Abstract
Introduction
Discrepancies of 5-24% between superior vena cava oxygen saturation (ScvO2) and mixed venous oxygen saturation (SvO2) have been reported in patients with severe heart failure. Thenar muscle tissue oxygenation (StO2) measured with near-infrared spectroscopy (NIRS) during arterial occlusion testing decreases slower in sepsis/septic shock patients (lower StO2 deoxygenation rate). The StO2 deoxygenation rate is influenced by dobutamine. The aim of this study was to determine the relationship between the StO2 deoxygenation rate and the ScvO2-SvO2 discrepancy in patients with severe left heart failure and additional sepsis/septic shock treated with or without dobutamine.
Methods
Fifty-two patients with severe left heart failure due to primary heart disease with additional severe sepsis/septic shock were included. SvO2 and ScvO2 were compared to the thenar muscle StO2 before and during arterial occlusion.
Results
SvO2 correlated significantly with ScvO2 (Pearson correlation 0.659, P = 0.001), however, Bland Altman analysis showed a clinically important difference between both variables (ScvO2-SvO2 mean 72 ± 8%, ScvO2-SvO2 difference 9.4 ± 7.5%). The ScvO2-SvO2 difference correlated with plasma lactate (Pearson correlation 0.400, P = 0.003) and the StO2 deoxygenation rate (Pearson correlation 0.651, P = 0.001). In the group of patients treated with dobutamine, the ScvO2-SvO2 difference correlated with plasma lactate (Pearson correlation 0.389, P = 0.011) and the StO2 deoxygenation rate (Pearson correlation 0.777, P = 0.0001).
Conclusions
In patients with severe heart failure with additional severe sepsis/septic shock the ScvO2-SvO2 discrepancy presents a clinical problem. In these patients the skeletal muscle StO2 deoxygenation rate is inversely proportional to the difference between ScvO2 and SvO2; dobutamine does not influence this relationship. When using ScvO2 as a treatment goal, the NIRS measurement may prove to be a useful non-invasive diagnostic test to uncover patients with a normal ScvO2 but potentially an abnormally low SvO2.
Trial Registration
NCT00384644 ClinicalTrials.Gov.
Introduction
Maintenance of adequate oxygen delivery (DO2) is essential to preserve organ function, because a sustained low DO2 leads to organ failure and death [1]. Low cardiac output states (cardiogenic, hypovolemic and obstructive types of shock), anemic and hypoxic hypoxemia are characterized by a decreased DO2 but a preserved oxygen extraction ratio. In distributive shock, the oxygen extraction capability is altered so that the critical oxygen extraction ratio is typically decreased [2]. Measurement of mixed venous oxygen saturation (SvO2) from the pulmonary artery is used for calculations of oxygen consumption and has been advocated as an indirect index of tissue oxygenation and a prognostic predictor in critically ill patients [3-6]. However, catheterization of the pulmonary artery is costly, has inherent risks and its usefulness remains under debate [7,8].
Not surprisingly the monitoring of central venous oxygen saturation (ScvO2) was suggested as a simpler and cheaper assessment of global DO2 to oxygen consumption ratio [1,2].
A concern with ScvO2 compared with mixed venous oxygen saturation (SvO2) is that it may not accurately reflect global hypoxia, because organs with capillary beds that drain into the inferior vena cava or coronary sinus will not be involved in this measurement. Healthy resting individuals have a ScvO2 that is slightly lower than the SvO2 [3]. In heart failure and shock, however, this situation is reversed. Most authors attribute this pattern to changes in the distribution of cardiac output that occur in periods of haemodynamic instability. In shock states, blood flow to the splanchnic and renal circulations fall, while flow to the heart and brain is maintained due to redistribution of blood away from the mesenteric and renal vascular beds and additional right heart dysfunction [4]. Discrepancies of 5 to 24% have been reported [5-7,9].
Near infrared spectroscopy (NIRS) is a technique used for continuous, non-invasive, bedside monitoring of tissue oxygen saturation (StO2) [8,10].
We have previously shown that skeletal muscle StO2 does not estimate SvO2 in patients with severe left heart failure and additional severe sepsis or septic shock. However, in patients with severe left heart failure without additional severe sepsis or septic shock, StO2 values could be used for fast noninvasive SvO2 estimation; the trend of StO2 may be substituted for the trend of SvO2 [8].
We have also shown that thenar skeletal muscle StO2 during stagnant ischemia (deoxygenation rate during arterial occlusion test) decreases slower in septic shock patients compared with patients with severe sepsis or localized infection or healthy volunteers [10].
Impaired skeletal muscle microcirculation, especially impaired deoxygenation rate during arterial occlusion test, was recently detected in patients with chronic heart failure. Dobutamine, but not levosimendan, partially reversed this impairment [11].
