipulated groups (α ≤ 0.05).
The disruptive effects of donepezil on accuracy in GX and intact males were similar to those on response rate, in that there was a significant interaction of factors (dose × hormone status) in the acquisition (F5,74 = 6.24, p < 0.001) and performance (F5,76 = 2.90, p < 0.02; Figure 1, lower panels) components, and subsequent analyses indicated an overall difference in sensitivity. For example, even though error-increasing effects occurred in GX and intact males in the acquisition [intact: (F5,45 = 6.42, p < 0.001); GX: (F4,27 = 12. 50, p < 0.001)] and performance [intact: (F5,46 = 7.25, p < 0.001); GX: (F4,28 = 5.87, p < 0.002)] components, GX males were more sensitive to the error-increasing effects of donepezil than intact males. This was particularly evident at 3.2 mg/kg of donepezil, where one-way ANOVA tests indicated that the percentage of errors for both the acquisition (F1,11 = 11.83, p < 0.001) and performance (F1,12 = 22.47, p < 0.001) components was higher for GX males than for intact males. Interestingly, the ED50values of the dose-effect curves were also more than 2-fold lower for GX males than for intact males (2.3 and 2.5 mg/kg in the acquisition and performance components, respectively, for GX males, and 5.6 mg/kg in both components for intact males).
As shown in Figure 2, GX + T and intact males responded in a similar manner after control (saline) injections and after donepezil administration, even though exogenous testosterone replacement increased hormone titers to levels greater than those for intact males (i.e. supraphysiological levels; cf. Table 1). The similarity in responding of GX + T and intact males was also indicated by a two-way ANOVA, which revealed only a significant main effect of dose (acquisition rate: F5,83 = 9.94, p < 0.001; acquisition error: F5,78 = 9.18, p < 0.001; performance rate: F5,83 = 9.16, p < 0.001; performance error F5,79 = 10.09, p < 0.001). Specifically, 3.2 and 5.6 mg/kg of donepezil disrupted response rate in the acquisition component (p < 0.05), and 5.6 mg/kg of donepezil disrupted response rate in the performance component (p < 0.05), for both GX + T and intact males. With regard to the percentage of errors, only 5.6 mg/kg of donepezil significantly increased percent errors in the acquisition component compared to saline (p < 0.05), whereas both 3.2 and 5.6 mg/kg of donepezil increased percent errors in the performance component (p < 0.05). Although the ED50 values were not identical, there was less than a 2-fold difference in the ED50values of the GX + T group compared to those of the intact group.
Fig. 2.
Effects of donepezil on responding under a multiple schedule of repeated acquisition and performance of response sequences in male rats that were either gondally intact or gonadectomized with testosterone replacement. Circles represent intact males (Intact) and triangles represent gonadectomized males after testosterone replacement (GX + T). The mean data from the acquisition components are represented by the unfilled symbols, whereas the mean data from the performance components are represented by the filled symbols. For additional details, see legend for Figure 1.
While exogenous estradiol replacement restored titers in GX males to supraphysiological levels (cf. Table 1), GX + E males were more sensitive to the rate-decreasing effects of donepezil than intact males (Figure 3, upper panels). This was indicated by significant main effects for dose in each component (acquisition: F(5,89) = 9.73, p<0.001; performance: F(5,89) = 8.69, p<0.001) and for hormone in each component (acquisition: (F(1,89) = 5.68, p<0.02; performance: F(1,89) = 6.39, p<0.02). This difference in sensitivity was also indicated by a leftward shift in the dose-effect curves for response rate in the GX + E males relative to the curves obtained in the intact males, and by the lowest ED50values for response rate among all of the groups (cf., Table 2).
Fig. 3.
Effects of donepezil on responding under a multiple schedule of repeated acquisition and performance of response sequences in male rats that were either gonadally intact or gonadectomized with estradiol replacement. Circles represent intact males (Intact) and diamonds represent gonadectomized males after estradiol replacement (GX + E). The mean data from the acquisition components are represented by the unfilled symbols, whereas the mean data from the performance components are represented by the filled symbols. For additional details, see legend for Figure 1.
Table 2.
