Abstract
Patients who survive severe sepsis often display severely compromised immune function. One hallmark of such immune suppression in septic patients is an impaired delayed-type hypersensitivity (DTH) response, manifested by a loss of skin testing to recall Ags. Because sepsis induces significant apoptosis in lymphoid and myeloid cells, and apoptotic cells are themselves tolerogenic, we tested the hypothesis that suppression of DTH is mediated by tolerogenic properties of the apoptotic cells generated during sepsis. Mice subjected to cecal ligation and puncture demonstrated a loss of DTH for the 7 d following cecal ligation and puncture; however, the immune response returned to normal by day 10. Blocking sepsis-induced apoptosis via Bcl-2 overexpression or Bim deficiency prevented the loss of DTH. Importantly, injection of apoptotic cells into Bim−/− mice prevented an effective DTH response, thereby suggesting a causal link between apoptotic cells and immune suppression. Surprisingly, when TRAIL null mice were examined, we found that these animals had significant apoptosis but retained their DTH responses. Further studies revealed that apoptotic cells generated during sepsis induced a CD8+ regulatory T cell that suppressed DTH by TRAIL production. These results establish a link between apoptotic cells and immune suppression during sepsis and suggest TRAIL may be a viable therapeutic target for boosting the adaptive immune response following sepsis.
Sepsis is the leading cause of death in most intensive care units (1, 2). Patients with sepsis are severely immunosuppressed, making it difficult to control the primary infection and predisposing them to secondary nosocomial infections (3, 4). A number of studies in animals and humans suggest that immune defects may be critical to the pathogenesis and subsequent mortality in sepsis (5, 6). It is well documented that cell loss by apoptosis depletes critical components of the immune system, but there is also a functional loss of immunity (7). For example, depressed phagocytic function by macrophages (Mφ) and neutrophils impairs the clearance of microorganisms (8). There are also defects in dendritic cells (DCs) (9), which play a pivotal role in both innate and acquired immunity (10, 11). Increases in immunosuppressive cytokines (e.g., IL-10) (12) and CD4+ regulatory T cells (Tregs) (13, 14) have been observed during sepsis, as well as the loss of MHC Ag expression (15). Experiments using mouse models of sepsis demonstrate that defects in the APC compartment suppress T cell-mediated responses (10). Other studies suggest that a loss of proinflammatory Th1-type T cell responses and a shift toward an anti-inflammatory Th2 T cell response takes place during sepsis (12, 16).
Patients with sepsis have defects in the delayed-type hypersensitivity (DTH) response, as illustrated by their failure to respond to skin testing with Ags to which previous exposure is known to have occurred (17, 18). DTH is predominately mediated by CD4+ T cells (19–24), and any (or all) of the mechanisms of immune suppression listed above could account for the loss of this type of immunity. That multiple immunosuppressive mechanisms have been detected in sepsis suggests the complex nature of immune dysfunction following septic insult, and unraveling these mechanisms will have important consequences for the design of rational treatments.
It is well established that sepsis induces significant apoptosis in lymphoid and myeloid cells (7, 25, 26). It was originally thought that apoptosis was a cell’s final act and that dead cells were quickly removed and remained silent to the immune response. However, there is now a significant literature that demonstrates apoptotic cells are not passive and can significantly affect immunity (for review, see Refs. 23, 27). Current thought is that apoptotic cell death and the handling of these cells by the immune system provides a mechanism whereby self-tolerance can be maintained, either through deletion or active immune regulation. Furthermore, exposure of the immune system to large numbers of apoptotic cells can induce suppression of immunity (19, 21). Suppression is induced by the engulfment of dead cells by DCs (21, 28) and is mediated by several mechanisms including production of immunosuppressive cytokines by phagocytic cells (29), deletion of T cells (30), the induction of immune deviation (e.g., Th1-Th2 shift) (22), and the activation of CD8+ Tregs (19).
Recently, we demonstrated that immune suppression (also called immune tolerance) generated by injection of apoptotic cells also involves TRAIL. We found that presentation of apoptotic cells via DCs induces CD8+ Tregs that make TRAIL. These immunosuppressive CD8+Tregs were generated because Ag presented to CD8+ T cells in the presence of apoptotic cells did not prime CD4+ T cell help (19), a phenomenon similar to what has been termed helpless CTLs (31). Production of TRAIL inhibits immunity by directly suppressing the function of CD4+ T cells undergoing Ag-specific activation. The tolerance induced by the i.v. delivery of apoptotic cells is often called infectious tolerance, because it can be transferred to nontolerant recipients using T cells from tolerant individuals (23). Because a septic insult exposes the immune system to a significant number of apoptotic cells (7), we tested the idea that sepsis-induced apoptosis may be related to natural suppressive mechanisms in place to deal with apoptotic cells. Our results show that suppression of DTH following sepsis is the result of the presence of apoptotic cells that induce CD8+ Tregs that mediate suppression by producing TRAIL.