The aim of current study was to combine our previous findings. We tested the hypothesis that in patients with severe left heart failure and additional sepsis/septic shock the skeletal muscle deoxygenation rate during an arterial occlusion test could predict a ScvO2-SvO2 discrepancy. The second aim was to explore the effect of dobutamine treatment on any ScvO2-SvO2 discrepancy.
Materials and methods
Patients
The study protocol was approved by the National Ethics Committee of Slovenia; informed consent was obtained from all patients or their relatives. The study was performed between October 2004 and June 2007.
After initial hemodynamic resuscitation according to early goal-directed therapy [12] and Surviving Sepsis Campaign guidelines [13], transthoracic echocardiography for the assessment of left ventricular volume, ejection fraction (Simpson's rule) and valvular function was performed in all patients admitted to our ICU (Hewlett-Packard HD 5000, Hewlett Packard, Andover, MA, USA) by experienced ICU doctors (HM and MP) trained in echocardiography.
In patients with primary heart disease, low cardiac output, and no signs of hypovolemia, a right heart catheterization with a pulmonary artery floating catheter (Swan-Ganz CCOmboV CCO/SvO2/CEDV, Edwards Lifesciences, Irvine, CA, USA) was performed following a decision of the treating physician. The site of insertion was confirmed by the transducer waveform, the length of catheter insertion, and chest radiography. Systemic arterial pressure was measured invasively using radial or femoral arterial catheterization. Consecutive patients with severe left heart failure due to primary heart disease (left ventricular systolic ejection fraction below 40%, pulmonary artery occlusion pressure above 18 mmHg) and additional severe sepsis/septic shock were included in our study. Severe sepsis and septic shock were defined according to the 1992 American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) consensus conference definitions [14]. Patients with heart failure confirmed by echocardiography without sepsis/septic shock were excluded. Patients with cachexia were not included.
Patients were divided into two groups depending on treatment with dobutamine or not.
All patients received standard treatment of localized infection, severe sepsis and septic or cardiogenic shock including: source control, fluid infusion, catecholamine infusion, organ failure replacement and/or support therapy, intensive control of blood glucose and corticosteroid substitution therapy according to current Surviving Sepsis Campaign Guidelines [13]. Mechanically ventilated patients were sedated with midazolam and/or propofol infusion. Paralytic agents were not used.
Measurements
Skeletal muscle oxygenation
Thenar muscle StO2 was measured non-invasively by NIRS (25 mm Probe, InSpectra™, Hutchinson Technology Inc., West Highland Park Drive NE, MN, USA) [8,10,15]. Maximal thenar muscle StO2 was located by moving the probe over the thenar prominence. StO2 was continuously monitored and stored onto a computer using InSpectra™ software. The average of StO2 changing over a 15 second span was used. The arterial occlusion test was performed as previously reported [10]: StO2 was monitored before and during (StO2 deoxygenation rate) upper limb ischemia until StO2 decreased to 40%. Upper limb ischemia was induced by rapid automatic pneumatic cuff inflation (to 260 mmHg) placed above the elbow.
Severity of disease
Sepsis-related Organ Failure Assessment (SOFA) score was calculated at the time of each measurement to assess the level of organ dysfunction [16]. Dobutamine and norepinephrine requirement represented the dose of drug during the StO2 measurement. Use of an intra-aortic balloon pump during the ICU stay is reported.
Plasma lactate concentration was measured using an enzymatic colorimetric method (Lactate, Roche Diagnostics, Hoffman-La Roche, Basel, Switzerland) at the time of each StO2 measurement.
Laboratory analysis
Blood was withdrawn from the superior vena cava approximately 2 cm above the right atrium and from the pulmonary artery at the time of each StO2 measurement to determine ScvO2 (%) and SvO2 (%), respectively. In view of known problems arising during sampling from the pulmonary artery, including the possibility of contaminating arterial blood with pulmonary capillary blood, all samples from this site were withdrawn over 30 seconds, using a low-negative pressure technique, without inflating the balloon. A standard volume of 1 mL of blood was obtained from each side after withdrawal of dead-space blood and flushing fluid. All measurements were made using a cooximeter (RapidLab 1265, Bayer HealthCare, Leverkusen, Germany).
Data analysis
A sample size of 41 patients was estimated for a correlation coefficient of 0.6 with a desired power o f0.95 and alpha of 0.01 (SigmaPlot 2004 for Windows, version 9.01 SyStat Software, Inc., Chicago, IL, USA).
Data was expressed as mean ± standard deviation (SD). The Mann Whitney non-parametric test was used to compare groups. A P value of less than 0.05 was considered statistically significant. The Pearson correlation test was applied to determine correlation (SPSS 10.0 for Windows™, SPSS Inc., Chicago, IL, USA). In order to compare ScvO2 and SvO2 we calculated bias, systemic disagreement between measurements (mean difference between two measurements), precision and the random error in measuring (SD of bias) [17]. The 95% limits of agreement were arbitrarily set following Bland and Altman as the bias ± two SD.