Effective dose for decreasing response rate by 50% or increasing the percentage of errors by 50% in gonadally intact, gonadectomized, testosterone-replaced, and estradiol-replaced male rats responding under a behavioral procedure with both acquisition and performance components (i.e., ED50s).
| ED50 (mg/kg) | Acquisition Component | Performance Component | ||
|---|---|---|---|---|
| Group | Response Rate | Percent Error | Response Rate | Percent Error |
| Intact | 4.2 | 5.6 | 4.2 | 5.6 |
| GX | 2.9 | 2.3 | 3.0 | 2.5 |
| GX + T | 3.1 | 5.1 | 3.3 | 4.8 |
| GX +E | 2.4 | 1.8 | 2.4 | 1.8 |
Intact = gonadally intact, GX = gonadectomized, GX + T = testosterone-replaced, and GX + E = estradiol-replaced.
Figure 3 shows that GX + E males were also more sensitive to the error-increasing effects of donepezil than intact males (lower panels) as indicated by significant main effects for dose and hormone in the acquisition component (F5,82 = 5.82, p < 0.001 and F1,12 = 21.75, p < 0.001, respectively), and the significant interaction of these two factors in the performance component (F 5,83 = 2.44, p =< 0.05). More specifically, in the acquisition components, GX + E males had higher percentages of error under control conditions (data above S), and higher error levels after each of the doses of donepezil compared to intact males. Administration of the two highest doses of donepezil (3.2 and 5.6 mg/kg) significantly increased the percentages of error compared to saline administration (p < 0.05) in both GX + E and intact males. In the performance component, error levels under control conditions were not significantly different, but the error-increasing effects at 0.56, 1, 1.8 and 3.2 mg/kg of donepezil were higher in GX + E males than in intact males. Compared to control injections, both 3.2 and 5.6 mg/kg of donepezil produced a significant increase in the frequency of errors in GX + E males (p < 0.05), whereas only the 5.6 mg/kg dose significantly increased percent errors in intact males (p < 0.05). The overall difference in sensitivity was also reflected in the ED50values, which were 3-fold lower for the GX + E males than the intact males.
The similarity of the effects of donepezil in GX and GX + E males is shown in Figure 4, and is reflected in the significant main effect of dose in both components (acquisition: F(5,78) = 25.39, p < 0.001; performance: F(5,78) = 21.70, p < 0.001; Figure 4, upper panels) and lack of significance for hormone or for the interaction of hormone and dose. Both the 3.2- and 5.6-mg/kg doses of donepezil in GX and GX + E males significantly decreased response rate compared to controls in the acquisition and performance components (p < 0.05). As shown in Table 2, the ED50 values for the dose-effect curves were also comparable, indicating the similarity of these two manipulations on the effects of donepezil.
Fig. 4.
Effects of donepezil on responding under a multiple schedule of repeated acquisition and performance of response sequences in male rats that were either gonadectomized or gonatectomized with estradiol replacement. Squares represent males after gonadectomy (GX) and diamonds represent gonadectomized males after estradiol replacement (GX + E). The mean data from the acquisition components are represented by the unfilled symbols, whereas the mean data from the performance components are represented by the filled symbols. For additional details, see legend for Figure 1.
One of the few significant differences between GX + E and GX males occurred in the acquisition component,s where GX + E males were more sensitive to the error-increasing effects of donepezil than GX males. This was revealed by a two-way ANOVA indicating significant main effects for dose (F 5,66 = 7.36, p < 0.001) and for hormone (F 1,61 = 4.27, p < 0.05). In the performance component, the effects of donepezil on percent errors were only dependent on dose in the performance component (F5,67 = 5.68, p < 0.001). Percent errors in both the acquisition and performance components were significantly higher than in control (p < 0.05) after administration of the 3.2 mg/kg dose of donepezil; too few animals responded after 5.6 mg/kg to allow for comparisons with responding after control injections.
The cumulative records in Figure 5 show the within-session pattern of effects of donepezil on responding under the multiple schedule. The hormonal status of the three representative male rats depicted was intact, GX, and GX + E. When saline was administered to each of these animals (first through third rows), the pattern of responding was similar; however, compared to responding for the intact male, the GX + E male had somewhat slower response rates in each component as indicated by the longer record, and a higher rate of errors, particularly during the first acquisition component. In general, the pattern of responding for each animal was reflected in that animal's daily, non-injection or baseline response pattern (not shown).
Fig. 5.
