Abstract
Seasonal and pandemic influenza are frequently complicated by bacterial infections, causing additional hospitalization and mortality. Secondary bacterial respiratory infection can be subdivided into combined viral/bacterial pneumonia and post-influenza pneumonia, which differ in their pathogenesis. During combined viral/bacterial infection, the virus, the bacterium and the host interact with each other. Post-influenza pneumonia may, at least in part, be due to resolution of inflammation caused by the primary viral infection. These mechanisms restore tissue homeostasis but greatly impair the host response against unrelated bacterial pathogens. In this review we summarize the underlying mechanisms leading to combined viral/bacterial infection or post-influenza pneumonia and highlight important considerations for effective treatment of bacterial pneumonia during and shortly after influenza.
Background on influenza pandemics
Influenza A virus is one of the most prevalent pathogens, causing respiratory illness every winter [1]. These influenza outbreaks are usually associated with mild symptoms, such as fever, headache, sore throat, sneezing and nausea, accompanied by decreased activity and food intake [2]. Nevertheless, influenza virus still accounts for 250,000 to 500,000 deaths each year and this number may increase due to the recently emerged H1N1 pandemic influenza strain [3].
Influenza virus evolves rapidly because of a high mutation rate and may escape acquired immunity [4]. This antigenic drift is the major reason why outbreaks of influenza occur every winter. In addition, the segmented genome of influenza virus also increases the risk of recombination of two or more influenza strains [4]. These major changes in the viral genome, also referred to as antigenic shift, could lead to a pandemic outbreak of influenza [5]. Although influenza virus itself can lead to severe pneumonia, mortality is most often caused by complications of the infection or by pre-existing conditions, such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis or cardiovascular disease [6-9]. Viruses are well known to cause exacerbations of asthma and chronic obstructive pulmonary disease, but the association between influenza virus and cardiovascular disease is less clear. Nevertheless, epidemiological studies indicate that the incidence of myocardial infarction and stroke correlates with the incidence of influenza [10], while influenza vaccination has been shown to reduce the risk of these cardiovascular events. Whether these epidemiological findings correlate with the pro-thrombotic state observed during influenza virus infection is still unclear [11].
Epidemiology of secondary bacterial pneumonia
Bacterial superinfection is a common cause of influenza-related hospitalization of otherwise healthy individuals [12]. Primary influenza virus infection may lead to lower respiratory tract symptoms, but secondary bacterial infections during and shortly after recovery from influenza virus infection are a much more common cause of pneumonia. Although pandemic strains are usually more pathogenic than seasonal influenza strains, the excess mortality rates during pandemics is mainly caused by secondary bacterial pneumonia [13]. Retrospective analysis of post-mortem lung tissue of individuals that died from the 1918 pandemic influenza strain indicated that most of these people also had a bacterial infection. Also, during the influenza pandemic of 1957 more than two-thirds of fatal cases were associated with bacterial pneumonia [14]. Bacteria such as Staphylococcus aureus and Haemophilus influenzae are known to cause post-influenza pneumonia, but Streptococcus pneumoniae is the most prominent pathogen involved [15]. A recent report on the new H1N1 influenza strain indicates that 29% of fatal H1N1 cases between May 2009 and August 2009 in the United States were associated with a secondary bacterial infection [16], which is markedly less than for previous influenza pandemics [17,18]. In addition to S. aureus and S. pneumoniae, Streptococcus pyogenes was also frequently isolated [16,18]. Primary infections with these pathogens are usually less severe than secondary infections. The incidence of invasive pneumococcal disease closely correlates with the influenza season [19], and pneumococcal vaccination not only results in an overall reduced number of pneumonia cases, it also leads to markedly reduced cases of virus-associated pneumonia [20]. Although secondary bacterial pneumonia has been described for other respiratory viruses as well, the morbidity and mortality is much lower than observed for influenza [21,22].
Pathogenesis of bacterial pneumonia with influenza
Bacterial respiratory infection during influenza virus infection can be divided into combined viral/bacterial pneumonia or secondary bacterial infection following influenza. Clinical symptoms do not distinguish between bacterial and viral pneumonia early in the course of disease, rendering early clinical distinction a challenge. Critically ill patients with viral pneumonia present with bilateral interstitial infiltrates on the chest radiograph indistinguishable from bacterial pneumonia [23]. Other markers of inflammation are also not specific. Distinction between viral and bacterial pneumonia by microbiological and/or molecular techniques, however, is highly relevant in terms of initiating antimicrobial therapy, as 32% of patients with viral pneumonia develop a concomitant bacterial pneumonia [23]. Secondary bacterial infections following influenza are more easily recognized clinically compared to combined viral/bacterial pneumonia since these bacterial infections tend to occur during the recovery phase from influenza [24]. Epidemio logical studies indicate that individuals infected with influenza virus are most susceptible to secondary bacterial pneumonia between 4 and 14 days after the onset of influenza symptoms [25].