Materials and Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 Trail−/− mice were obtained from Amgen (Seattle, WA) (32), Bcl-2-transgenic (Tg) mice (human MHC H-2k promoter) were obtained from Dr. Irving Weissman (Stanford University, Palo Alto, CA) (33), and Bim−/− mice were obtained from Andreas Strasser (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) (34). All of these transgenic and knockout strains of mice are on the C57BL/6 background. Littermates or in-house–bred C57BL/6 wild-type mice were used as controls for all transgenic and knockout strain experiments. Groups consisted of at least five mice, and experiments were repeated at least twice to confirm results. All animal procedures were performed according to National Institutes of Health guidelines and approved by Washington University Institutional Animal Care and Use Committee (St. Louis, MO).
Sepsis model: cecal ligation and puncture
The cecal ligation and puncture (CLP) model was used to induce intra-abdominal peritonitis as described previously (7, 25, 35). These earlier studies from our laboratory include positive blood cultures for polymicrobial organisms (aerobic and anaerobic bacteria) from CLP, but not sham-operated mice. Mice were anesthetized with isoflurane, and an abdominal incision was performed. The cecum was identified, ligated, and punctured twice with a 27-gauge needle. The abdomen was closed in two layers, and 1 ml 0.9% saline was administered s.c. This level of injury typically results in 25–30% mortality as detected on day 4 of the experiments. No further mortality was observed during the course of the experiments performed in this study. Sham-operated mice were treated identically, except the cecum was not ligated or punctured.
DTH response to trinitrophenol
Mice were immunized with 0.1 ml 10 mM 2,4,6 trinitrobenzene sulfonic acid (TNBS) s.c. Four days later, mice were challenged with 0.033 ml 10 mM TNBS in PBS in the right and 0.033 ml PBS in the left footpad. Measurements were taken 24 h postinjection by a masked observer. Values are expressed as immune response to trinitrophenol (TNP) in micrometers (± SE) and represent the difference between the right (Ag challenge) and left footpad (PBS challenge). Background values represent the difference between the challenged and unchallenged footpad in unimmunized mice.
Quantification of apoptosis
TUNEL staining was performed to identify apoptotic cells (36, 37). Mice were killed, and spleens were harvested 24 h following CLP or sham surgery. A single-cell suspension was prepared, and the cells were fixed in 1% paraformaldehyde for 30 min at room temperature. Following washing, cells were permeabilized by treatment with 90% methanol for 30 min on ice. Apoptosis was quantified by flow cytometry using the APO-BRDU kit (Phoenix Flow Systems, San Diego, CA) according to the manufacturer’s instructions. Cells were then stained with anti-CD3 mAb (CD3-FITC, BD Pharmingen, San Diego, CA), and TUNEL+ CD3+ cells were identified by flow cytometry using an FACScan (BD Biosciences, San Jose, CA).
Quantification of T cells, Mφ, and DCs
Total viable cell counts per spleen were determined via the Vi-Cell counter (Beckman Coulter, Fullerton, CA) by trypan blue exclusion. The percentages of individual cell phenotypes were determined via flow cytometric analysis. The absolute cell counts for each splenic subset population were calculated by using the following equation: count of subset = total splenic count × percent of subset/100. Cell percentages were determined by staining for CD4-PE (eBioscience, San Diego, CA), CD8-FITC (BD Pharmingen), CD11b-FITC (BD Pharmingen), and F4/80-PE (eBioscience) to identify macrophages or CD11c-PE (BD Pharmingen) and MHC class II (MHC II-PE, BD Pharmingen) to identify DCs.
T cell isolation and adoptive transfer
T cell populations were obtained using Easy Sep Mouse CD4/CD8 T Cell enrichment kit(s) (Stem Cell Technologies, Vancouver, British Columbia, Canada) as per manufacturer’s instructions. Cell populations were used only when purity was >95% as determined by flow cytometry using anti-CD4 and anti-CD8 Abs. Adoptive transfers were performed as described (19) using purified T cells from sham or CLP mice 4 d following sham or CLP. Following purification, recipient mice received CD4+CD8+ or CD4− CD8− cells equivalent to one donor to one recipient.
CD8 T cell depletion
Anti-CD8 (clone 2.43) Ab for in vivo deletion was purified from hybridoma supernatants (19). Mice received daily doses of 100 μg i.v. beginning 3 d prior to CLP or sham surgery. This treatment results in complete elimination of CD8+ T cells as determined by flow cytometry (19, 31).
