Abstract
Introduction
Dead space negatively influences carbon dioxide (CO2) elimination, particularly at high respiratory rates (RR) used at low tidal volume ventilation in acute respiratory distress syndrome (ARDS). Aspiration of dead space (ASPIDS), a known method for dead space reduction, comprises two mechanisms activated during late expiration: aspiration of gas from the tip of the tracheal tube and gas injection through the inspiratory line - circuit flushing. The objective was to study the efficiency of circuit flushing alone and of ASPIDS at wide combinations of RR and tidal volume (VT) in anaesthetized pigs. The hypothesis was tested that circuit flushing and ASPIDS are particularly efficient at high RR.
Methods
In Part 1 of the study, RR and VT were, with a computer-controlled ventilator, modified for one breath at a time without changing minute ventilation. Proximal dead space in a y-piece and ventilator tubing (VDaw, prox) was measured. In part two, changes in CO2 partial pressure (PaCO2) during prolonged periods of circuit flushing and ASPIDS were studied at RR 20, 40 and 60 minutes-1.
Results
In Part 1, VDaw, prox was 7.6 ± 0.5% of VT at RR 10 minutes-1 and 16 ± 2.5% at RR 60 minutes-1. In Part 2, circuit flushing reduced PaCO2 by 20% at RR 40 minutes-1 and by 26% at RR 60 minutes-1. ASPIDS reduced PaCO2 by 33% at RR 40 minutes-1 and by 41% at RR 60 minutes-1.
Conclusions
At high RR, re-breathing of CO2 from the y-piece and tubing becomes important. Circuit flushing and ASPIDS, which significantly reduce tubing dead space and PaCO2, merit further clinical studies.
Introduction
In acute respiratory distress syndrome, severe obstructive lung disease, and at increased intracranial pressure it may be important to maintain adequate CO2 exchange at low tidal volume ventilation (LTVV). LTVV will otherwise lead to respiratory acidosis. To uphold CO2 elimination, increased respiratory rate (RR) may then be applied [1]. At high RR, when dead space as a fraction of tidal volume increases, dead space reduction may be called for. A first step is to reduce the volume of connectors and humidifiers. A further step may be expiratory flushing of airways, later denoted tracheal gas insufflation (TGI) [2,3]. TGI is associated with problems related to humidification of the injected gas and of local effects of the jet stream at the tip of the tracheal tube. TGI will also disturb monitoring of ventilation. Therefore, a new technique, aspiration of dead space (ASPIDS) was developed and tested [4-6]. ASPIDS comprises two mechanisms, which are simultaneously activated late during expiration. One is aspiration of gas from the tip of the tracheal tube that is performed through a special lumen of the tracheal tube or through a catheter ending close to the tip of the tracheal tube. The other mechanism is gas injection through the inspiratory line, Circuit Flushing. Circuit Flushing compensates for the volume of aspirated gas and fills the inspiratory system with fresh gas. Before the ensuing inspiration, ASPIDS brings the interface between expired gas and fresh gas down to the tip of the tracheal tube.
After an ordinary expiration without ASPIDS or Circuit Flushing, CO2 is present at the start of inspiration in the Y-piece, in adjacent parts of the inspiratory tube and also in the expiratory tube. A volume of CO2 representing about 20 to 24 ml of alveolar gas is re-inspired from that zone during the inspiration [7,8]. It was reasoned that Circuit Flushing alone might clear this volume of CO2, thereby reducing dead space.
No systematic study has previously been performed to analyze how a wide range of RR and tidal volume (VT) combinations affects re-inspiration of dead space gas from the Y-piece and adjacent tubing. To what extent Circuit Flushing in itself contributes to the effects of ASPIDS at different RR and VT has not been studied. The objective of this study was to quantify re-inspiration from the Y-piece and adjacent parts of tubing at ordinary and increased RR and to examine the extent at which Circuit Flushing alone explains positive effects of ASPIDS at different combinations of RR and VT. The hypothesis was tested that ASPIDS and Circuit Flushing are particularly efficient at high RR.
Materials and methods
The Ethics Board of Animal Research of Lund University approved the study. Five pigs of Swedish native breed weighing 19 to 23 kg were premedicated with xylazine (2 mg·kg-1), ketamine (15 mg·kg1) and atropine (0.5 mg). Anaesthesia was maintained by continuous intravenous infusion of fentanyl (60 μg·kg-1·h-1), midazolam (0.7 mg·kg-1·h-1), and ketamine (7 mg·kg-1·h-1). Paralysis was avoided to allow judgement of anaesthesia depth during the experiments. However, no muscular movements were observed. Initially the animals were hydrated with 1,000 ml Ringer-acetate (600 ml·h-1) followed by dextran at 200 ml·h-1. A femoral artery catheter was used for blood gas sampling (Radiometer ABL725, Copenhagen, Denmark) and blood pressure monitoring (HP 78353A). Mean arterial pressure (MAP) and pulse rate (HR) were monitored. Body temperature was maintained constant.