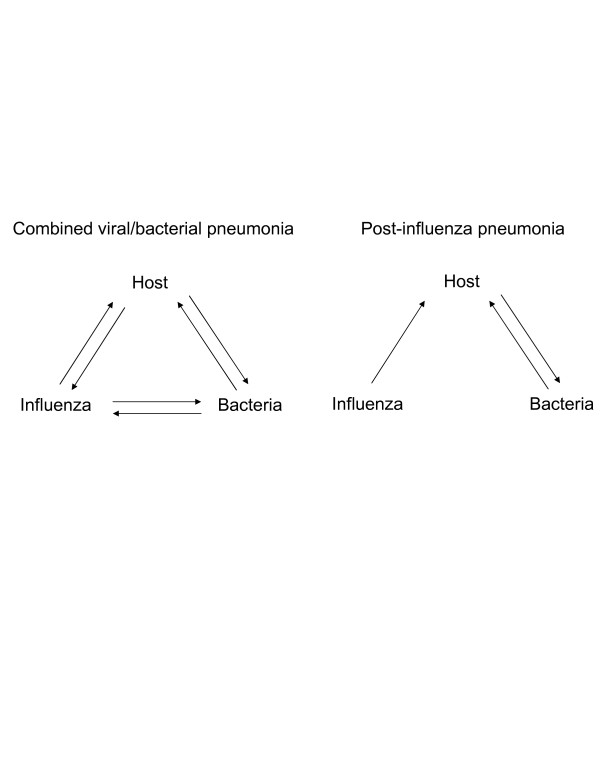
Although the incidence of a secondary bacterial infection does not show a clear distinction between combined viral/bacterial pneumonia and secondary bacterial infection following influenza, the processes leading to severe bacterial pneumonia in conjunction with influenza virus infections are multifactorial and differ between early and late bacterial infection. During combined viral/bacterial infection, the virus not only interacts with the host response, it also interacts with bacterial-induced inflammation, increasing bacterial colonization and outgrowth as well as viral replication (Figure 1). Conversely, the host response to both pathogens will affect viral replication and bacterial growth [26,27]. From a mechanistic point of view, post-influenza pneumonia is less complicated than combined viral/bacterial pneumonia, since the virus has been cleared (Figure 1). The pathogenesis of post-influenza pneumonia involves virus-induced changes to the host [28,29]. These differences are important to take into consideration when studying the mechanisms of secondary bacterial complications and may also have an impact on therapeutic strategies to be followed when patients are hospitalized for influenza complicated by pneumonia.
Figure 1.
Complexity of combined viral/bacterial and post-influenza pneumonia. Severe bacterial pneumonia following influenza can be subdivided into combined viral/bacterial (left) and post-influenza pneumonia (right). During combined viral/bacterial pneumonia, the virus, the bacteria and the host all interact with each other. The severity of post-influenza pneumonia is due to virus-induced changes to the host that affect the course of bacterial infection.
The severity of combined viral/bacterial infection or post-influenza pneumococcal pneumonia is classically attributed to influenza-induced damage to the airway epithelium, which leads to increased colonization of bacteria at the basal membrane [30]. Influenza virus preferentially infects and replicates in airway epithelial cells, leading to the induction of an antiviral process in order to eradicate the virus. Besides limiting viral replication by means of transcriptional and translational inhibition, epithelial cells are instructed to undergo apoptosis [31]. The apoptotic bodies containing the virus are subsequently removed by (alveolar) macrophages [32]. Major drawbacks of this antiviral mechanism include not only the increased risk of bacterial colonization, but also enhanced invasion by bacteria. In addition to epithelial injury, mucociliary clearance has recently been shown to be impaired during influenza virus infection, leading to an enhanced burden of S. pneumoniae already at 2 hours after bacterial challenge [33].
Over the past few years it has become increasingly clear that epithelial injury is not the only factor that contributes to the severe outcome resulting from bacterial complications during influenza infection [27-29,33,34]. Mouse studies have revealed additional mechanisms that play a critical role in either combined viral/bacterial infection or post-influenza pneumococcal pneumonia (summarized in Table 1). Most mouse models that are currently used focus on combined viral/bacterial pneumonia (bacterial challenges up to 7 days after influenza) [25,33-35], while other models are used to investigate post-influenza pneumonia [28,29] (bacterial challenges ranging from 14 days up to 35 days after influenza infection).
Table 1.
