Abstract
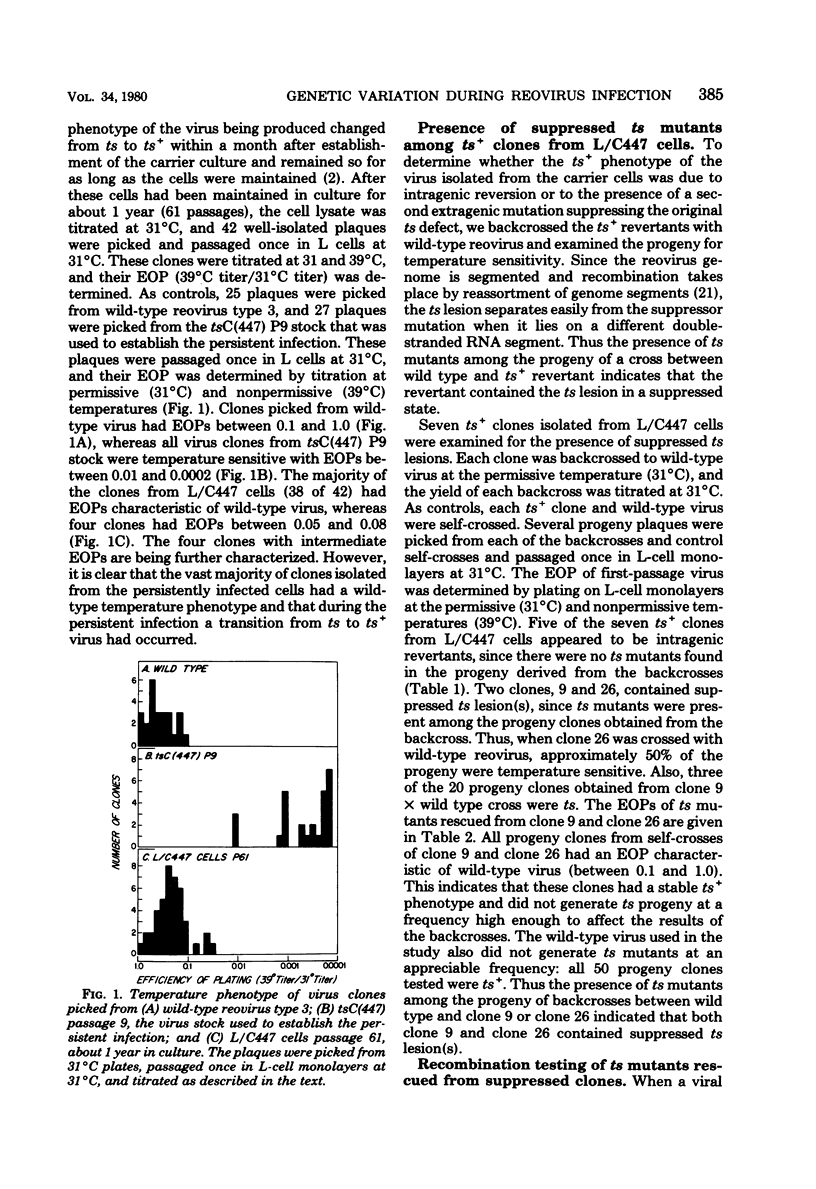
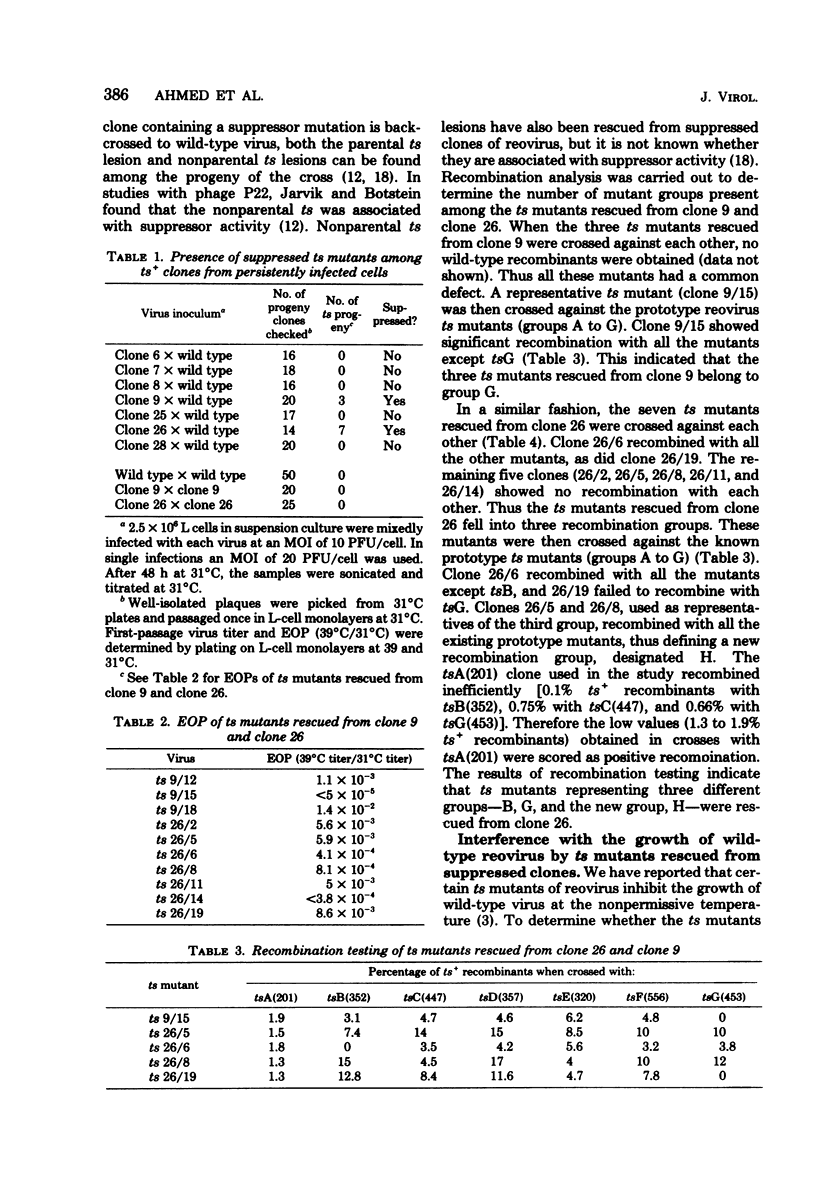
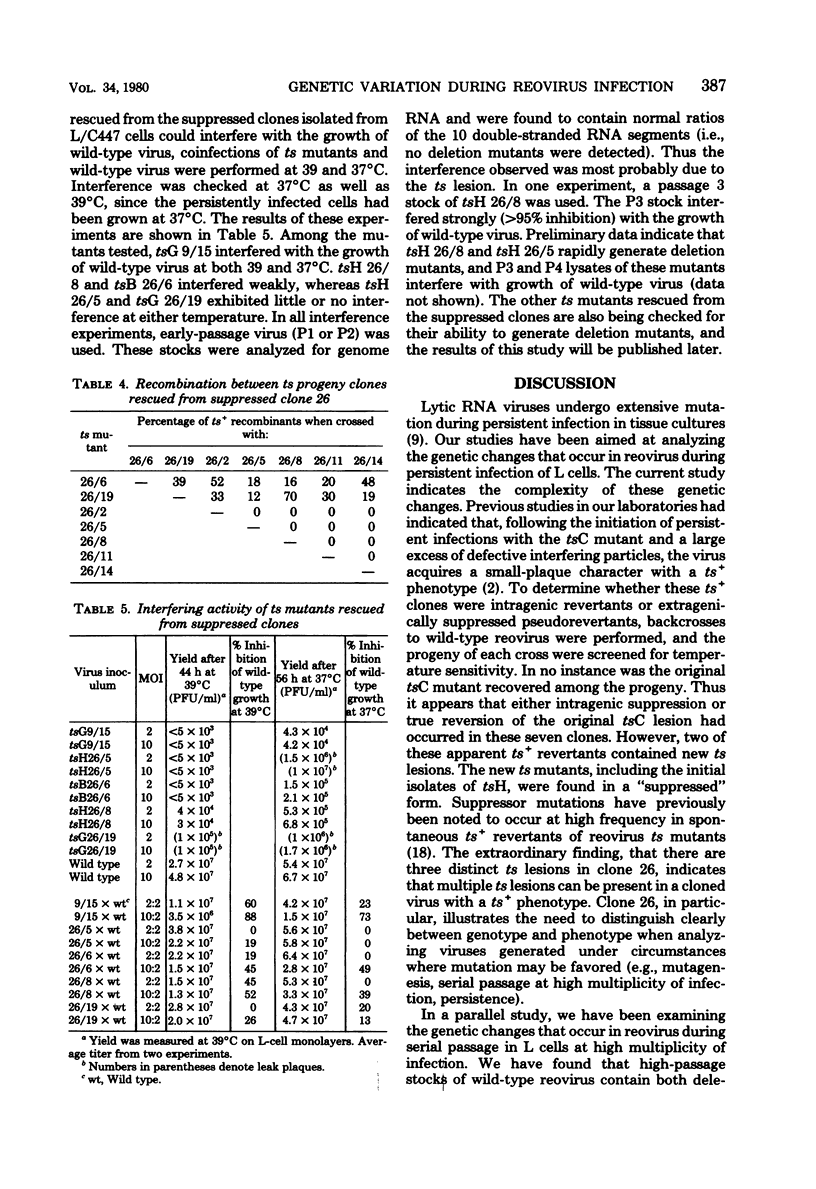
Persistent reovirus infection of L cells was established with a serially passaged stock of temperature-sensitive (ts) mutant C(447) containing greater than 90% defective interfering particles. Within a month after establishment of the carrier culture, the ts mutant was replaced by virus that expressed the wild-type (ts+) temperature phenotype (R. Ahmed and A. F. Graham, J. Virol. 23:250-262, 1977). To determine whether the ts+ phenotype of the virus was due to intragenic reversion or to the presence of an extragenic mutation suppressing the original ts defect, several clones were backcrossed to wild-type reovirus, and the progeny of each cross were screened for temperature sensitivity. The results indicated that the original tsC lesion had reverted. However, in two of the seven clones examined, new ts lesions were found. These new ts lesions appeared phenotypically as ts+ due to the presence of extragenic suppressor mutations. Temperature-sensitive mutants representing three different groups were rescued from one suppressed clone, indicating that this ts+ clone contained multiple ts lesions. Among the ts mutants rescued were the initial isolates of a new recombination group which we have designated H. Some of the ts mutants rescued from the suppressed clones are capable of interfering with the growth of wild-type reovirus and may play a role in maintaining the carrier state. The results of this study show that persistently infected L cells contain a genetically heterogeneous population of reovirus even though all virus clones express the ts+ phenotype. It is thus critical to distinguish between genotype and phenotype when analyzing viruses that emerge during persistent infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Chakraborty P. R., Fields B. N. Genetic variation during lytic reovirus infection: high-passage stocks of wild-type reovirus contain temperature-sensitive mutants. J Virol. 