Results
During the study period (20 months), 2,121 patients were admitted to the 15-bed university center internal medicine ICU. In that period 151 right heart catheterizations were performed. The final sample of 52 patients was reached after exclusion of 65 patients with heart failure without sepsis/septic shock, 24 patients who did not have heart failure, 2 patients for whom consent was not given and 8 patients for whom NIRS measurements were not performed. The detailed description of our selected population is given in Table 1. Patients were all mechanically ventilated.
Table 1.
Description of patients
| Parameter | All (n = 52) |
Treatment with dobutemine (n = 43) |
Treatment without dobutamine (n = 9) |
P value |
|---|---|---|---|---|
| Age (years) | 68 ± 13 | 68 ± 14 | 69 ± 8 | 0.8 |
| Female (n) | 7 | 5 | 2 | 0.6 |
| Heart disease | ||||
| Ischemic heart disease (n) | 42 | 36 | 6 | 0.4 |
| Aortic stenosis (n) | 6 | 4 | 2 | 0.6 |
| Dilated cardiomyopathy (n) | 1 | 1 | 0 | 0.9 |
| Myocarditis (n) | 3 | 2 | 1 | 0.6 |
| Echocardiography | ||||
| LVEF (%) | 28 ± 5 | 25 ± 8 | 29 ± 9 | 0.1 |
| LVEDD (cm) | 5.8 ± 0.9 | 5.8 ± 0.7 | 6.0 ± 0.9 | 0.2 |
| Severe mitral regurgitation (n) | 26 | 22 | 4 | 0.8 |
| Cause of infection | ||||
| Pneumonia (n) | 45 | 38 | 7 | 0.6 |
| Urosepsis (n) | 5 | 4 | 1 | 0.9 |
| Other (n) | 2 | 1 | 1 | 0.7 |
| SOFA score | 12.2 ± 2.5 | 12. ± 2.2 | 12.6 ± 2.6 | 0.8 |
| ICU stay (days) | 9 ± 4 | 9 ± 6 | 9 ± 5 | 0.9 |
| ICU survival (%) | 48 | 47 | 55 | 0.8 |
LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; SOFA, Sequential Organ Failure Assessment.
Intra-aortic balloon pumps were inserted in patients who were treated with percutaneous coronary intervention and stent implantation after primary cardiac arrest due to ST-elevation myocardial infarction (STEMI; n = 42) and cardiogenic shock. Patients with STEMI after cardiac arrest were treated with medically induced hypothermia for 24 hours. During the ICU stay and before study inclusion they all developed pneumonia. All other patients were admitted to the ICU primarily because of sepsis or septic shock.
Forty-three patients were treated with dobutamine. There was no difference between patients treated with or without dobutamine in additional hemodynamic support (Table 2). Patients treated with dobutamine had a lower cardiac index (Table 3) and a higher procalcitonin value (Table 4).
Table 2.
Treatment of patients
| Treatment | All (n = 52) |
Treatment with dobutemine (n = 43) |
Treatment without dobutamine (n = 9) |
P value |
|---|---|---|---|---|
| Norepinephrine (mg/h, n) | 0.09 ± 0.10 (43) | 0.08 ± 0.11 (37) |
0.04 ± 0.06 (9) |
0.1 |
| Dobutamine (μg/kg/min) | - | 0.47 ± 0.25 | - | - |
| Levosimendan (n) | 23 | 17 | 6 | 0.2 |
| IAPB (n) | 20 | 15 | 5 | 0.3 |
| Mechanical ventilation(n) | 52 | 43 | 9 | 1.0 |
| FiO2 | 0.72 ± 0.22 | 0.73 ± 0.23 | 0.71 ± 0.23 | 0.8 |
FiO2, fractional inspired oxygen; IAPB, intra-aortic balloon pump.
Table 3.
Hemodynamic data in patients with heart failure and additional sepsis treated with and without dobutamine
| Hemodynamic data | All (n = 52) |
Treatment with dobutemine (n = 43) |
Treatment without dobutamine (n = 9) |
P value |
|---|---|---|---|---|
| HR (bpm) | 113 ± 20 | 113 ± 20 | 114 ± 21 | 0.8 |
| SAP (mmHg) | 118 ± 21 | 117 ± 20 | 124 ± 27 | 0.9 |
| DAP (mmHg) | 74 ± 22 | 76 ± 22 | 66 ± 21 | 0.4 |
| PAPs (mmHg) | 57 ± 14 | 56 ± 13 | 57 ± 16 | 0.9 |
| PAPd (mmHg) | 28 ± 8 | 27 ± 8 | 29 ± 7 | 0.4 |
| CVP (mmHg) | 16 ± 5 | 16 ± 5 | 15 ± 5 | 0.8 |
| DO2 (ml/kg/min) | 406 ± 128 | 391 ± 134 | 470 ± 121 | 0.1 |
| VO2 (ml/kg/min) | 118 ± 42 | 116 ± 43 | 126 ± 38 | 0.5 |
| PAOP (mmHg) | 23 ± 7 | 24 ± 7 | 22 ± 8 | 0.7 |
| CI (L/min/m2) | 2.5 ± 0.7 | 2.4 ± 0.7 | 2.9 ± 0.6 | 0.03 |
| SvO2 (%) | 67 ± 10% | 66 ± 10 | 71 ± 7 | 0.2 |
| ScvO2 (%) | 77 ± 8% | 77 ± 7 | 78 ± 10 | 0.6 |
Bold: statistically significant difference, P < 0.05.