Predisposing factors identified for combined viral/bacterial pneumonia and/or post-influenza pneumonia
| Factors associated with combined viral/bacterial infection | Factors associated with post-influenza pneumonia | |
|---|---|---|
| Viral factors | Viral neuraminidase [37,38] | Not involved, that is, virus is cleared [28,29] |
| PB1-F2 [39] | ||
| Bacterial factors | Pneumococcal surface protein A [40] | Unknown |
| Mechanical factors (host) | Epithelial injury [30] | Unknown |
| Mucociliary velocity [33] | ||
| Immune cells (host) | Neutrophil function [34,47,49,51,57] | Neutrophil function [28] |
| Neutrophil recruitment [52,53,55] | Neutrophil recruitment [29] | |
| Neutrophil apoptosis [48,54] | ||
| Macrophages [57,58] | ||
| Monocytes [57] | ||
| Cytokines/chemokines (host) | IFN-γ [59] | IL-10 [28] |
| IFN-α/β [53] | ||
| KC [53] | ||
| MIP-2 [53] | ||
| Pattern recognition receptors (host) | MARCO [59] | TLR2 [29] |
| TLR4 [29] | ||
| TLR5 [29] | ||
| Metabolic enzymes (host) | Unknown | Indoleamine 2,3-dioxygenase [61] |
Abbreviations: IFN, interferon; IL, interleukin; KC, keratinocyte-derived chemokine; MARCO, macrophage receptor with collagenous structure; MIP, macrophage inflammatory protein; TLR, Toll-like receptor
Viral factors contributing to secondary bacterial complications
Several viral factors have been identified as critical for the development of secondary bacterial pneumonia. Viral neuraminidase has been shown to enhance bacterial growth as well as bacterial dissemination in a mouse model for secondary pneumococcal pneumonia. Studies with recombinant influenza strains containing different neuraminidase genes indicate that neuraminidase activity correlates with increased adhesion of pneumococci to airway epithelial cells, which could be reversed by adding neuraminidase inhibitors [36]. Influenza strains with relatively high neuraminidase activity, such as the 1957 pandemic influenza strain, were associated with an increased incidence of pneumococcal pneumonia and higher mortality rates in mice after bacterial challenge [37]. In addition, mice treated with neuraminidase inhibitors for up to 5 days after viral exposure showed markedly increased survival rates. Nevertheless, neuraminidase inhibitors were only partially protective in this model for bacterial complications following influenza virus infection [38].
In addition to neuraminidase, PB1-F2, a pro-apoptotic protein expressed by most influenza A strains, has been implicated in the pathogenesis of secondary bacterial pneumonia as well. Mice infected with viral strains lacking PB1-F2 were largely protected against secondary bacterial complications. In line with this, mice infected with a viral strain that expresses the PB1-F2 protein from the 1918 pandemic influenza strain appeared to be highly susceptible to pneumococcal pneumonia [39]. Since PB1-F2 did not have an impact on bacterial loads and since it has been implicated in the pathogenesis of primary infection with influenza virus, it may be concluded that PB1-F2 induces lung pathology during viral infection, which may enhance the inflammatory response to a secondary challenge. The underlying mechanism of PB1-F2-induced lung pathology is largely unknown.
Bacterial factors contributing to secondary bacterial pneumonia
Bacterial components that contribute to secondary bacterial pneumonia have been poorly investigated. In contrast to viral neuraminidase, bacterial neuraminidase has not been implicated in combined viral/bacterial pneumonia or post-influenza pneumonia [34,37,40]. The fact that bacterial neuraminidase does not contribute to enhanced replication of influenza is most likely due to poor enzymatic activity compared to viral neuraminidase and the strict sialic acid substrate requirements of bacterial neuraminidase.
In contrast, pneumococcal surface protein A (PspA) has been shown to increase bacterial colonization in mice infected with influenza virus [40]. PspA is known to interfere with complement-mediated phagocytosis and lactoferrin-mediated killing. However, it is also identified as a virulence factor for primary pneumococcal pneumonia [41]. As such, PspA seems to have a limited contribution to the severe outcome of bacterial pneumonia with influenza. Similarly, pneumococcal hyaluro nidase has been identified as a virulence factor for primary pneumococcal pneumonia, but did not have an impact on pneumococcal pneumonia following influenza [40].
S. pneumoniae has been shown to bind to the platelet-activating factor receptor (PAFR) through phosphatidyl-choline in the bacterial cell wall [42], which has been suggested to increase colonization of bacteria and/or to mediate transition from the lung to the blood [43]. The impact of this interaction was further investigated using PAFR knockout mice [44,45] and pharmacological inhibitors of PAFR [35]. Although influenza virus has been shown to upregulate the expression of PAFR [43], no studies have identified a more pronounced role for it in secondary pneumococcal pneumonia compared to primary pneumococcal infection [35,44,45]. PAFR appears to mediate invasive pneumococcal disease during primary and secondary pneumococcal pneumonia, while colonization within the lung seems to be dependent on the bacterial strain [43-45].
In conclusion, there is little evidence that bacterial virulence plays an important role in the pathogenesis of secondary pneumococcal pneumonia after influenza. Protease activity by S. aureus has been shown to increase the virulence of influenza A virus in mice by cleaving virus hemagglutinin. However, protease inhibitors have not been further investigated in models of secondary bacterial pneumonia [46].