Statistics
Statistical analysis was performed using Student t test; p < 0.01 was considered significant.
Results
Loss and recovery of the DTH response following sepsis
DTH is predominantly a CD4+ T cell-mediated reaction to Ags presented to the immune system on APCs (e.g., DCs) via MHC II (19, 23). We have published extensively on the DTH response in other systems (19–23) and therefore undertook studies to understand this immune reaction in a mouse model of sepsis. Mice were subjected to CLP and immunized to the hapten TNP by s.c. injection of TNBS at various days following surgery. Responses in septic mice were compared with mice that had undergone a sham operation. Data in Fig. 1 demonstrate that for the first 7 d after CLP, mice were not able to mount a DTH response; however, by day 10, the DTH response returned to a normal level of intensity.
FIGURE 1.
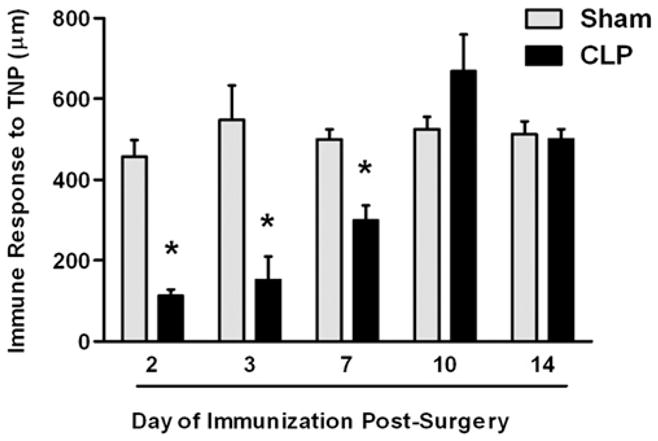
DTH response during sepsis. C57BL/6 mice were subjected to CLP or sham surgery. On various days postsurgery, the mice were immunized s.c. with TNBS. After an additional 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right (Ag challenge) and left footpad (PBS challenge). The data are reported as immune response to TNP. *Significant difference from sham-treated mice measured on the same day (p < 0.01).
Role of apoptosis in DTH loss during sepsis
There are a number of explanations for the loss of DTH reactivity following CLP, including the deletion (or dysfunction) of important APCs (10, 11), the loss of potential effector T cells (38), the failure of T cells to respond in the septic environment (36), and the induction of regulatory cells (13, 14). As a first step in analyzing the mechanism of sepsis-induced suppression of DTH, we determined the extent to which DTH responsiveness was related to profound cellular apoptosis (38). We did this by examining the DTH response in septic Bcl2-Tg (human Bcl2 driven by the MHC H-2k promoter) and Bim−/− mice. These strains do not show significant loss of lymphoid or myeloid cells following CLP, which gives them a survival advantage compared to wild-type animals (35, 39). These mice were subjected to CLP or sham operation and 4 d later immunized with TNBS s.c. Four days following immunization, the mice were challenged with TNBS and DTH measured 24 h later. The results demonstrate that Bcl2-Tg (Fig. 2A) as well as the Bim−/− mice (Fig. 2B) did not lose their response to the immunizing Ag. Because these mice do not have significant apoptosis following CLP (35, 39), these data suggest a correlation between cell depletion by apoptosis and the loss of cell-mediated immunity as measured by the DTH reaction.
FIGURE 2.
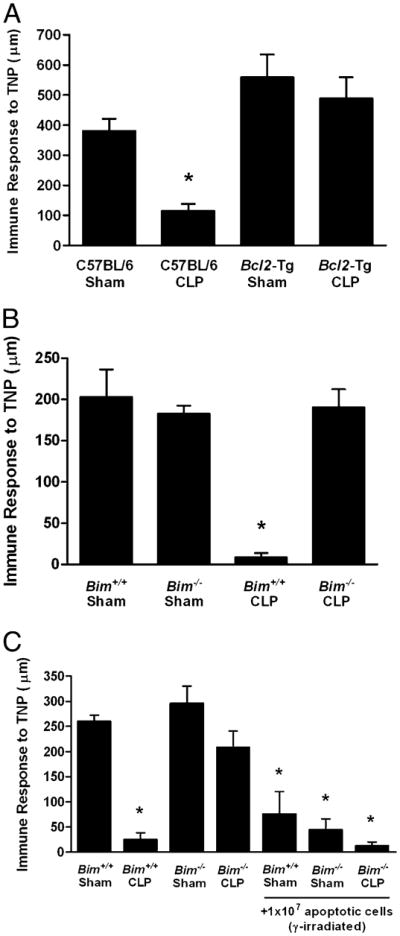
Role of cellular apoptosis in the DTH response following sepsis. Bcl2-Tg or littermate C57BL/6 mice (A) or Bim−/− and Bim+/+ (C57BL/6 littermates) mice (B) were subjected to CLP or sham surgery. Four days postsurgery, mice were immunized s.c. with TNBS. After an additional 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right (Ag challenge) and left footpad (PBS challenge). The data are reported as immune response to TNP. C, Bim−/− mice and Bim+/+ (C57BL/6 littermates) were subjected to CLP or sham surgery. Two days later, mice were given 107 γ-irradiated apoptotic spleen cells i.v. After an additional 2 d (4 d postsurgery), mice were immunized s.c. with TNBS. After an additional 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. *Significant difference from C57BL/6 or Bim+/+ control mice (p < 0.01).