1980 Apr;34(1):285–287. doi: 10.1128/jvi.34.1.285-287.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Graham A. F. Persistent infections in L cells with temperature-sensitive mutants of reovirus. J Virol. 1977 Aug;23(2):250–262. doi: 10.1128/jvi.23.2.250-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P. R., Ahmed R., Fields B. N. Genetics of reovirus: the relationship of interference to complementation and reassortment of temperature-sensitive mutants at nonpermissive temperature. Virology. 1979 Apr 15;94(1):119–127. doi: 10.1016/0042-6822(79)90442-2. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral RNA. Virology. 1972 Dec;50(3):799–809. doi: 10.1016/0042-6822(72)90434-5. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Gould E. A., Linton P. E. The production of a temperature-sensitive persistent measles virus infection. J Gen Virol. 1975 Jul;28(1):21–28. doi: 10.1099/0022-1317-28-1-21. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Jarvik J., Botstein D. Conditional-lethal mutations that suppress genetic defects in morphogenesis by altering structural proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2738–2742. doi: 10.1073/pnas.72.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Ito Y., Shimokata K., Nishiyama Y., Nagata I. Temperature-sensitive virus derived from BHK cells persistently infected with HVJ (Sendai virus). J Virol. 1975 Jan;15(1):55–63. doi: 10.1128/jvi.15.1.55-63.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Small plaque variants of simian virus 40 from a persistent infection of rhesus monkey kidney cells. Virology. 1979 Aug;97(1):201–206. doi: 10.1016/0042-6822(79)90388-x. [DOI] [PubMed] [Google Scholar]