Host factors contributing to secondary bacterial pneumonia
Most studies on the mechanism underlying bacterial pneumonia following influenza have focused on impaired host defense against secondary infection with an unrelated pathogen. Influenza virus infection has been shown to impair neutrophil function at multiple levels [28,34,47-54]. Initial studies indicated that influenza virus reduces chemotaxis and chemokinesis of neutrophils in vitro and in vivo [55], which appeared to be strain-dependent in subsequent studies with patients infected with influenza virus [52]. In addition to this direct inhibitory mechanism, a recent study identified type I interferon (IFN), an antiviral cytokine, as an important factor in the downregulation of relevant chemokines, such as keratinocyte-derived chemokine and macrophage inflammatory protein 2, thereby inhibiting the migration of neutrophils [53]. However, several studies reported increased, rather than reduced, numbers of neutrophils after secondary bacterial challenge in mice infected with influenza virus [28,34,56]. The increased number of neutrophils may correlate with higher bacterial loads in these models of secondary bacterial pneumonia. The higher bacterial loads might be explained by a reduced phagocytic capacity of neutrophils [28,34,45,57,58]. In vitro studies with ultraviolet irradiated and heat killed influenza virus indicated that the reduction in phagocytic capacity is mediated, at least in part, by viral neuraminidase activity [58]. Nevertheless, the impaired effector function is still present after the virus has been cleared [28], indicating that host factors contribute to impaired bacterial killing. IL-10 production is synergistically enhanced in mice infected with S. pneumoniae during viral infection [38,56] as well as after clearance [28] of influenza virus. Inhibition of IL-10 markedly improved survival in a mouse-model for post-influenza pneumococcal pneumonia, which was associated with reduced bacterial loads. The role of IL-10 in combined viral/bacterial pneumonia seems to be limited, since IL-10 knockout mice did not show an improved response to secondary bacterial infection [59]. It should be noted, however, that IL-10 knockout mice respond differently to primary viral infection as well, leading to a more pronounced proinflammatory state [60]. Together, these findings not only illustrate the complexity of secondary bacterial pneumonia, they also stress that combined viral/bacterial infection is intrinsically different from post-influenza pneumonia.
The tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase (IDO) has been shown to enhance IL-10 levels in a mouse model for post-influenza pneumococcal pneumonia [61]. Inhibition of IDO, which is expressed during the recovery phase of influenza infection, reduced bacterial loads during secondary, but not primary, pneumococcal infection. Despite a clear reduction in bacterial loads as well as markedly reduced levels of IL-10 and TNF-α, it did not have an impact on survival. It is unlikely, therefore, that IDO predisposes for bacterial pneumonia by means of enhancing IL-10 production. Recent observations in our laboratory indicate that local IDO activity induces apoptosis of neutrophils during bacterial infection of the airways (submitted for publication). IDO-mediated apoptosis, which has been extensively studied for T lymphocytes, is particularly mediated by metabolites such as kynurenine and 3-hydroxy anthranilic acid, rather than depletion of tryptophan. Tryptophan metabolites have been implicated in monocyte and macrophage apoptosis as well [62,63]. Together, these data indicate that IDO functions as a natural mechanism to remove inflammatory cells. This mechanism to resolve inflammation prevents excessive damage to the airways after viral infection, but increases the susceptibility to secondary bacterial pneumonia.
In addition to neutrophils, macrophages and monocytes [58,64] have also been shown to have a reduced phagocytic capacity during influenza infection. IFN-γ has been shown to play a critical role in macrophage dysfunction through downregulation of 'macrophage receptor with collagenous structure' (MARCO) expression on alveolar macrophages [65]. MARCO can be classified as a scavenger receptor involved in the innate recognition and subsequent killing of bacteria. MARCO knockout mice have been shown to be more susceptible to pneumococcal pneumonia, which was associated with higher bacterial loads, enhanced lung pathology and increased mortality rates [63]. Although other factors that mediate opsonization or phagocytosis of bacteria have been extensively studied for primary bacterial pneumonia [66-68], their roles in either combined viral/bacterial pneumonia or post-influenza pneumonia are largely unknown.
Knowledge about the role of other pattern recognition receptors, such as Toll-like receptors (TLRs), is limited. A recent study indicated that influenza virus infection resulted in sustained desensitization of TLRs for up to 6 weeks after influenza virus infection [29]. Mice exposed to influenza virus exert a poor response to lipopolysaccharide, lipoteichoic acid and flagellin, ligands for TLR4, TLR2 and TLR5, respectively, as reflected by reduced neutrophil numbers in bronchoalveolar lavage fluid. These data are supported by the fact that TLR2 knockout mice were equally susceptible to secondary bacterial pneumonia following influenza virus infection compared to wild-type mice [69]. It is worth noting that TLR4 can compensate for a defect in TLR2 during primary pneumococcal pneumonia [70]. In addition to TLR desensitization, CD200R expression has been proposed to impair the host response towards bacteria during influenza virus infection [71]. Although CD200- CD200R interactions have been shown to negatively regulate inflammation through induction of IDO [72], its role in secondary bacterial pneumonia has not been investigated yet.