The simplest explanation for the results in Fig. 2A and 2B is that sepsis-induced apoptosis depletes the cells important for adaptive immunity (e.g., T cells and APCs), thereby preventing any response to foreign Ag. Consequently, mice that do not lose DTH (e.g., Bim−/− mice) do not suffer the loss of critical cell populations. However, it is well established that apoptotic cells are not passive participants in immunity, but are capable of modifying the immune response following their engulfment by APCs (19, 21, 27, 29, 30). Therefore, we tested the idea that apoptotic cells might play a more active role in the suppressed immunity observed during sepsis. C57BL/6 or Bim−/− mice underwent sham or CLP surgery, and 48 h later, they were given a large number of apoptotic cells i.v. [107 γ-irradiated syngeneic spleen cells (19, 21, 40)]. Two days later, they were immunized with TNBS s.c., and 4 d later, the mice were challenged with TNBS. The DTH measurements were taken 24 h later. Data in Fig. 2C show that wild-type mice fail to mount a DTH response following CLP, whereas immunity is maintained in Bim−/− mice (similar to Fig. 2B). However, when apoptotic cells were given to the Bim−/− mice, significant suppression of DTH was observed. Thus, apoptotic cells can induce suppression of immunity (or tolerance) in mice resistant to both sepsis-induced apoptosis and loss of DTH reactivity, suggesting an active role for dead cells in the induction of immune suppression.
TRAIL and immune suppression during sepsis
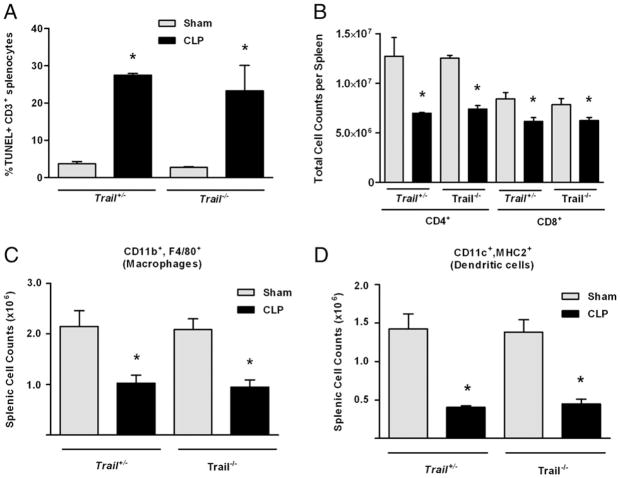
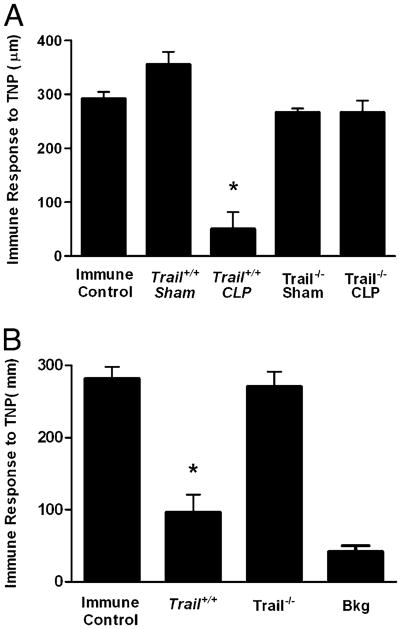
Our previous studies demonstrated that suppression induced by apoptotic cells was mediated by a TRAIL-expressing CD8+ T cell population (19). This CD8+ Treg suppresses immunity via TRAIL secretion that potently inhibits CD4+ T cell function. In these published studies, apoptotic cells could not induce tolerance in Trail−/− mice because their CD8+ Tregs could not produce TRAIL. Because data in Fig. 2 suggest a role for apoptotic cells in immune tolerance during sepsis, we examined the effect of sepsis on apoptosis and DTH responses in Trail−/− mice. Wild-type and Trail−/− mice were subjected to CLP or sham operation, and evidence of apoptotic T cells by TUNEL staining and loss of key T cell and APC populations (specifically, Mφ and DCs) in the spleen was evaluated 24 h later. Fig. 3A shows there was an equivalent amount of CD3+ T cell apoptosis in wild-type and Trail−/− mice. This was reflected in the loss of CD4+ and CD8+ T cells, which was equivalent in both strains (Fig. 3B). In addition, both Mφ (CD11b+, F4/80+; Fig. 3C) and DCs (CD11c+, MHC II+; Fig. 3D) were depleted at similar levels in Trail+/− and Trail−/− mice. When we examined the DTH response in CLP-treated Trail−/− mice, these mice displayed a robust DTH response (Fig. 4A), despite having substantial T cell apoptosis in lymphoid organs (Fig. 3A, 3B) and significant reductions in other important cell populations (Fig. 3C, 3D).