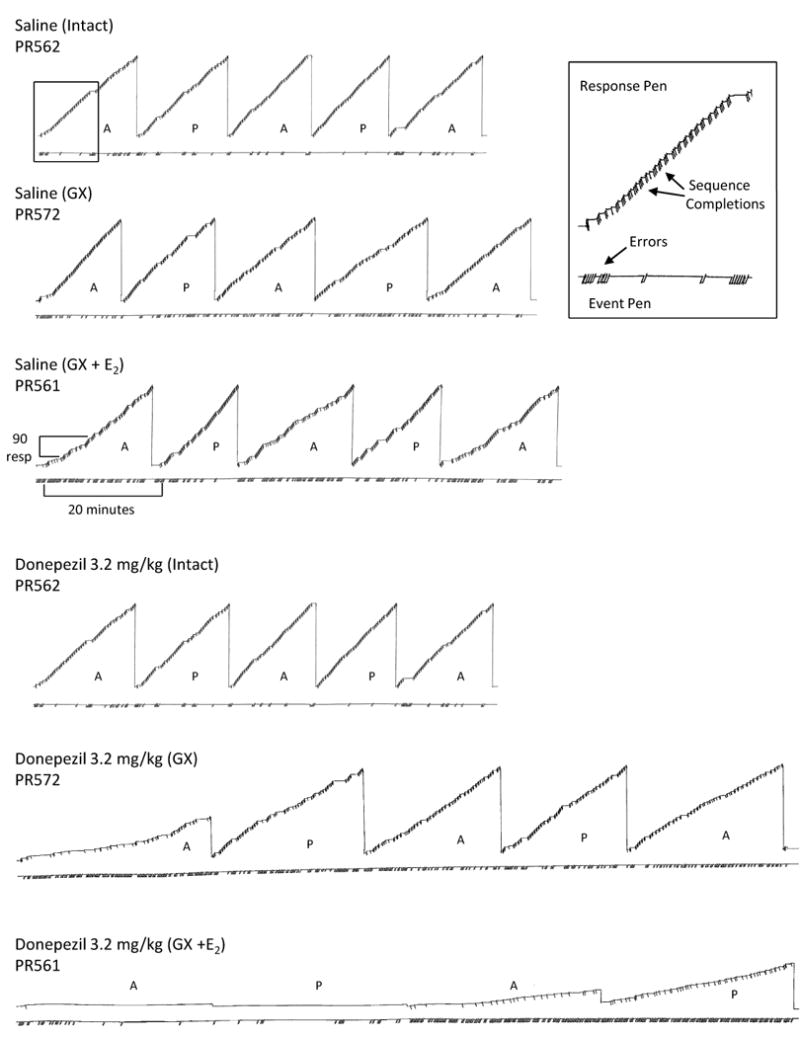
Representative cumulative records for three individual animals (PR561, PR562, and PR572) demonstrating the within-session effects produced by saline (rows one through three) and 3.2 mg/kg of donepezil (rows four through six) on responding in the acquisition (A) and performance (P) components of a multiple schedule when rats were either gonadally intact (Intact), gonadectomized (GX), or gonadectomized with estradiol replacement (GX + E). In each record (see inset), the response pen stepped upward with each correct response and was deflected downward each time the three-response sequence was completed. A food pellet was delivered after every three completions of the sequence. The response pen reset at the completion of each component. Downward deflections of the event pen (below each record) indicate incorrect responses in both components and the pen was deflected for the duration of the 5-sec timeout. Each session began with an acquisition component, which alternated with a performance component after 40 reinforcers or 20 min, whichever occurred first. Each session terminated after 200 reinforcers or 80 min, whichever occurred first.
The cumulative record in the fourth row shows that the within-session pattern of responding after 3.2 mg/kg of donepezil was similar to that obtained after saline administration in the intact male. Briefly, acquisition of the response sequence occurred in the initial acquisition component shortly after the start of the session and responding in the subsequent acquisition components was comparable to that obtained in the performance components. Unlike the effects of 3.2 mg/kg in the intact male, this dose in the GX male (row 5) markedly decreased response rate and increased errors in both the acquisition and performance components when compared with saline administration. The final record (row 6) illustrates the effects of 3.2 mg/kg of donepezil in a GX + E male. Compared to saline administration, this particular dose markedly decreased response rates and increased errors in the acquisition and performance components. Response rates were largely decreased by an increase in the amount of pausing, particularly in the first two components where the subject completed only one response sequence and each component ended after 20 minutes rather than after 40 reinforcements as in the saline sessions. When responding recovered, the error rate was also markedly increased and there was little evidence of sequence acquisition.