- P'ringle C. R., Shirodaria P. V., Cash P., Chiswell D. J., Malloy P. Initiation and maintenance of persistent infection by respiratory syncytial virus. J Virol. 1978 Oct;28(1):199–211. doi: 10.1128/jvi.28.1.199-211.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1972 Feb;9(2):200–206. doi: 10.1128/jvi.9.2.200-206.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Fields B. N. Revertants of temperature-sensitive mutants of reovirus: evidence for frequent extragenic suppression. Virology. 1979 Jan 15;92(1):155–167. doi: 10.1016/0042-6822(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., White R. M., Fields B. N. Suppression of the temperature-sensitive phenotype of a mutant of reovirus type 3. Science. 1977 Jan 28;195(4276):406–407. doi: 10.1126/science.831284. [DOI] [PubMed] [Google Scholar]
- Sekellick M. J., Marcus P. I. Persistent infection. II. Interferon-inducing temperature-sensitive mutants as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1979 May;95(1):36–47. doi: 10.1016/0042-6822(79)90399-4. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Ramig R. F., Mustoe T. A., Fields B. N. A genetic map of reovirus. 1. Correlation of genome RNAs between serotypes 1, 2, and 3. Virology. 1978 Jan;84(1):63–74. doi: 10.1016/0042-6822(78)90218-0. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Koshelnyk K. A., Stollar V. Temperature-sensitive virus from Aedes albopictus cells chronically infected with Sindbis virus. J Virol. 1974 Feb;13(2):439–447. doi: 10.1128/jvi.13.2.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Rustigian R., Stallcup K. C., Byers K. B., Winston S. H., Fields B. N. Measles virus-specified polypeptide synthesis in two persistently infected HeLa cell lines. J Virol. 1979 Sep;31(3):677–684. doi: 10.1128/jvi.31.3.677-684.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