FIGURE 3.
Apoptosis and cell loss in Trail−/− mice. A, Trail+/− or Trail+/+ (C57BL/6 littermates) were subjected to CLP or sham surgery. Twenty-four hours later, spleens were harvested, and TUNEL stains were performed and analyzed by flow cytometry on gated CD3+ cells. Results are reported as % TUNEL+ CD3+ splenocytes. B–D, Trail−/− or Trail+/− littermates were subjected to CLP or sham surgery. The number of CD4+ and CD8+ CD3+ T cells (B), splenic macrophages (CD11b+, F4/80+) (C), and DCs (CD11c+, MHC II+) (D) were quantified by flow cytometry. *Significant reduction versus Trail+/− mice.
FIGURE 4.
TRAIL regulates DTH in naive and sensitized CLP mice. A, Trail−/− or Trail+/+ (C57BL/6 littermates) were subjected to CLP or sham surgery. Four days postsurgery, mice were immunized s.c. with TNBS. After an additional 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. B, Trail−/− or Trail+/+ (C57BL/6 littermates) were immunized s.c. with TNBS. Four days later, they were subjected to CLP. After an additional 3 d, they were challenged with TNBS in the right and PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right (Ag challenge) and left footpad (PBS challenge). The data are reported as immune response to TNP and are compared with an immune control that received only s.c. immunization. *Significant difference from immune control mice measured on the same day (p < 0.01).
The loss of cell-mediated immunity is an important marker for assessing the overall health of the immune system following injury or infection. In fact, skin reactivity to recall Ags is a general test for immune suppression (18). Consequently, we examined the role of TRAIL in the recall response in the present system. Mice were immunized s.c. with TNBS, and 4 d later, they were subjected to CLP. After an additional 3 d, the mice were challenged with TNBS in the footpad, and the DTH measurements were taken 24 h later. Data in Figure 4B show that Trail+/+ mice that had undergone CLP lost the DTH response, whereas CLP-treated Trail−/− mice did not. Thus, the loss of a recall Ag response is also regulated by TRAIL.
Induction of TRAIL-expressing CD8+ Tregs during sepsis
Our data suggest a link between apoptotic cells produced during sepsis and the suppression of the DTH response. However, in Trail−/− mice, there was substantial T cell apoptosis and APC loss, but the DTH reaction was normal (Figs. 3, 4A). An interesting possibility that would reconcile these findings would be that the apoptotic cells actively induced DTH suppression through the action of TRAIL-expressing CD8+ T cells. Immune regulation via this mechanism would also be consistent with the data we have presented thus far and would explain our results in the Trail−/− mice. We tested this hypothesis with the experiments presented in Fig. 5. C57BL/6 mice were depleted of CD8+ T cells and then underwent sham or CLP surgery. Four days later, they were tested for DTH as described above. These data (Fig. 5A) demonstrate that whereas depletion of CD8+ T cells had no effect on the DTH response in sham-operated mice, it reversed the suppression of DTH observed in septic (CLP) animals. Thus, CD8+ T cells are essential for the loss of DTH in sepsis.
FIGURE 5.