Figure 6 depicts the differences in AChE activity levels expressed as a percent control of the gonadally intact group. Due to a significant interaction of hormone status and brain region (F5,71 = 4.24, p < 0.005), paired t-tests were used to determine differences in AChE activity for each corresponding brain. Gonadectomized males had higher levels of AChE activity in the striatum and hippocampus than intact males (p < 0.05). In contrast, GX males were found to have lower levels of AChE activity in the midbrain than intact males (p < 0.05). For the remaining three regions (prefrontal cortex, hypothalamus, and cortex), AChE activity did not differ significantly between GX and intact males.
Figure 6.
Acetylcholinesterase (AChE) activity levels for six brain regions. Bars represent AChE activity levels (mean + S.E.M.) of gonadectomized males (GX; n=6) expressed as a percent control of the gonadally intact group. The asterisks reflect significant differences in AChE activity levels between the intact and GX males for that particular brain area (α ≤ 0.05). PFC=prefrontal cortex; STR=striatum; HIP=hippocampus; CTX=cortex; HYPO=hypothalamus; MID=midbrain.
Discussion
Previous studies involving donepezil largely examined the behavioral effects of low doses to determine its facilitative effects on learning and memory. Many of these studies involved groups of rodents that were compromised in some manner because donepezil is putatively regarded to have nootropic effects. More specifically, in these studies that frequently involved aged, pharmacologically-impaired or lesioned rodents, low doses of donepezil generally enhanced memory and improved responding on spatial and working memory tasks, while larger doses were ineffective or produced impairments (van der Staay and Bouger, 2005; Wise et al., 2007); for review see Yuede et al., 2007; Yoo et al., 2007). In contrast, only a small number of studies have investigated the effects of donepezil on uncompromised rodents, although many of the reported effects of donepezil were similar. For instance, chronic administration of donepezil (0.5 mg/kg then 0.2 mg/kg) in uncompromised male rats was shown to enhance acquisition and performance on several spatial and nonspatial memory and attention tasks (Cutuli et al., 2008). Doses of 0.3 and 1 mg/kg of donepezil were also reported to decrease the number of errors emitted in a two-phase radial-arm maze procedure compared to control in male rats (Wise et al., 2007). However, similar doses of donepezil (0.03-1 mg/kg) did not have an effect on uncompromised male rats responding on the delayed matching-to-position (DMTP) task, a nonspatial, operant task (Poorheidari et al., 1998). Thus, while donepezil can improve responding in both compromised and uncompromised animals, its effects appear to be somewhat task specific and it does not uniformly improve all learning and memory tasks (e.g., spatial versus operant).
Unlike many of the previous studies involving donepezil, the present study examined the effects of comparatively high doses of this drug to gauge the interactive effects of the cholinergic system with gonadal hormones. In this study involving intact and hormonally-manipulated male rats, low doses of donepezil had little or no effect compared with the respective control injections for each group of males. Although the absence of effects at low doses could have been due to a floor effect, as animals were trained to achieve low levels of errors, these data do complement and extend previous findings with other cholinesterase inhibitors such as physostigmine (Howard and Pollard, 1983) in this behavioral paradigm. As the doses of donepezil administered increased, dose-dependent rate-decreasing and error-increasing effects occurred in both the acquisition and performance components of the multiple schedule. Similarly, disruptive effects on responding in repeated-acquisition procedures have been reported for other cholinesterase inhibitors in both rats (Howard and Pollard, 1983) and monkeys (Anger and Setzer, 1979; Frederick et al., 1995), and this would seem to indicate that there is a threshold above which an increase in ACh is disruptive to behavior.