CI, cardiac index; CVP, central venous pressure; DAP, diastolic arterial pressure; DO2, delivery of oxygen; HR, heart rate; PAOP, pulmonary artery occlusion pressure; PAPd, diastolic pulmonary arterial pressure; PAPs, systolic pulmonary arterial pressure; SAP, systolic arterial pressure; SvO2, mixed venous hemoglobin saturation; ScvO2, central venous oxygen saturation; VO2, oxygen consumption.
Table 4.
Laboratory data
| Laboratory data | All (n = 52) |
Treatment with dobutemine (n = 43) |
Treatment without dobutamine (n = 9) |
P value |
|---|---|---|---|---|
| Core temperature (°C) | 38.0 ± 0.9 | 37.9 ± 0.87 | 38.2 ± 0.92 | 0.5 |
| Lactate (mmol/l) | 3.5 ± 3.0 | 3.6 ± 3.3 | 3.0 ± 1.7 | 0.4 |
| CRP (mg/l) | 127 ± 78 | 124 ± 65 | 154 ± 120 | 0.6 |
| PCT (mg/l) | 6.2 ± 6.1 | 7.2 ± 6.3 | 2.5 ± 4.2 | 0.01 |
| Leucocytes (*109/l) | 14.0 ± 5.4 | 13.8 ± 5.3 | 15.4 ± 6.3 | 0.5 |
| Hemoglobin (g/L) | 11.6 ± 1.5 | 11.6 ± 1.6 | 11.6 ± 1.0 | 0.9 |
| Creatinine | 198 ± 160 | 162 ± 142 | 231 ± 182 | 0.1 |
| Sodium (mmol/L) | 144 ± 12 | 144 ± 11 | 147 ± 14 | 0.8 |
| Arterial blood gal analysis | ||||
| pH | 7.35 ± 0.09 | 7.35 ± 0.08 | 7.33 ± 0.09 | 0.6 |
| pCO2 (kPa) | 4.7 ± 1.0 | 4.6 ± 1.0 | 5.3 ± 0.8 | 0.06 |
| pO2 (kPa) | 15.3 ± 5.4 | 14.6 ± 4.8 | 18.5 ± 7.4 | 0.1 |
| HCO3 (mmol/L) | 20.6 ± 5.6 | 20.4 ± 6.1 | 21.5 ± 3.9 | 0.5 |
| BE(mEq/l) | -5.1 ± 6.4 | -5.4 ± 6.9 | -4.2 ± 4.8 | 0.5 |
| SatHbO2 (%) | 97 ± 3% | 97 ± 2 | 98 ± 3 | 0.4 |
Bold: statistically significant difference, P < 0.05.
BE, base excess; CRP, C-reactive protein; HCO3, bicarbonate; PCT, procalcitonin; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; SatHbO2, hemoglobin oxygen saturation.
Thenar StO2 before (basal StO2) and during the vascular occlusion test is presented in Table 5. There was no difference between patients treated with and without dobutamine in NIRS data.
Table 5.
NIRS data of skeletal muscle tissue oxygenation (StO2) during vascular occlusion test in patients with heart failure and additional sepsis
| NIRS data | All (n = 52) |
Treatment with dobutemine (n = 43) |
Treatment without dobutamine (n = 9) |
P value |
|---|---|---|---|---|
| Basal StO2 (%) | 89 ± 8 | 88 ± 8 | 92 ± 6 | 0.1 |
| StO2 deoxygenation rate (%/min) |
-12.6 ± 4.9 | -12.7 ± 5.2 | -12.6 ± 4.6 | 0.9 |
NIRS, near-infrared spectroscopy; StO2, skeletal muscle tissue oxygenation.