Taken together, these host factors contributing to severe post-influenza pneumonia all relate to altered innate immune mechanisms that are supposed to resolve or dampen virus-induced inflammation and related tissue damage. It should be noted that most studies have been performed using mouse models for combined viral/bacterial pneumonia or post-influenza bacterial pneumonia and require confirmation in humans.
Current treatment options
Vaccination against influenza has been shown to reduce mortality rates during influenza epidemics [73]. Seasonal influenza epidemics are primarily caused by antigenic drift (that is, single-point mutations that are caused by the high mutation rate of influenza virus strains). Although single-point mutations occur at random, genetic changes can be predicted in advance [74]. These predictions provide the opportunity to develop vaccines to prevent seasonal influenza and therefore also the risk of secondary bacterial infections. Vaccination of elderly patients has been shown to reduce hospitalizations by 52%. In contrast to seasonal influenza, pandemic influenza, such as caused by the recently emerged H1N1 strain [3,75], results from antigenic shift. It is hard to predict when these changes occur and which strains are involved. It is virtually impossible, therefore, to develop vaccines directed against pandemic influenza strains in advance. Vaccines against new influenza strains only become available when the vaccine has been validated extensively.
Besides vaccination, treatment options to prevent a complicated course of influenza is to inhibit viral replication with antiviral agents, such as amantadine (Symmetrel®), or neuraminidase inhibitors, such as oseltamivir (Tamiflu®) and zanamivir (Relenza®). These agents have been shown to reduce influenza-related symptoms [76-78], but their efficacy against bacterial complications remains to be determined [79]. Viral neuraminidase has been shown to be involved in the enhanced response to bacteria in a mouse model for post-influenza pneumococcal pneumonia [37]. Moreover, mice treated with neuraminidase inhibitors were less susceptible to secondary bacterial infections. However, neuraminidase inhibitors did not completely prevent mortality in mice with influenza complicated by bacterial pneumonia, which may relate to the relatively small time-window in which neuraminidase inhibitors can reduce viral replication [80]. In addition, the efficacy of neuraminidase inhibitors in established viral/bacterial pneumonia was not tested. Rimantadine, an amantadine analogue, did not improve mortality in mice with postinfluenza pneumococcal pneumonia [33]. The efficacy of these inhibitors in the treatment of bacterial complications in humans has not been established yet. These approaches mainly focus on the prevention of secondary bacterial pneumonia.
Patients with community-acquired pneumonia who demonstrate or have demonstrated signs and symptoms of illness compatible with influenza in the days or weeks before should be empirically treated with antibiotics targeting S. pneumoniae and S. aureus in order to cover the most common pathogens causing the most severe secondary infections, and coverage of H. influenzae is also recommended [81]. Appropriate antimicrobial agents therefore include cefotaxime, ceftriaxone and respiratory fluoroquinolones. As mentioned above, combined infection needs to be confirmed by microbiological and molecular techniques. When samples from respiratory tract are proven culture negative, antibiotics can be stopped. Treatment targeted at methicillin-resistant S. aureus (by vancomycin or linezolid) should be limited to patients with confirmed infection or a compatible clinical presentation (shock and necrotizing pneumonia) [80]. Of note, mouse studies indicate that ampicillin treatment is insufficient to prevent mortality in a model for secondary bacterial pneumonia, while the bacteriostatic protein synthesis inhibitors clindamycin or azithromycin improve the outcome after streptococcal pneumonia in influenza-infected mice [82]. This protective effect is likely mediated by inhibition of toxin release [82], but it may be associated with the anti-inflammatory properties of these latter antimicrobial agents as well [83,84]. Although ampicillin alone did not have an impact on survival in influenza-infected mice with secondary pneumococcal pneumonia, it did improve mortality rates in mice previously treated with oseltamivir compared to mice treated with oseltamivir alone [37].
Future perspectives
Secondary bacterial complications are the result of an altered host response due to influenza virus infection. Most factors that have been identified to play a critical role in post-influenza pneumococcal pneumonia are in fact mechanisms to prevent excessive inflammation and/or to promote resolution of inflammation, which are initiated to restore tissue homeostasis after clearance of the primary infection. At the same time, these mechanisms greatly impair the host response towards secondary unrelated pathogens. Cytokines and chemokines appear to play a critical role in dampening virus-induced immunopathology. IFN-γ and IL-10 have been shown to alter macrophage and neutrophil function, respectively, while type I IFN seems to impair neutrophil recruitment after secondary bacterial infection. In addition, IDO expression is induced by proinflammatory cytokines such as TNF-α, IFN-γ, IL-12 and IL-18, leading to apoptosis of inflammatory cells. Although the contribution of these mediators needs to be confirmed in humans, targeting cytokines may be an alternative approach to trigger an effective host response to bacteria. Although it is practically not feasible to neutralize these inflammatory mediators as prophylactic treatment to prevent secondary bacterial pneumonia in all influenza-infected subjects, it may be a useful approach in hospitalized subjects, especially those that are admitted to the intensive care unit.