While there is considerable evidence indicating that gonadal hormones can mediate learning and memory through the cholinergic system (e.g. Gibbs et al., 1997; Luine, 1985; Daniel and Dohanich, 2001; Gibbs and Pfaff, 1992; Singh et al., 1994), the interactive effects of donepezil and the gonadal hormones on learning and memory have only recently received attention (Luine et al., 2002; Gibbs et al., 2009; Buccafusco et al., 2003). For example, two studies utilizing female rats investigated whether donepezil could overcome deficits in learning and memory induced by ovariectomy. Chronic administration of 1 mg/kg enhanced performance of ovariectomized females responding in an object recognition and an object placement task (Luine et al., 2002), whereas 3 mg/kg administered chronically enhanced acquisition of a DMTP T-maze task in aged ovariectomized female rats, but only when administered in conjunction with estradiol (Gibbs et al., 2009). To our knowledge, the present study is the first to demonstrate a differential sensitivity to the acute effects of donepezil in hormonally-manipulated male rats. Contrary to the effects of donepezil in female rats, the effects of donepezil in male rats were largely disruptive at 3.2 mg/kg and were more potent in GX males than in intact or GX+T males. In fact, a greater than two-fold difference in the ED50 values for response rate and percent of errors occurred in both components of the multiple schedule between these groups of males. Interestingly, GX + E males had a higher baseline rate of errors in the acquisition component and displayed greater sensitivity to the rate-decreasing and error-increasing effects of donepezil in both components of the multiple schedule task than intact males. This difference in sensitivity was also evident in the ED50 values of the dose-effect curves for both response rate and the percentage of errors. For instance, there was a greater than two-fold difference in the ED50 values for percent of errors in both the acquisition and performance components between GX + E and intact males. Thus, testosterone appears to attenuate the disruptive effects of donepezil, possibly by dampening the increase in ACh that results from donepezil's inhibition of AChE activity. Furthermore, exogenous estradiol may interact with donepezil to increase ACh to levels above those required that exert disruptive effects, at least in males, while estradiol replacement has been shown to restore ACh to basal levels in female ovariectomized rats (Mitsushima et al., 2009).
Further study may be necessary to address the possibility that the behavioral effects of donepezil resulted from the supraphysiological levels of testosterone and estradiol that occurred as a result of hormone replacement. Although the size and length of the hormone capsules (i.e., dose) was previously shown to maintain physiological titers of testosterone (Butler et al., 2001; Woodson & Balleine, 2002) and estradiol (Pinilla et al., 1999) in male rats, these capsules produced substantially higher hormone titers in the present study. Nonetheless, GX + T and intact males responded in a similar manner after donepezil administration, and GX males were more sensitive to the rate-decreasing and error-increasing effects of donepezil than both GX + T and intact males. The data are less clear with regard to estradiol, because GX + E males were more sensitive to the rate-decreasing and error-increasing effects of donepezil than intact males, but similar in sensitivity compared to GX males (except in the acquisition components where GX + E males were more sensitive to the error-increasing effects of donepezil). Together, these data suggest than the supraphysiological effects of testosterone are less problematic for the behavioral effects of donepezil than the supraphysiological effects of estradiol in male rats. The possibility also exists that the overexpression of hormone in vivo in this experiment may have provided an insight into the interaction of the gonadal hormones and the cholinergic system that would not have been possible otherwise at physiological levels, in a manner similar to the way a cellular “overexpression” system can provide insight into previously uncharacterized second messenger pathways (c.f. Leonard et al. 2008).
The present study also demonstrated differences in AChE activity levels, a marker commonly used to assess cholinergic function, between GX and gonadally intact male rats in several brain regions that mediate various learning and memory processes. Previously, knowledge regarding the interactive effects of the gonadal hormones and AChE activity in males has been limited to brain regions such as the cerebral cortex, preoptic suprachiasmatic area, and medial preopotic area (Libertun et al., 1973; Commins and Yahr, 1984; James and Kanungo, 1978), as well as the nuclei in the horizontal limb of the diagonal band bed and in the stria terminalis (Luine and McEwen, 1983). In these earlier studies gonadectomy reduced AChE activity, whereas testosterone and estradiol replacement restored AChE activity to basal levels relative to that of GX males. In contrast, the present study indicated that GX males had higher activity levels of AChE in the striatum and the hippocampus compared to that of intact males (activity in the prefrontal cortex was also higher in GX males but only approached statistical significance). Thus, while a loss of testosterone due to gonadectomy can decrease AChE activity levels in various areas of the brain, AChE activity levels in brain areas integral to learning and memory are increased by gonadectomy. Because both facilitative and disruptive effects of donepezil on learning and memory are assumed to result from an increase in ACh at cholinergic receptors, due to the inhibition of AChE activity, the relative increase in AChE activity levels in GX males compared to intact males likely reflects an overall increase in cholinergic activity (and most likely an increase in ACh release). This increase, in turn, strongly suggests that the presence of testosterone in intact males may suppress cholinergic activity and help explain why GX males show a higher sensitivity to donepezil's effects than did intact males.