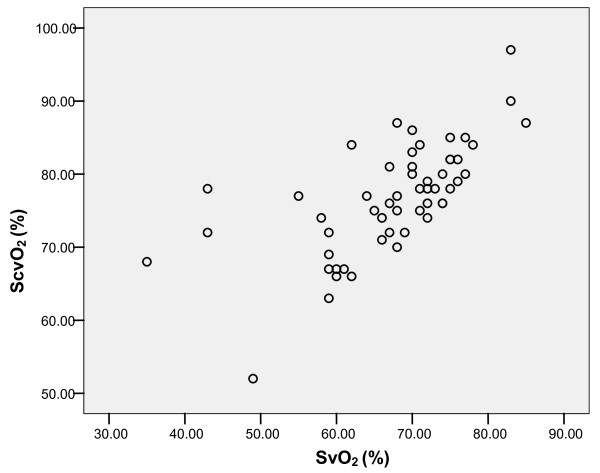
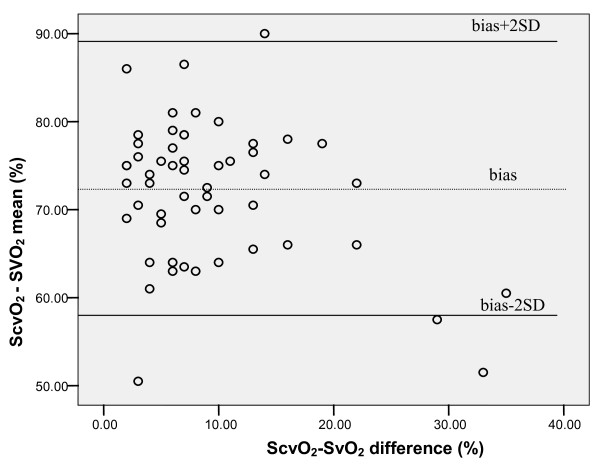
SvO2 correlated significantly with ScvO2 (Pearson correlation 0.659, P = 0.001; Figure 1); however, Bland Altman analysis showed a clinically important difference between both variables (ScvO2-SvO2 mean 72 ± 8%, ScvO2-SvO2 difference 9.4 ± 7.5%; Figure 2).
Figure 1.
Correlation between mixed venous (SvO2) and central venous saturation (ScvO2) in patients with heart failure and additional sepsis/septic shock. Pearson correlation 0.659, P = 0.001.
Figure 2.
Bland Altman analysis of clinically important difference between mixed venous (SvO2) and central venous saturation (ScvO2) in patients with heart failure and additional sepsis/septic shock. ScvO2-SvO2 mean 72 ± 8%, Scv-Svo2 difference 9.4 ± 7.5%.
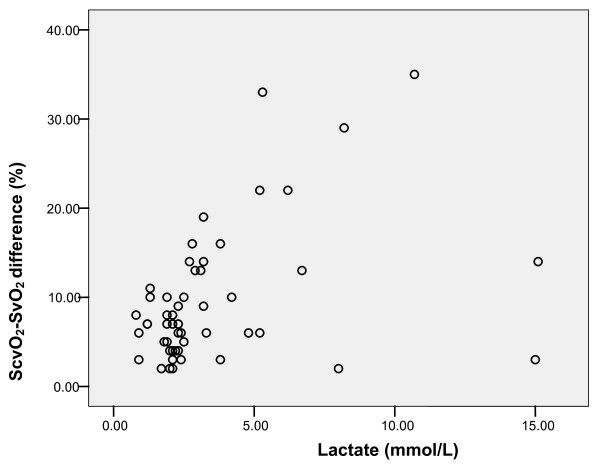
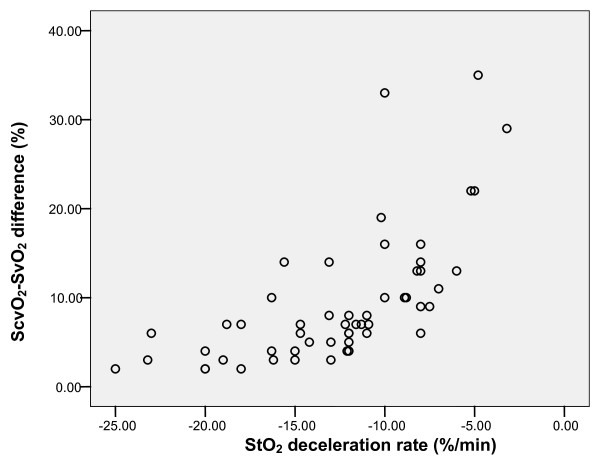
The ScvO2-SvO2 difference correlated with plasma lactate (Pearson correlation 0.400, P = 0.003; Figure 3) and StO2 deoxygenation rate (Pearson correlation 0.651, P = 0.001; Figure 4).
Figure 3.
Correlation of mixed venous (SvO2) and central venous saturation (ScvO2) difference with plasma lactate (mmol/L). Pearson correlation 0.400, P = 0.003.
Figure 4.
Correlation of central venous saturation (ScvO2) central venous saturation (SvO2) difference with skeletal muscle tissue oxygenation (StO2) deceleration rate. Pearson correlation 0.651, P = 0.001.
In the group of patients treated with dobutamine the ScvO2-SvO2 difference correlated with plasma lactate (Pearson correlation 0.389, P = 0.011) and StO2 deoxygenation rate (Pearson correlation 0.777, P = 0.0001).
In a small group of patients (n = 9) treated without dobutamine the ScvO2-SvO2 difference correlated with the StO2 deoxygenation rate (Pearson correlation 0.673, P = 0.033); however, there was no correlation between the ScvO2-SvO2 difference and plasma lactate (Pearson correlation 0.503, P = 0.139).