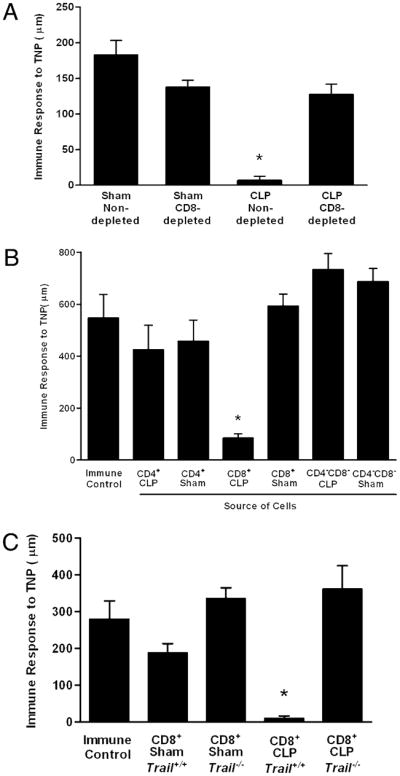
CD8+ T cells suppress DTH. A, C57BL/6 mice were depleted of CD8+ T cells by i.v. injection of 100 μg anti-CD8 Ab beginning 3 d prior to CLP or sham surgery. Four days postsurgery, mice were immunized to the hapten TNP by s.c. injection TNBS. After 4 additional days, mice were challenged with TNBS in the right footpad and PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right footpad (Ag challenge) and left footpad (PBS challenge). The data are reported as immune response to TNP. *Significant difference from sham nondepleted mice measured on the same day (p < 0.01). B, C57BL/6 mice were subjected to CLP or sham surgery. Four days following surgery, spleens were isolated, and CD4+ and CD8+ T cells were purified by negative selection. Mice received an equivalent number (one donor into one recipient) of cells i.v. On the day of cell transfer, mice were immunized s.c. with TNBS. After an additional 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right (Ag challenge) and left footpad (PBS challenge). *Significant difference from immune control mice measured on the same day (p < 0.05). C, Trail−/− or Trail+/+ (C57BL/6 littermates) were subjected to CLP or sham surgery. Four days following surgery, spleens were isolated, and CD4+ and CD8+ T cells were purified by negative selection. Mice (Trail+/+) received an equivalent number (one donor into one recipient) of cells i.v. On the day of cell transfer, mice were immunized s.c. with TNBS. After an additional 4 d, mice were challenged with TNBS in the right and PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right (Ag challenge) and left footpad (PBS challenge). The data are reported as immune response to TNP. *Significant difference from immune control mice measured on the same day (p < 0.05).
Some forms of tolerance mediated by T cells are termed infectious because they can be transferred from tolerant animals to nontolerant recipients using purified T cell populations (21, 23). This was explored in the present system by the experiment presented in Fig. 5B. Mice underwent CLP or a sham operation, and 4 d later, the spleens were harvested. CD4+ T cells, CD8+ T cells, and the non-T cell populations (CD4−, CD8−) were isolated, and these purified cells were then transferred to naive recipient mice (Trail+/+) that were immediately sensitized with TNBS via s.c injection. After 4 additional days, the mice were challenged with TNBS and the DTH measured 24 h later. These data demonstrate that whereas no cells from sham-operated animals could transfer tolerance, CD8+ T cells from septic animals transferred tolerance to the recipient mice. Neither CD4+ T cells nor the non-T cell population (CD4−CD8−) from septic mice was able to transfer tolerance. The experiment in Fig. 5C explored the mechanism of adoptive tolerance using CD8+ T cells from wild-type and Trail−/− mice, and the results show that the transfer of wild-type CD8+ T cells from CLP-operated animals suppressed DTH, whereas the same population derived from CLP-operated Trail−/− mice did not. We conclude from our data that during sepsis, apoptotic cells induce a TRAIL-expressing CD8+ T cell that suppresses CD4+ T cell mediated-immunity.
Discussion
The loss of cell-mediated immunity is an important marker for assessing the overall health of the immune system following injury or infection. Indeed, skin reactivity to recall Ags is a general test for immune suppression in trauma and surgical patients (18), as individuals who are nonresponsive typically have severe defects in both the innate and acquired arms of the immune response. In addition, injury has profound suppressive effects on the development of an immune response when Ags are introduced posttrauma (36). We have undertaken studies to explore the effect of CLP-induced sepsis on the DTH response in naive animals as well as individuals previously sensitized to an Ag. We observed that the ability to respond to the immunizing Ag is rapidly lost following CLP, but returns to normal levels by 10 d postsurgery. Although this result may not be surprising in light of the many published reports of myeloid and lymphoid cell apoptosis (12, 38) and APC dysfunction from sepsis (10, 11), the novel finding of the data presented in this study is that the apoptotic cells resulting from the septic event actively induce a population of TRAIL-expressing CD8+ T cells that mediate immune suppression. Thus, our data point to an additional mechanism of immune suppression and further emphasize the complexity of the immune regulation following sepsis. The data also suggest that the TRAIL/TRAIL receptor system may be a viable therapeutic target to boost acquired immune responses to combat nosocomial infections in critically ill patients.