In summary, gonadal hormones differentially affected the potency with which donepezil disrupted male rats responding under a complex operant procedure requiring the acquisition and performance of response sequences. Gonadectomized males were more sensitive to the effects of donepezil, as compared to intact and GX + T males, while GX + E males demonstrated the highest sensitivity of all four groups. Also, GX males had higher AChE activity levels in several areas of the brain integral to learning and memory compared to intact males. Taken together, these data suggest that testosterone may ordinarily dampen the activity of the cholinergic system in males, an effect opposite to that of estrogen in females. These findings are important, as donepezil is frequently used in the symptomatic treatment of mild to moderate dementia associated with Alzheimer's disease (Shigeta and Homma, 2001; Sugimoto, 2001). Alzheimer's disease is the most common cause of dementia and predominately affects those over 65 years of age (Hebert et al., 1995; Evans et al., 1989), a time when gonadal hormones have generally reached their nadir.
Acknowledgments
The authors would like to sincerely thank Dr. Johnny Porter for assistance with the brain dissections, Dr. Patricia Molina for the generous use of her microplate reader, and Mr. Russell Amato and Ms. Jessie Sutton for their technical assistance with these experiments.
This study was supported, in part, by Grant AA009803 (P.J.W.) from the National Institute on Alcohol Abuse and Alcoholism and Grant DA019625 (P.J.W.) from the National Institute on Drug Abuse.
References
- Anger WK, Setzer JV. Effects of oral and intramuscular carbaryl administrations on repeated chain acquisition in monkeys. J Toxicol Environ Health. 1979;5:793–808. doi: 10.1080/15287397909529790. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008;54:244–252. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Pontecorvo MJ, Flicker C. The cholinergic hypothesis: a historical overview, current perspective, and future directions. Ann N Y Acad Sci. 1985;444:332–358. doi: 10.1111/j.1749-6632.1985.tb37600.x. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ, Stone JD, Terry AV. Sex dimorphisms in the cognitive-enhancing action of the Alzheimer's drug donepezil in aged Rhesus monkeys. Neuropharmacology. 2003;44:381–389. doi: 10.1016/s0028-3908(02)00378-7. [DOI] [PubMed] [Google Scholar]
- Butler PC, Mills RH, Bloch GJ. Inhibition of lordosis behavior in male and female rats by androgens and progesterone. Hormones and Behavior. 2001;40:384–395. doi: 10.1006/hbeh.2001.1703. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Acetylcholinesterase activity in the sexually dimorphic area of the gerbil brain: sex differences and influences of adult gonadal steroids. J Comp Neurol. 1984;224:123–131. doi: 10.1002/cne.902240111. [DOI] [PubMed] [Google Scholar]
- Cutuli D, Foti F, Mandolesi L, De Bartolo P, Gelfo F, Federico F, Petrosini L. Cognitive performance of healthy young rats following chronic donepezil administration. Psychopharmacology (Berl) 2008;197:661–673. doi: 10.1007/s00213-008-1084-0. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Brauner IN, Moerschbaecher JM. Estrogen improves response accuracy and attenuates the disruptive effects of delta9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behav Neurosci. 2002;116:989–998. [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Dohanich G. Gonadal steroids, learning and memory. In: Pfaff D, Arnold A, Etgen A, Farback S, Rubin R, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2002. pp. 265–327. [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- Fadda F, Cocco S, Stancampiano R. Hippocampal acetylcholine release correlates with spatial learning performance in freely moving rats. Neuroreport. 2000;11:2265–2269. doi: 10.1097/00001756-200007140-00040. [DOI] [PubMed] [Google Scholar]
- Frederick DL, Schulze GE, Gillam MP, Paule MG. Acute effects of physostigmine on complex operant behavior in rhesus monkeys. Pharmacol Biochem Behav. 1995;50:641–648. doi: 10.1016/0091-3057(94)00358-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW. Effects of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Exp Neurol. 1992;116:23–39. doi: 10.1016/0014-4886(92)90173-n. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA. Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res. 1997;749:143–146. doi: 10.1016/s0006-8993(96)01375-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Beckett LA, Albert MS, Pilgrim DM, Chown MJ, Funkenstein HH, Evans DA. Age-specific incidence of Alzheimer's disease in a community population. JAMA. 1995;273:1354–1359. [PubMed] [Google Scholar]