Conclusion
Influenza may be complicated by bacterial pneumonia. It is important to consider the time interval between viral and bacterial infection. At present, antibiotic treatment appears to be the only therapeutic option for postinfluenza pneumonia. Further insight into the underlying mechanisms in combined viral/bacterial infection and post-influenza pneumonia may provide new targets for the treatment of these complicated infections.
Abbreviations
IDO: indoleamine 2,3-dioxygenase; IFN: interferon; IL: interleukin; MARCO: macrophage receptor with collagenous structure; PAFR: platelet-activating factor receptor; PspA: pneumococcal surface protein A; TLR: Toll-like receptor; TNF: tumor necrosis factor.
Note
This article is part of a review series on Influenza, edited by Steven Opal. Other articles in the series can be found online at http://ccforum.com/series/influenza
Competing interests
The authors declare that they have no competing interests.
See related commentary by Wunderink, http://ccforum.com/content/14/3/150
Contributor Information
Koenraad F van der Sluijs, Email: kvandersluijs@amc.uva.nl.
Tom van der Poll, Email: t.vanderpoll@amc.uva.nl.
René Lutter, Email: r.lutter@amc.uva.nl.
Nicole P Juffermans, Email: n.p.juffermans@amc.uva.nl.
Marcus J Schultz, Email: m.j.schultz@amc.uva.nl.
References
- Monto AS. Epidemiology of influenza. Vaccine. 2008;26:D45–48. doi: 10.1016/j.vaccine.2008.07.066. [DOI] [PubMed] [Google Scholar]
- Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5:718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Molecular evolution of influenza viruses. Virus Genes. 1995;11:209–215. doi: 10.1007/BF01728660. [DOI] [PubMed] [Google Scholar]
- Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002;20:3068–3087. doi: 10.1016/S0264-410X(02)00254-2. [DOI] [PubMed] [Google Scholar]
- Glezen WP. Asthma, influenza, and vaccination. J Allergy Clin Immunol. 2006;118:1199–1206. doi: 10.1016/j.jaci.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Mallia P, Johnston SL. Influenza infection and COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:55–64. doi: 10.2147/copd.2007.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Saiman L. Pulmonary infections in patients with cystic fibrosis. Semin Respir Infect. 2002;17:47–56. doi: 10.1053/srin.2002.31690. [DOI] [PubMed] [Google Scholar]
- Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 2008;130:304–309. doi: 10.1016/j.ijcard.2008.04.044. [DOI] [PubMed] [Google Scholar]
- Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- Keller TT, Sluijs KF van der, de Kruif MD, Gerdes VE, Meijers JC, Florquin S, Poll T van der, van Gorp EC, Brandjes DP, Büller HR, Levi M. Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ Res. 2006;99:1261–1269. doi: 10.1161/01.RES.0000250834.29108.1a. [DOI] [PubMed] [Google Scholar]
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers JF, Masurel N, Mulder J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet. 1958;2:1141–1143. doi: 10.1016/S0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- de Roux A, Ewig S, García E, Marcos MA, Mensa J, Lode H, Torres A. Mixed community-acquired pneumonia in hospitalised patients. Eur Respir J. 2006;27:795–800. doi: 10.1183/09031936.06.00058605. [DOI] [PubMed] [Google Scholar]
- Grabowska K, Höberg L, Penttinen P, Svensson A, Ekdahl K. Occurrence of invasive pneumococcal disease and number of excess cases due to influenza. BMC Infect Dis. 2006;6:58. doi: 10.1186/1471-2334-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–1074. [PubMed] [Google Scholar]
- Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, Vugia D, Harriman K, Matyas B, Glaser CA, Samuel MC, Rosenberg J, Talarico J, Hatch D. California Pandemic (H1N1) Working Group. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med. 2010;38:e91–7. doi: 10.1097/CCM.0b013e3181c92eeb. [DOI] [PubMed] [Google Scholar]
- Khater F, Moorman JP. Complications of influenza. South Med J. 2003;96:740–743. doi: 10.1097/01.SMJ.0000084984.13843.C0. [DOI] [PubMed] [Google Scholar]
- Oliveira EC, Marik PE, Colice G. Influenza pneumonia: a descriptive study. Chest. 2001;119:1717–1723. doi: 10.1378/chest.119.6.1717. [DOI] [PubMed] [Google Scholar]
- Talbot TR, Poehling KA, Hartert TV, Arbogast PG, Halasa NB, Edwards KM, Schaffner W, Craig AS, Griffin MR. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am J Med. 2005;118:285–291. doi: 10.1016/j.amjmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Patel J, Faden H, Sharma S, Ogra PL. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. Int J Pediatr Otorhinolaryngol. 1992;23:15–23. doi: 10.1016/0165-5876(92)90075-Z. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA. Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- Madhi SA, Klugman KP. Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M, Clezy K, Lindley R, Pearce R. Pandemic influenza: clinical issues. Med J Aust. 2006;185:S44–S47. doi: 10.5694/j.1326-5377.2006.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Jakab GJ. Mechanisms of bacterial superinfections in viral pneumonias. Schweiz Med Wschr. 1985;115:75–86. [PubMed] [Google Scholar]