The mechanisms by which apoptosis is induced in sepsis remain poorly defined; however, there is substantial evidence demonstrating that both intrinsic and extrinsic pathways are involved (37, 41). Protection from apoptosis by blocking the mitochondrial pathway (intrinsic) by overexpression of Bcl2 (or loss of Bim) as well as the demonstration blocking the Fas/FasL-mediated pathway (extrinsic) (42, 43) emphasize this point. Whatever the ultimate pathway to cell death in sepsis, it is clear that the immune system is exposed to substantial numbers of apoptotic cells that must be disposed of by a compromised phagocytic system. Additionally, the presence of dead cells is clearly detrimental to the overall health of the individual, as demonstrated by studies showing that injection of apoptotic cells can decrease survival if administered to septic mice (40). Our studies show that as the immune system deals with large numbers of apoptotic cells in sepsis, it invokes an important immunoregulatory mechanism, perhaps to manage the large number of self-Ags revealed following injury. Unfortunately, this mechanism impairs a critical arm of the immune response necessary for general health and may predispose recovering individuals to secondary infections.
TRAIL is one of several proteins within the TNF superfamily that can induce apoptosis. TRAIL has received its greatest attention in the cancer field, as early studies revealed TRAIL potently induced apoptosis in tumor cells but not normal cells (44). There is now substantial evidence, however, demonstrating that TRAIL can induce death in a number of normal cells during cellular activation, including CD4+ (19) and CD8+ T cells (31) and neutrophils (45). Interestingly, the effect of TRAIL loss on DTH in sepsis is not related to apoptosis induced by TRAIL, as normal and Trail−/− mice display equivalent levels of T cell death following CLP. Recent studies have also shown that TRAIL is functional in a variety of physiological systems (31, 46, 47). Although Trail−/− mice do not have an overt phenotype (32), studies with infectious agents and autoimmune models clearly show TRAIL plays an important role in controlling the extent of immune reactions and can function as an effector molecule in certain disease states (48–51). These results, along with our results presented in this paper, suggest TRAIL is a key immunoregulatory molecule involved in controlling immunity, perhaps preventing potential autoimmunity following injury or infection.
In our system, TRAIL-mediated regulation of the immune system appears to be short-lived. Specifically, we found that suppressed DTH responses were evident 7 d after CLP, but the immune response returned to normal by 10 d post CLP. The transient nature of the tolerance is consistent with what has been observed in systems in which TRAIL-producing CD8+ T cells have been reported (19, 31). In these studies, the production of TRAIL not only inhibited CD4+ T cell responses, but also culminated in the suicide of the TRAIL-producing cells.
The loss of immune responses following septic injury is well documented, and a number of mechanisms have been described. Results presented in this study demonstrate that there is an additional mechanism whereby the handling of apoptotic cells by the immune system induces active immunoregulation. Although identification of these mechanisms in human (including the presence of CD8+ Tregs) has yet to be accomplished, our results highlight the complexity of immunoregulation during sepsis. Should the importance in human patients be confirmed, such results would reveal a potential site of therapeutic intervention [i.e., that preventing TRAIL-mediated immune suppression could improve adaptive immune responses (such as DTH), leading to increased responses to secondary infections].
Acknowledgments
This work was supported by National Institutes of Health Grants EY06765 (to T.A.F.), EY015570 (to T.A.F.), EY02687 (Department of Ophthalmology and Visual Sciences Core Grant), GM44118 (to R.S.H.), GM55194 (to R.S.H.), AI077565 (to T.S.G.); a University of Iowa Carver Medical Research Initiative Grant (to T.S.G.); a Department of Ophthalmology and Visual Sciences grant from Research to Prevent Blindness, New York, NY; and the Macular Vision Research Foundation (to T.A.F.).