Discussion
Our study confirmed the hypothesis that the skeletal muscle StO2 deoxygenation rate correlates (or is inversely proportional) to the ScvO2-SvO2 difference in patients with severe heart failure with additional sepsis/septic shock. This relation between the StO2 deoxygenation rate and the ScvO2-SvO2 difference was also present in patients treated with or without dobutamine. We also showed that these patients have a clinically considerable ScvO2-SvO2 discrepancy. Monitoring of ScvO2 is a simpler and cheaper assessment of global DO2 to oxygen consumption ratio, but its use as a treatment goal in patients with severe heart failure with additional sepsis/septic shock is questionable.
The high StO2/low SvO2 seen in patients with severe sepsis and septic shock suggests blood flow redistribution. Thenar muscle StO2 correlates with central venous oxygen saturation that is measured in a mixture of blood from the head and both arms [18]. In healthy resting individuals the ScvO2 is slightly lower than the SvO2 [3]. Blood in the inferior vena cava has a high oxygen content because the kidneys do not utilise much oxygen but receive a high proportion of the cardiac output [19]. Blood in the inferior vena cava blood has a higher oxygen content than blood from the upper body and the SvO2 is thus greater than the ScvO2.
This relation changes in periods of cardiovascular instability. Scheinman and colleagues performed the earliest comparison of ScvO2 and SvO2 in both hemodynamically stable and shocked patients [5]. In stable patients, ScvO2 was similar to SvO2. In patients with a failing heart, ScvO2 was slightly higher than SvO2 and in patients with shock the difference between SvO2 and ScvO2 was even more expressed (47.5% ± 15.11% vs. 58.0% ± 13.05%, respectively, P < 0.001). Lee and colleagues described similar findings [20]. Other more detailed studies in mixed groups of critically ill patients designed to test if the ScvO2 measurements could substitute the SvO2 showed problematically large confidence limits [6] and poor correlation between the two values [7].
Most authors attribute this pattern to changes in the distribution of cardiac output that occur in periods of hemodynamic instability. In shock states, blood flow to the splanchnic and renal circulations falls, while flow to the heart and brain is maintained [21]. This results in a fall in the oxygen content of blood in the inferior vena cava. As a consequence, in shock states the normal relation is reversed and ScvO2 is greater than SvO2 [5]. Therefore, when using ScvO2 or StO2 as a treatment goal, the relative oxygen consumption of the superior vena cava system may remain stable, while the oxidative metabolism of vital organs, such as the splanchnic region, may reach a level where a flow-limited oxygen consumption is achieved, together with a marked decrease in oxygen saturation. In this situation skeletal muscle StO2 provides a false favorable impression of an adequate body perfusion, because of the inability to detect organ ischemia in the lower part of the body.
In our study, three patients with septic shock had skeletal muscle StO2 of 75% or less (under the lower boundary of 95% confidence interval for the mean of StO2 in controls); they were all in septic shock (lactate value above 2.5 mmol/L) with a low cardiac index below 2.0 L/min/m2. These patients were probably in an early under-resuscitated phase of septic shock. The low quantity of septic patients with low StO2 did not allow statistical comparison of StO2 and SvO2/SvO2 in these types of patients. Additional research is necessary to study muscle skeletal StO2 in under resuscitated septic patients.
Our data are supported by previous work by Boekstegers and colleagues who measured the oxygen partial pressure distribution in bicep muscle [22]. They found low peripheral oxygen availability in cardiogenic shock compared with sepsis. In cardiogenic shock the skeletal muscle oxygen partial pressure correlated with systemic oxygen delivery (r = 0.59, P < 0.001) and systemic vascular resistance (r = 0.74, P < 0.001). No correlation was found between systemic oxygen transport variables and the skeletal muscle partial oxygen pressure in septic patients. These measurements were performed in the most common cardiovascular state of sepsis in contrast to hypodynamic shock, which is only present in the very final stage of sepsis or in patients without adequate volume replacement [23]. In a following study the same authors have shown that even in the final state of hypodynamic septic shock leading to death, the mean muscle partial oxygen pressure did not decrease to below 4.0 kPa before circulatory standstill [24].
A recent study confirmed the use of NIRS and the arterial occlusion test in the assessment of peripheral muscle microcirculation impairment in patients with congestive heart failure [11]. This impairment of microcirculation was partially reversed by infusion of the inotropic agent dobutamine but not by levosimendan. In chronic heart failure patients, dobutamine increases cardiac output and improves tissue perfusion, which leads to improvement of endothelial function and tissue oxygenation. It was demonstrated that short-term (72 hours) and short-term intermittent (for five hours, biweekly) administration of dobutamine has a sustained beneficial effect on vascular endothelial function for two weeks or longer and after four months, respectively [25,26]. Despite this effect of dobutamine on endothelial function in patients with chronic heart failure, we have not detected any difference in StO2 deoxygenation in our mixed population of patients with left heart failure and additional sepsis/septic shock treated with or without dobutamine. Sepsis/septic shock-related microvascular changes and the lack of inclusion of end-stage heart failure patients in our study are probably causes for discrepancy between the results of our study and the study performed by Nanas and colleagues [11].
