Abstract
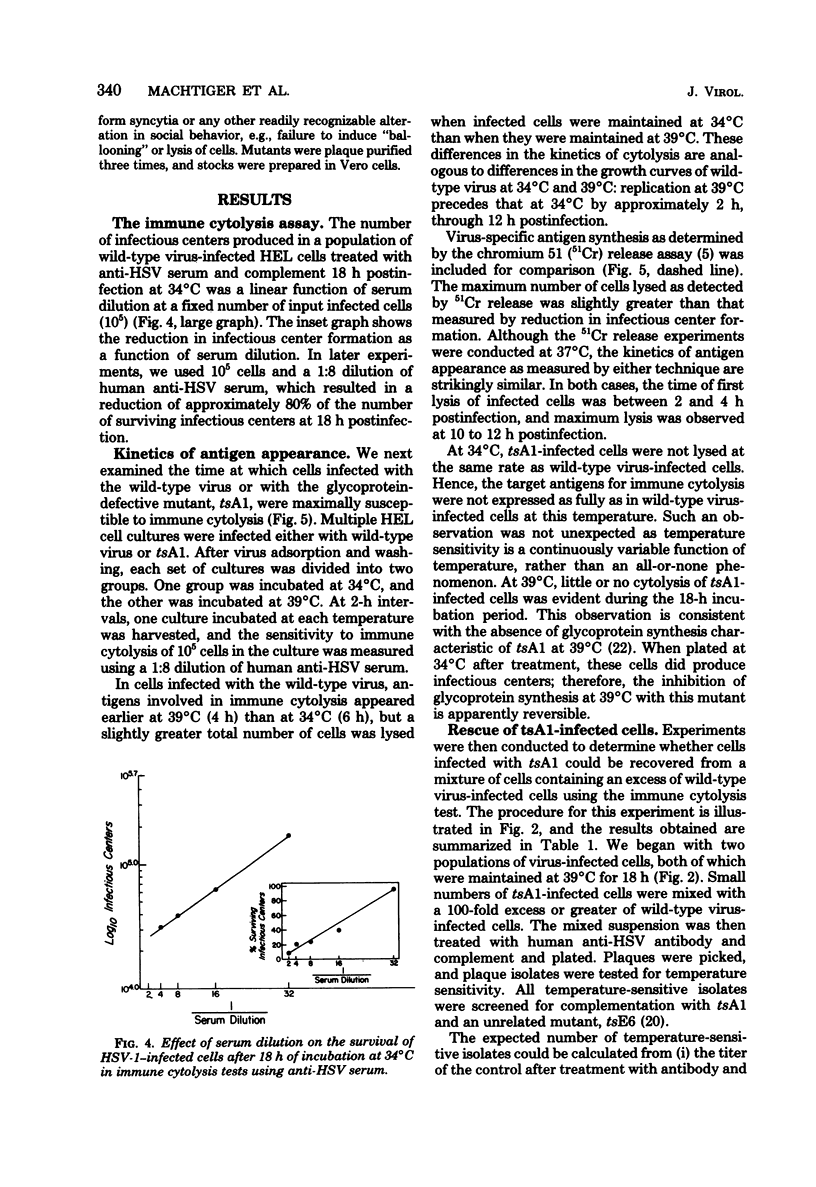
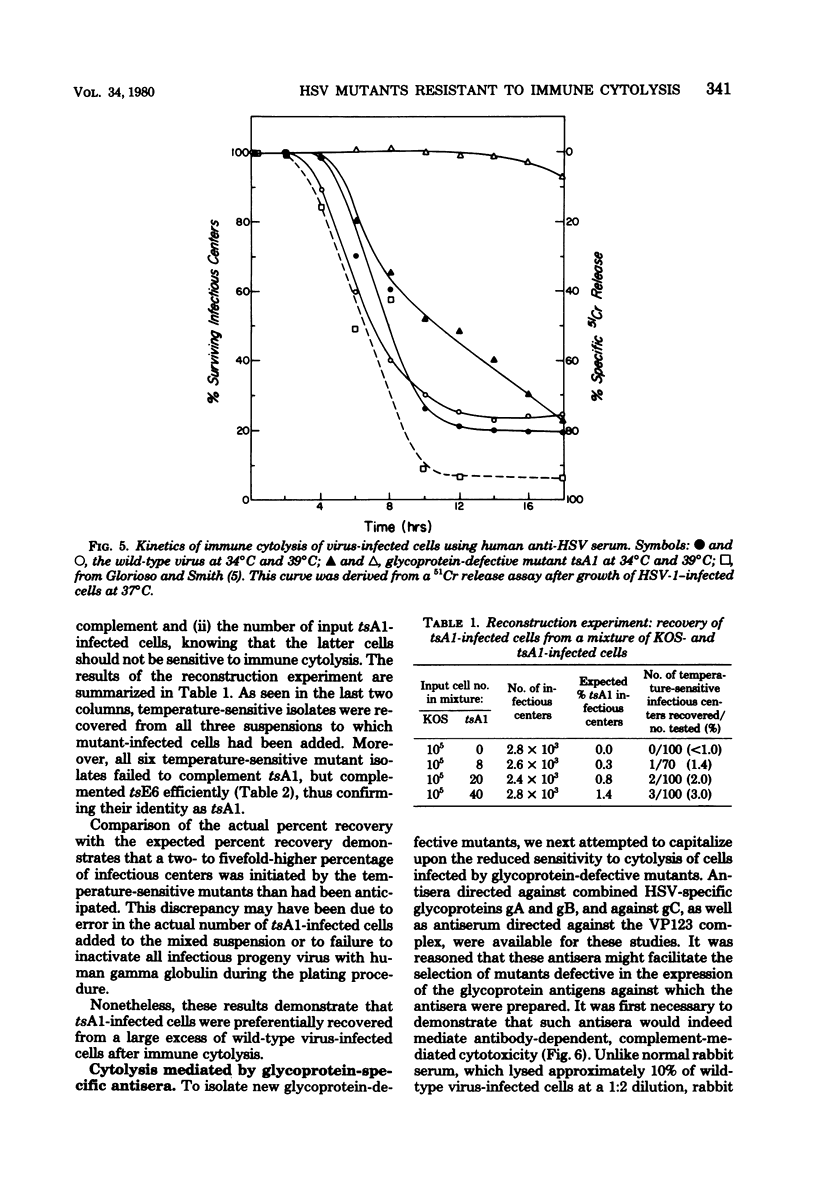
Immune cytolysis mediated by antibody and complement is directed against components of the major herpes simplex virus (HSV) glycoprotein complex (molecular weight, 115,000 to 130,000), comprised of gA, gB, and gC, and against glycoprotein gD—all present on the surfaces of infected cells. Tests with a temperature-sensitive (ts) mutant of HSV-1 (tsA1) defective in glycoprotein synthesis at the nonpermissive temperature (39°C) demonstrated that over 90% of mutant-infected cells maintained at 39°C and treated with antibody and complement were not lysed, presumably due to the absence of viral glycoproteins on the surface of infected cells at this temperature. Furthermore, a small number of tsA1-infected cells could be detected among a large excess of wild-type virus-infected cells by virtue of their failure to be lysed at 39°C by antibody and complement. Making use of the involvement of viral glycoproteins in immune cytolysis and the ability of cells infected with glycoprotein-defective mutants to escape cytolysis, we sought mutants defective in the expression of individual viral glycoproteins. For this purpose, antisera directed against the VP123 complex and against the gC and combined gA and gB glycoprotein subcomponents of this complex were first tested for their ability to lyse wild-type virus-infected cells in the presence of complement. Wild-type virus-infected cells were lysed after treatment with each of the three antisera, demonstrating that the gC glycoprotein and the combined gA and gB glycoproteins can act as targets in the immune cytolysis reaction. Next, these antisera were used to select for mutants which were resistant to immune cytolysis. Cells infected with wild-type virus which had been mutagenized with 2-aminopurine and incubated at 39°C were treated with one of the three types of antisera (anti-VP123 complex, anti-gC, or anti-gAgB) and lysed by the addition of complement. Cells which survived immune cytolysis were plated, and virus in the resulting plaques was isolated. Plaque isolates were tested for temperature sensitivity of growth and altered cytopathic effects in cell culture at 34°C (the permissive temperature) and 39°C. A total of 73 mutants was isolated in this manner. Selection with glycoprotein-specific antisera resulted in a 2- to 16-fold enrichment for mutants compared with “mock” -selected mutants using normal rabbit serum. Phenotypically, 24 mutants were temperature sensitive for growth, 27 were partially temperature sensitive, and 22 were not temperature sensitive but exhibited markedly altered cytopathic effects at both permissive and nonpermissive temperatures. Nine mutants of each phenotype (temperature sensitive, partially temperature sensitive, and non-temperature sensitive) were selected at random for confirmatory immune cytolysis tests with the antisera used in their selection. Cells infected with eight of the nine mutants were shown to be significantly more resistant to immune cytolysis at the nonpermissive temperature than were the mock-selected mutants or the wild-type virus from which they were derived.