Abbreviations used in this paper
- CLP
cecal ligation and puncture
- DC
dendritic cell
- DTH
delayed-type hypersensitivity
- Mφ
macrophage
- MHC II
MHC class II
- Tg
transgenic
- TNBS
2,4,6 trinitrobenzene sulfonic acid
- TNP
trinitrophenol
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SL. Deaths: final data for 1998. Natl Vital Stat Rep. 2000;48:1–105. [PubMed] [Google Scholar]
- 3.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 4.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Huber-Lang MS, Younkin EM, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Curnutte JT, Erickson R, Ward PA. Complement-induced impairment of innate immunity during sepsis. J Immunol. 2002;169:3223–3231. doi: 10.4049/jimmunol.169.6.3223. [DOI] [PubMed] [Google Scholar]
- 9.Guisset O, Dilhuydy MS, Thiébaut R, Lefèvre J, Camou F, Sarrat A, Gabinski C, Moreau JF, Blanco P. Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Med. 2007;33:148–152. doi: 10.1007/s00134-006-0436-7. [DOI] [PubMed] [Google Scholar]
- 10.Wen H, Dou Y, Hogaboam CM, Kunkel SL. Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood. 2008;111:1797–1804. doi: 10.1182/blood-2007-08-106443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flohé SB, Agrawal H, Schmitz D, Gertz M, Flohé S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol. 2006;79:473–481. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- 12.Ayala A, Chung CS, Song GY, Chaudry IH. IL-10 mediation of activation-induced TH1 cell apoptosis and lymphoid dysfunction in polymicrobial sepsis. Cytokine. 2001;14:37–48. doi: 10.1006/cyto.2001.0848. [DOI] [PubMed] [Google Scholar]
- 13.Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, Vonderfecht SL, Na S. Adoptive transfer of in vitro-stimulated CD4+ CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol. 2005;174:7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- 14.Venet F, Pachot A, Debard AL, Bohé J, Bienvenu J, Lepape A, Monneret G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25− lymphocytes. Crit Care Med. 2004;32:2329–2331. doi: 10.1097/01.ccm.0000145999.42971.4b. [DOI] [PubMed] [Google Scholar]
- 15.Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, Faivre V, Boval B, Payen D. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37:2746–2752. doi: 10.1097/CCM.0b013e3181ab858a. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 17.Christou NV, Meakins JL, Gordon J, Yee J, Hassan-Zahraee M, Nohr CW, Shizgal HM, MacLean LD. The delayed hypersensitivity response and host resistance in surgical patients. 20 years later. Ann Surg. 1995;222:534–546. doi: 10.1097/00000658-199522240-00011. discussion 546–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meakins JL, Christou NV, Bohnen J, MacLean LD. Failure of delayed hypersensitivity skin testing to predict postoperative sepsis and mortality. Br Med J (Clin Res Ed) 1982;285:1207–1208. doi: 10.1136/bmj.285.6349.1207-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith TS, Kazama H, VanOosten RL, Earle JK, Jr, Herndon JM, Green DR, Ferguson TA. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178:2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 20.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson TA, Stuart PM, Herndon JM, Griffith TS. Apoptosis, tolerance, and regulatory T cells—old wine, new wineskins. Immunol Rev. 2003;193:111–123. doi: 10.1034/j.1600-065x.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 24.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 27.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 32.Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R, Gliniak B. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur J Immunol. 2002;32:2246–2254. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 34.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 36.Unsinger J, Herndon JM, Davis CG, Muenzer JT, Hotchkiss RS, Ferguson TA. The role of TCR engagement and activation-induced cell death in sepsis-induced T cell apoptosis. J Immunol. 2006;177:7968–7973. doi: 10.4049/jimmunol.177.11.7968. [DOI] [PubMed] [Google Scholar]
- 37.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss RS, I, Karl E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 39.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 40.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci USA. 2003;100:6724–6729. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 42.Ayala A, Lomas JL, Grutkoski PS, Chung S. Fas-ligand mediated apoptosis in severe sepsis and shock. Scand J Infect Dis. 2003;35:593–600. doi: 10.1080/00365540310015656. [DOI] [PubMed] [Google Scholar]
- 43.Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74:344–351. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 44.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 45.Renshaw SA, Parmar JS, Singleton V, Rowe SJ, Dockrell DH, Dower SK, Bingle CD, Chilvers ER, Whyte MK. Acceleration of human neutrophil apoptosis by TRAIL. J Immunol. 2003;170:1027–1033. doi: 10.4049/jimmunol.170.2.1027. [DOI] [PubMed] [Google Scholar]
- 46.Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 47.Lee HO, Herndon JM, Barreiro R, Griffith TS, Ferguson TA. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J Immunol. 2002;169:4739–4744. doi: 10.4049/jimmunol.169.9.4739. [DOI] [PubMed] [Google Scholar]
- 48.Zheng SJ, Jiang J, Shen H, Chen YH. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J Immunol. 2004;173:5652–5658. doi: 10.4049/jimmunol.173.9.5652. [DOI] [PubMed] [Google Scholar]
- 49.Zheng SJ, Wang P, Tsabary G, Chen YH. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest. 2004;113:58–64. doi: 10.1172/JCI200419255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Brincks EL, Kucaba TA, Legge KL, Griffith TS. Influenza-induced expression of functional tumor necrosis factor-related apoptosis-inducing ligand on human peripheral blood mononuclear cells. Hum Immunol. 2008;69:634–646. doi: 10.1016/j.humimm.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]