It is known that progressive chronic heart failure leads to cardiac cachexia and decreased resting energy expenditure, both of which are worst outcome predictors [27]. Previously, we have shown that in these patients metabolism is changed to the predominant utilization of lipids [28]. However, these changes happen in stages of advanced chronic heart failure, while on the other hand in patients without cachexia the resting energy expenditure is increased proportionally to a higher New York Heart Association class [29]. No patients with cardiac cachexia were included in our study. The effects of dobutamine on skeletal muscle metabolism in patients with chronic heart failure were studied by magnetic resonance spectroscopy, which indicated that dobutamine has the ability to increase cardiac output and limb blood flow, although it does not improve oxygen delivery to the working muscle of the patients [30]. Increased resting blood flow can result in increased oxyhemoglobin content in muscle leading to increased basal StO2 but the StO2 deoxygenation rate should stay unchanged if the metabolic rate remains constant.
Conclusions
In patients with severe heart failure with additional sepsis/septic shock, there is a clinically important discrepancy between ScvO2 and SvO2. However, with the use of arterial occlusion testing and measurement of the skeletal muscle deoxygenation rate, we can predict the ScvO2-SvO2 difference and determine adequate monitoring. Dobutamine use did not change this relation. Applying these findings in practice, in a patient with severe left heart failure, first perform arterial occlusion testing to determine the StO2 deoxygenation rate. If it is high (not prolonged as seen in sepsis/septic shock), estimate the SvO2 by using basal StO2. In the case of a prolonged skeletal muscle StO2 deoxygenation rate, look for additional sepsis, and the deoxygenation rate can estimate discrepancy between the ScvO2 and SvO2.
Key messages
• In patients with severe left heart failure and additional severe sepsis or septic shock the ScvO2-SvO2 discrepancy is clinically important.
• The skeletal muscle StO2 deoxygenation rate estimates the ScvO2-SvO2 discrepancy in patients with severe left heart failure with additional severe sepsis or septic shock.
Abbreviations
DO2: systemic oxygen delivery; NIRS: near infrared spectroscopy; SOFA: Sepsis-related Organ Failure Assessment Score; ScvO2: central venous oxygen saturation; SD: standard deviation; STEMI: ST-elevation myocardial infarction; StO2: tissue oxygen consumption; SvO2: mixed venous oxygen saturation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HM contributed to original observation, conception, design, acquisition of data, analysis and interpretation, and drafting the manuscript. MP contributed to conception, design, acquisition of data, analysis and interpretation, and drafting the manuscript.
Contributor Information
Hugo Možina, Email: hugon.mozina@mf.uni-lj.si.
Matej Podbregar, Email: matej.podbregar@guest.arnes.si.
Acknowledgements
The study was partly supported by Grant for Ministry of science and technology, Slovenia and Research projects of University Centre Ljubljana, Slovenia. We thank Timotej Jagric, PhD from Department for Quantitative Economic Analysis, Faculty of Economics and Business, University of Maribor, Slovenia for statistical advice.
References
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Reinhart K, Kuhn HJ, Hartog C, Bredle DL. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med. 2004;30:1572–1578. doi: 10.1007/s00134-004-2337-y. [DOI] [PubMed] [Google Scholar]
- Barratt-Boyes Bg, Wood EH. The oxygen saturation of blood in vena cava, right heart chambers and pulmonary vessels of healthy subjects. J Lab Clin Med. 1957;50:93–106. [PubMed] [Google Scholar]
- Lee J, Wright F, Barber R. Central venous oxygen saturation in shock: a study in men. Anesthesiology. 1972;36:472–478. doi: 10.1097/00000542-197205000-00012. [DOI] [PubMed] [Google Scholar]
- Scheinman MM, Brown MA, Rapaport E. Critical assesment of use of central venous oxygen saturation as a mirror of mixed venous oxygen saturation in severly ill cardiac patients. Circulation. 1969;40:165–172. doi: 10.1161/01.cir.40.2.165. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Mayall RM. Importance of the sampling site for measurement of mixed venous oxygen saturation in shock. Crit Care Med. 1998;26:1356–1360. doi: 10.1097/00003246-199808000-00020. [DOI] [PubMed] [Google Scholar]