- Hironaka N, Tanaka K, Izaki Y, Hori K, Nomura M. Memory-related acetylcholine efflux from rat prefrontal cortex and hippocampus: a microdialysis study. Brain Res. 2001;901:143–150. doi: 10.1016/s0006-8993(01)02338-1. [DOI] [PubMed] [Google Scholar]
- Howard J, Pollard G. Effects of d-amphetamine, Org 2766, scopolamine and physostigmine on repeated acquisition of four-response chains in rat. Drug Dev Res. 1983;3:37–48. [Google Scholar]
- James TC, Kanungo MS. Effect of sex steroids on choline acetyltransferase and acetylcholinesterase of cerebral hemisphere of male rats of various ages. Biochim Biophys Acta. 1978;538:205–211. doi: 10.1016/0304-4165(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Kasa P, Rakonczay Z, Gulya K. The cholinergic system in Alzheimer's disease. Prog Neurobiol. 1997;52:511–535. doi: 10.1016/s0301-0082(97)00028-2. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Sato A, Yoshizawa M, Fujii T, Fujimoto K, Suzuki T. Effects of the centrally acting cholinesterase inhibitors tetrahydroaminoacridine and E2020 on the basal concentration of extracellular acetylcholine in the hippocampus of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:523–528. doi: 10.1007/BF00173022. [DOI] [PubMed] [Google Scholar]
- Kelleher R. Chaining and conditioned reinforcement. In: Honig WK, editor. Operant behavior: areas of research and application. New York: Appleton-Century-Crofts; 1966. pp. 160–212. [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Leanza G, Muir J, Nilsson OG, Wiley RG, Dunnett SB, Bjorklund A. Selective immunolesioning of the basal forebrain cholinergic system disrupts short-term memory in rats. Eur J Neurosci. 1996;8:1535–1544. doi: 10.1111/j.1460-9568.1996.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Lehmann O, Bertrand F, Jeltsch H, Morer M, Lazarus C, Will B, Cassel JC. 5,7-DHT-induced hippocampal 5-HT depletion attenuates behavioural deficits produced by 192 IgG-saporin lesions of septal cholinergic neurons in the rat. Eur J Neurosci. 2002;15:1991–2006. doi: 10.1046/j.1460-9568.2002.02037.x. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Moerschbaecher JM, Winsauer PJ. Testosterone potentiates scopolamine-induced disruptions of nonspatial learning in gonadectomized male rats. Exp Clin Psychopharmacol. 2007;15:48–57. doi: 10.1037/1064-1297.15.1.48. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Moerschbaecher JM, Winsauer PJ. Estradiol replacement in gonadectomized male rats alters scopolamine-induced disruptions of nonspatial learning. Exp Clin Psychopharmacol. 2008;16:532–546. doi: 10.1037/a0013718. [DOI] [PubMed] [Google Scholar]
- Libertun C, Timiras PS, Kragt CL. Sexual differences in the hypothalamic cholinergic system before and after puberty: inductory effect of testosterone. Neuroendocrinology. 1973;12:73–85. doi: 10.1159/000122157. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN, McEwen BS. Sex differences in cholinergic enzymes of diagonal band nuclei in the rat preoptic area. Neuroendocrinology. 1983;36:475–482. doi: 10.1159/000123501. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Mohan G, Tu Z, Efange SM. Chromaproline and Chromaperidine, nicotine agonists, and Donepezil, cholinesterase inhibitor, enhance performance of memory tasks in ovariectomized rats. Pharmacol Biochem Behav. 2002;74:213–220. doi: 10.1016/s0091-3057(02)00988-7. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Wenk GL, Crawley JN. Analysis of galanin and the galanin antagonist M40 on delayed non-matching-to-position performance in rats lesioned with the cholinergic immunotoxin 192 IgG-saporin. Behav Neurosci. 1997;111:552–563. doi: 10.1037//0735-7044.111.3.552. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Pal SN, Marriott LK, Gold PE. Competition between memory systems: acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. J Neurosci. 2002;22:1171–1176. doi: 10.1523/JNEUROSCI.22-03-01171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Takase K, Funabashi T, Kimura F. Gonadal steroids maintain 24 h acetylcholine release in the hippocampus: organizational and activational effects in behaving rats. J Neurosci. 2009;29:3808–3815. doi: 10.1523/JNEUROSCI.5301-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerschbaecher JM, Boren JJ, Schrot J, Fontes JC. Effects of cocaine and d-amphetamine on the repeated acquisition and performance of conditional discriminations. J Exp Anal Behav. 