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A., Spear G. Transformation of hamster embryo fibroblasts by a specific fragment of the herpes simplex virus genome. Cell. 1978 Nov;15(3):993–1002. doi: 10.1016/0092-8674(78)90283-0. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Smith J. W. Immune interactions with cells infected with herpes simplex virus: antibodies to radioiodinated surface antigens. J Immunol. 1977 Jan;118(1):114–121. [PubMed] [Google Scholar]
- Glorioso J. C., Wilson L. A., Fenger T. W., Smith J. W. Complement-mediated cytolysis of HSV-1 and HSV-2 infected cells: plasma membrane antigens reactive with type-specific and cross-reactive antibody. J Gen Virol. 1978 Aug;40(2):443–454. doi: 10.1099/0022-1317-40-2-443. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Flannery V. L., Levy B., Schaffer P. A. Oncogenic transformation of primary hamster cells by herpes simplex virus type 2 (hsv-2) and an hsv-2 temperature-sensitive mutant. Int J Cancer. 1975 May 15;15(5):786–798. doi: 10.1002/ijc.2910150510. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Hydroxylapatite chromatography of protein-sodium dodecyl sulfate complexes. A new method for the separation of polypeptide subunits. J Biol Chem. 1972 Aug 25;247(16):5194–5198. [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris D. S., Courtney R. J., Schaffer P. A. Temperature-sensitive mutants of herpes simplex virus type 1 defective in transcriptional and post-transcriptional functions required for viral DNA synthesis. Virology. 1978 Oct 15;90(2):177–186. doi: 10.1016/0042-6822(78)90301-x. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Reed C. L., Cohen G. H., Rapp F. Detection of a virus-specific antigen on the surface of herpes simplex virus-transformed cells. J Virol. 1975 Mar;15(3):668–670. doi: 10.1128/jvi.15.3.668-670.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Carter V. C., Timbury M. C. Collaborative complementation study of temperature-sensitive mutants of herpes simplex virus types 1 and 2. J Virol. 1978 Sep;27(3):490–504. doi: 10.1128/jvi.27.3.490-504.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Courtney R. J., McCombs R. M., Benyesh-Melnick M. A temperature-sensitive mutant of herpes simplex virus defective in glycoprotein synthesis. Virology. 1971 Nov;46(2):356–368. doi: 10.1016/0042-6822(71)90037-7. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