- Martin C, Auffray JP, Badetti C, Perrin G, Papazian L, Gouin F. Monitoring of central venous oxygen saturation versus mixed venous oxygen saturation in critically ill patients. Intensive Care Med. 1992;18:101–104. doi: 10.1007/BF01705041. [DOI] [PubMed] [Google Scholar]
- Podbregar M, Mozina H. Skeletal muscle oxygen saturation does not estimate mixed venous oxygen saturation in patients with severe left heart failure and additional severe sepsis or septic shock. Crit Care. 2007;11:R6. doi: 10.1186/cc5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart K, Rudolph T, Bredle DL, Hannemann L, Cain SM. Comparison of central-venous to mixed-venous oxygen saturation during changes in oxygen supply/demand. Chest. 1989;95:1216–1221. doi: 10.1378/chest.95.6.1216. [DOI] [PubMed] [Google Scholar]
- Pareznik R, Knezevic R, Voga G, Podbregar M. Changes in muscle tissue oxygenation during stagnant ischemia in septic patients. Intensive Care Med. 2006;32:87–92. doi: 10.1007/s00134-005-2841-8. [DOI] [PubMed] [Google Scholar]
- Nanas S, Gerovasili V, Dimopoulos S, Pierrakos C, Kourtidou S, Kaldara E, Sarafoglou S, Venetsanakos J, Roussos C, Nanas J, Anastasiou-Nana M. Inotropic agents improve the peripheral microcirculation of patients with end-stage chronic heart failure. J Card Fail. 2008;14:400–406. doi: 10.1016/j.cardfail.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555. doi: 10.1007/s00134-004-2398-y. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Strahovnik I, Podbregar M. Measurment of skeletal muscle tissue oxygenation in critically ill. Signa Vitae. 2008;3:43–50. [Google Scholar]
- Vincent JL, Moreno R, Takala J, Willatts S, De Medonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurements. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Mesquida J, Masip J, Gili G, Artigas A, Baigorri F. Thenar oxygen saturation measured by near infrared spectroscopy as a noninvasive predictor of low central venous oxygen saturation in septic patients. Intensive Care Med. 2009;35:1106–1109. doi: 10.1007/s00134-009-1410-y. [DOI] [PubMed] [Google Scholar]
- Cargill W, Hickam J. The oxygen consumption of the normal and diseased human kidney. J Clin Invest. 1949;28:526–532. doi: 10.1172/JCI102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Wright F, Barber R, Stanley L. Central venous oxygen saturation in shock: a study in man. Anesthesiology. 1972;36:472–478. doi: 10.1097/00000542-197205000-00012. [DOI] [PubMed] [Google Scholar]
- Forsyth R, Hoffbrand B, Melmon K. Re-distribution of cardiac output during hemorrhage in the unanesthetized monkey. Circ Res. 1970;27:311. doi: 10.1161/01.res.27.3.311. [DOI] [PubMed] [Google Scholar]
- Boekstegers P, Weidenhoefer St, Pilz G, Werdan K. Peripheral oxygen availability within skeletal muscle in sepsis and septic shock: comparison to limited infection and cardiogenic shock. Infection. 1991;19:317–323. doi: 10.1007/BF01645355. [DOI] [PubMed] [Google Scholar]
- Parker MM, Parrillo JE. Septic shock: hemodynamics and pathogenesis. JAMA. 1983;250:3324–3327. doi: 10.1001/jama.250.24.3324. [DOI] [PubMed] [Google Scholar]
- Boekstegers P, Weidenhoefer, Kapsner T, Werdan K. Skeletal muscle partial pressure of oxygen in patients with sepsis. Crit Care Med. 1994;22:640–650. doi: 10.1097/00003246-199404000-00021. [DOI] [PubMed] [Google Scholar]
- Patel MB, Kaplan IV, Patni RN, Levy D, Strom JA, Shirani J, LeJemtel TH. Sustained improvement in flow-mediated vasodilation after short-term administration of dobutamine in patients with severe congestive heart failure. Circulation. 1999;99:60–64. doi: 10.1161/01.cir.99.1.60. [DOI] [PubMed] [Google Scholar]
- Freimark D, Feinberg MS, Matezky S, Hochberg N, Shechter M. Impact of short-term intermittent intravenous dobutamine therapy on endothelial function in patients with severe chronic heart failure. Am Heart J. 2004;148:878–882. doi: 10.1016/j.ahj.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- Podbregar M, Voga G. Effect of selective and nonselective beta-blockers on resting energy production rate and total body substrate utilization in chronic heart failure. J Card Fail. 2002;8:369–378. doi: 10.1054/jcaf.2002.130238. [DOI] [PubMed] [Google Scholar]
- Obisesan TO, Toth MJ, Donaldson K, Gottlieb SS, Fisher ML, Vaitekevicius P, Poehlman ET. Energy expenditure and symptom severity in men with heart failure. Am J Cardiol. 1996;77:1250–1252. doi: 10.1016/S0002-9149(96)00176-2. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Schwartz M, Ferraro N, Seestedt R, Chance B, Wilson JR. Effect of dobutamine on skeletal muscle metabolism in patients with congestive heart failure. Am J Cardiol. 1990;65:1121–1126. doi: 10.1016/0002-9149(90)90325-U. [DOI] [PubMed] [Google Scholar]