1979;31:127–140. doi: 10.1901/jeab.1979.31-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Fujita H, Kawata M. Effects of gonadectomy on immunoreactivity for choline acetyltransferase in the cortex, hippocampus, and basal forebrain of adult male rats. Neuroscience. 2002;109:473–485. doi: 10.1016/s0306-4522(01)00513-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Orsetti M, Casamenti F, Pepeu G. Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain Res. 1996;724:89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Seoane LM, Gonzalez L, Carro E, Aguilar E, Casanueva FF, Dieguez C. Regulation of serum leptin levels by gonadal function in rats. European Journal of Endocrinology. 1999;140:468–473. doi: 10.1530/eje.0.1400468. [DOI] [PubMed] [Google Scholar]
- Poorheidari G, Stanhope KJ, Pratt JA. Effects of the potassium channel blockers, apamin and 4-aminopyridine, on scopolamine-induced deficits in the delayed matching to position task in rats: a comparison with the cholinesterase inhibitor E2020. Psychopharmacology (Berl) 1998;135:242–255. doi: 10.1007/s002130050506. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Yamanishi Y, Yamatsu K. E2020-The pharmacology of a piperdine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic basis for Alzheimer therapy. Boston: Birkhauser; 2009. pp. 314–320. [Google Scholar]
- Shen J, Barnes CA, Wenk GL, McNaughton BL. Differential effects of selective immunotoxic lesions of medial septal cholinergic cells on spatial working and reference memory. Behav Neurosci. 1996;110:1181–1186. doi: 10.1037//0735-7044.110.5.1181. [DOI] [PubMed] [Google Scholar]
- Shigeta M, Homma A. Donepezil for Alzheimer's disease: pharmacodynamic, pharmacokinetic, and clinical profiles. CNS Drug Rev. 2001;7:353–368. doi: 10.1111/j.1527-3458.2001.tb00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Stancampiano R, Cocco S, Cugusi C, Sarais L, Fadda F. Serotonin and acetylcholine release response in the rat hippocampus during a spatial memory task. Neuroscience. 1999;89:1135–1143. doi: 10.1016/s0306-4522(98)00397-2. [DOI] [PubMed] [Google Scholar]
- Sugimoto H. Donepezil hydrochloride: a treatment drug for Alzheimer's disease. Chem Rec. 2001;1:63–73. doi: 10.1002/1528-0691(2001)1:1<63::AID-TCR9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Bouger PC. Effects of the cholinesterase inhibitors donepezil and metrifonate on scopolamine-induced impairments in the spatial cone field orientation task in rats. Behav Brain Res. 2005;156:1–10. doi: 10.1016/j.bbr.2004.05.010. [DOI] [PubMed] [Google Scholar]
- van Haaren F, van Hest A, Heinsbroek RP. Behavioral differences between male and female rats: effects of gonadal hormones on learning and memory. Neurosci Biobehav Rev. 1990;14:23–33. doi: 10.1016/s0149-7634(05)80157-5. [DOI] [PubMed] [Google Scholar]
- Vnek N, Kromer LF, Wiley RG, Rothblat LA. The basal forebrain cholinergic system and object memory in the rat. Brain Res. 1996;710:265–270. doi: 10.1016/0006-8993(95)01477-2. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer's disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging. 2004;21:453–478. doi: 10.2165/00002512-200421070-00004. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Bixler MA, Mele PC. Differential effects of ionizing radiation on the acquisition and performance of response sequences in rats. Neurotoxicology. 1995;16:257–269. [PubMed] [Google Scholar]
- Wise LE, Iredale PA, Stokes RJ, Lichtman AH. Combination of rimonabant and donepezil prolongs spatial memory duration. Neuropsychopharmacology. 2007;32:1805–1812. doi: 10.1038/sj.npp.1301297. [DOI] [PubMed] [Google Scholar]
- Woodson JC, Balleine BW. An assessment of factors contributing to instrument performance for sexual reward in the rat. Quarterly Journal of Experimental Psychology: Section B. 2002;55:75–88. doi: 10.1080/02724990143000199. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Valdovinos MG, Williams DC. Relevance of donepezil in enhancing learning and memory in special populations: a review of the literature. J Autism Dev Disord. 2007;37:1883–1901. doi: 10.1007/s10803-006-0322-8. [DOI] [PubMed] [Google Scholar]
- Yuede CM, Dong H, Csernansky JG. Anti-dementia drugs and hippocampal-dependent memory in rodents. Behav Pharmacol. 2007;18:347–363. doi: 10.1097/FBP.0b013e3282da278d. [DOI] [PMC free article] [PubMed] [Google Scholar]