- Jones WT, Menna JH, Wennerstrom DE. Lethal synergism induced in mice by influenza type A virus and type Ia group B streptococci. Infect Immun. 1983;41:618–623. doi: 10.1128/iai.41.2.618-623.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs KF van der, van Elden LJ, Nijhuis M, Schuurman R, Pater JM, Florquin S, Goldman M, Jansen HM, Lutter R, Poll T van der. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkowski MC, Puchelle E, Beck G, Jacquot J, Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986;134:1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- Brydon EW, Smith H, Sweet C. Influenza A virus-induced apoptosis in bronchiolar epithelial (NCI-H292) cells limits pro-inflammatory cytokine release. J Gen Virol. 2003;84:2389–2400. doi: 10.1099/vir.0.18913-0. [DOI] [PubMed] [Google Scholar]
- Fujimoto I, Pan J, Takizawa T, Nakanishi Y. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. J Virol. 2000;74:3399–3403. doi: 10.1128/JVI.74.7.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae . Am J Respir Cell Mol Biol. 2010;42:450–460. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine AM, Koeningsknecht V, Stark JM. Decreased pulmonary clearance of S. pneumoniae following influenza A infection in mice. J Virol Methods. 2001;94:173–186. doi: 10.1016/S0166-0934(01)00287-7. [DOI] [PubMed] [Google Scholar]
- McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae : characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–350. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae . J Infect Dis. 2003;187:1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis. 2005;192:249–257. doi: 10.1086/430954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis. 2004;190:519–526. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]
- McAuley JL, Hornung F, Boyd KL, Smith AM, McKeon R, Bennink J, Yewdell JW, McCullers JA. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe. 2007;2:240–249. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King QO, Lei B, Harmsen AG. Pneumococcal surface protein A contributes to secondary Streptococcus pneumoniae infection after influenza virus infection. J Infect Dis. 2009;200:537–545. doi: 10.1086/600871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AM, Paton JC. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000;68:133–140. doi: 10.1128/IAI.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- McCullers JA, Iverson AR, McKeon R, Murray PJ. The platelet activating factor receptor is not required for exacerbation of bacterial pneumonia following influenza. Scand J Infect Dis. 2008;40:11–17. doi: 10.1080/00365540701477568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs KF van der, van Elden LJ, Nijhuis M, Schuurman R, Florquin S, Shimizu T, Ishii S, Jansen HM, Lutter R, Poll T van der. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L194–L199. doi: 10.1152/ajplung.00050.2005. [DOI] [PubMed] [Google Scholar]
- Rijneveld AW, Weijer S, Florquin S, Speelman P, Shimizu T, Ishii S, Poll T van der. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J Infect Dis. 2004;189:711–716. doi: 10.1086/381392. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Klenk HD, Rott R. Inhibitory effect of a protease inhibitor, leupeptin, on the development of influenza pneumonia, mediated by concomitant bacteria. J Gen Virol. 1987;68:2039–2041. doi: 10.1099/0022-1317-68-7-2039. [DOI] [PubMed] [Google Scholar]
- McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun. 2006;74:6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelich G, White M, Hartshorn KL. Neutrophil survival is markedly reduced by incubation with influenza virus and Streptococcus pneumoniae : role of respiratory burst. J Leukoc Biol. 2001;69:50–56. [PubMed] [Google Scholar]
- Abramson JS, Hudnor HR. Effect of priming polymorphonuclear leukocytes with cytokines (granulocyte-macrophage colony-stimulating factor [GM-CSF] and G-CSF) on the host resistance to Streptococcus pneumoniae in chinchillas infected with influenza A virus. Blood. 1994;83:1929–1934. [PubMed] [Google Scholar]
- Cassidy LF, Lyles DS, Abramson JS. Depression of polymorphonuclear leukocyte functions by purified influenza virus hemagglutinin and sialic acid-binding lectins. J Immunol. 1989;142:4401–4406. [PubMed] [Google Scholar]
- Verhoef J, Mills EL, Debets-Ossenkopp Y, Verbrugh HA. The effect of influenza virus on oxygen-dependent metabolism of human neutrophils. Adv Exp Med Biol. 1982;141:647–654. doi: 10.1007/978-1-4684-8088-7_64. [DOI] [PubMed] [Google Scholar]
- Larson HE, Parry RP, Tyrrell DA. Impaired polymorphonuclear leucocyte chemotaxis after influenza virus infection. Br J Dis Chest. 1980;74:56–62. doi: 10.1016/0007-0971(80)90008-X. [DOI] [PubMed] [Google Scholar]
- Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamussi ML, White MR, Crouch E, Hartshorn KL. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999;93:2395–2403. [PubMed] [Google Scholar]
- Ruutu P, Vaheri A, Kosunen TU. Depression of human neutrophil motility by influenza virus in vitro . Scand J Immunol. 1977;6:897–906. doi: 10.1111/j.1365-3083.1977.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Smith MW, Schmidt JE, Rehg JE, Orihuela CJ, McCullers JA. Induction of pro- and anti-inflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp Med. 2007;57:82–89. [PMC free article] [PubMed] [Google Scholar]
- Abramson JS, Mills EL, Giebink GS, Quie PG. Depression of monocyte and polymorphonuclear leukocyte oxidative metabolism and bactericidal capacity by influenza A virus. Infect Immun. 1982;35:350–355. doi: 10.1128/iai.35.1.350-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets-Ossenkopp Y, Mills EL, van Dijk WC, Verbrugh HA, Verhoef J. Effect of influenza virus on phagocytic cells. Eur J Clin Microbiol. 1982;1:171–177. doi: 10.1007/BF02019619. [DOI] [PubMed] [Google Scholar]
- Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs KF van der, Nijhuis M, Levels JH, Florquin S, Mellor AL, Jansen HM, Poll T van der, Lutter R. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis. 2006;193:214–222. doi: 10.1086/498911. [DOI] [PubMed] [Google Scholar]
- Morita T, Saito K, Takemura M, Maekawa N, Fujigaki S, Fujii H, Wada H, Takeuchi S, Noma A, Seishima M. 3-Hydroxyanthranilic acid, an L-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-gamma. Ann Clin Biochem. 2001;38:242–251. doi: 10.1258/0004563011900461. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- Astry CL, Jakab GJ. Influenza virus-induced immune complexes suppress alveolar macrophage phagocytosis. J Virol. 1984;50:287–292. doi: 10.1128/jvi.50.2.287-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000;68:2286–2293. doi: 10.1128/IAI.68.4.2286-2293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F, Lee ME, Iannelli F, Pozzi G, Mitchell TJ, Read RC, Dockrell DH. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcgamma receptors correlates with intracellular bacterial load. J Infect Dis. 2003;188:1119–1131. doi: 10.1086/378675. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, Korfhagen TR. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol. 2000;165:3934–3940. doi: 10.4049/jimmunol.165.7.3934. [DOI] [PubMed] [Google Scholar]
- Dessing MC, Sluijs KF van der, Florquin S, Akira S, Poll T van der. Toll-like receptor 2 does not contribute to host response during postinfluenza pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2007;36:609–614. doi: 10.1165/rcmb.2006-0166OC. [DOI] [PubMed] [Google Scholar]
- Dessing MC, Florquin S, Paton JC, Poll T van der. Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell Microbiol. 2008;10:237–246. doi: 10.1111/j.1462-5822.2007.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T, Cavanagh MM. The innate immune rheostat: influence on lung inflammatory disease and secondary bacterial pneumonia. Biochem Soc Trans. 2009;37:811–813. doi: 10.1042/BST0370811. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173:3748–3754. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258–264. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Jin G, Zhu J, Zhou R. Using a mutual information-based site transition network to map the genetic evolution of influenza A/H3N2 virus. Bioinformatics. 2009;25:2309–2317. doi: 10.1093/bioinformatics/btp423. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron§ JM, Penn CR. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- Kim CU, Lew W, Williams MA, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen MS, Mendel DB, Tai CY, Laver WG, Stevens RC. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- Davies WL, Grunert RR, Haff RF, Mcgahen JW, Neumayer EM, Paulshock M, Watts JC, Wood TR, Hermann EC, Hoffmann CE. Antiviral activity of 1-Adamantanamine (Amantadine) Science. 1964;144:862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- Ruf BR, Szucs T. Reducing the burden of influenza-associated complications with antiviral therapy. Infection. 2009;37:186–196. doi: 10.1007/s15010-009-8241-1. [DOI] [PubMed] [Google Scholar]
- Crusat M, de Jong MD. Neuraminidase inhibitors and their role in avian and pandemic influenza. Antivir Ther. 2007;12:593–602. [PubMed] [Google Scholar]
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlström A, Boyd KL, English BK, McCullers JA. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J Infect Dis. 2009;199:311–319. doi: 10.1086/596051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaryo T, Oishi K, Yoshimine H, Tsuchihashi Y, Matsushima K, Nagatake T. Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob Agents Chemother. 2003;47:48–53. doi: 10.1128/AAC.47.1.48-53.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterre PF, Garber G, Levy H, Wunderink R, Kinasewitz GT, Sollet JP, Maki DG, Bates B, Yan SC, Dhainaut JF. PROWESS Clinical Evaluation Committee. Severe community-acquired pneumonia as a cause of severe sepsis: data from the PROWESS study. Crit Care Med. 2005;33:952–961. doi: 10.1097/01.CCM.0000162381.24074.D7. [DOI] [PubMed] [Google Scholar]