The animals were intubated with a 7.0 mm internal diameter tracheal tube connected to a ventilator (Servo Ventilator 900C, Siemens-Elema AB, Solna, Sweden). To minimize circuit dead space, the Y-piece was directly connected to the tracheal tube without swivel adaptor or humidifier. Ventilation was volume-controlled with square inspiratory flow pattern. At baseline, RR was 20 minutes-1, inspiratory time 33%, postinspiratory pause 5% and positive end-expiratory pressure (PEEP) 4 cmH2O. Below, RR is denoted RRnn, in which nn implies rate in minutes-1. The baseline minute ventilation (MV) was adjusted to achieve PaCO2 of 5 to 5.5 kPa. A mainstream CO2 analyser (CO2 Analyzer 930, Siemens-Elema, Solna, Sweden) was used to measure airway partial pressure of CO2 at the proximal end of the tracheal tube (PawCO2). The ventilator/computer system used for data recording and computer control of the ventilator has been described [9,10]. Signals from the ventilator and the CO2 analyzer representing flow rate, airway pressure and PawCO2 were sampled at 100 Hz. Compliance of the tracheal tube and ventilator tubing was measured in vitro. The system was tested for leakage. The animals were killed by an overdose of potassium chloride at the end of the experiment. There were no dropouts.
ASPIDS circuit
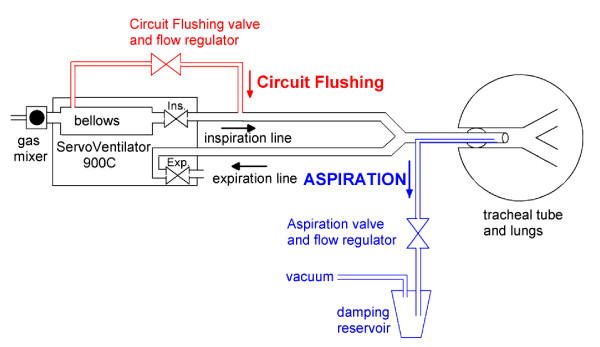
The ASPIDS system, comprising the Servo Ventilator 900C, an electronic control unit, and two valves, has been described in detail [5]. One valve, used for Aspiration, connects a vacuum source to the aspiration catheter (ID 2.5 mm, OD 2.9 mm) ending 2 cm proximal to the tip of the tracheal tube. The other, used for Circuit Flushing, connects the bellow of the ventilator to the inspiratory line, Figure 1. Aspiration and/or Circuit Flushing were performed over the last 30% of expiration time. Flow rate and volume for Aspiration and Circuit Flushing were adjustable. Aspiration volume was 5 to 10 ml lower than Circuit Flushing volume. The ASPIDS period is short at high RR. Therefore, Circuit Flushing flow rate, being 0.22 L·sec-1 at RR20 and 40, was increased to about 0.35 L·sec-1 at RR60 to assure that flushing and aspiration volumes were not less than 60 ml and sufficient to clear the tracheal tube.
Figure 1.
ASPIDS system. The Servo Ventilator 900C complemented by a system for Circuit Flushing (in red) and a system for aspiration of gas from the tip of the tracheal tube (in blue). During Circuit Flushing only the red valve is opening during the last third of the expiration period. During ASPIDS both red and blue valves are opening. A development suggested in the Discussion is to program the regular inspiratory flow regulating valve (Ins.) to perform Circuit Flushing without any extra tube or other hardware.
Protocol
After animal preparation, a stabilisation period at basal ventilation was allowed for 60 minutes to establish a steady state. The protocol had two parts. The experiment was performed with a previously described computer controlled ventilator [9].
Part 1: After the stabilisation period, the effect of different combinations of RR and VT on dead space from the Y-piece and adjacent parts of the ventilator tubing was analyzed without using ASPIDS or Circuit Flushing. At basal ventilation at RR20, single breaths were modified, with respect to RR and VT. Sequences of 10 breaths were recorded. The second and seventh breaths were modified under computer control. Between modified breaths were ordinary breaths. The combination of RR and VT was for each modified breath such that minute ventilation remained unchanged. For modified breaths RR was 10, 30, 40, 50 or 60 minutes-1 while VT was inversely modified. In randomized order, each RR-VT combination was recorded three times. Other parameters like PEEP were constant. The computer was programmed to modify single breaths at a time to allow comparisons with ordinary breaths within the same recording as in previous studies [10-12].
In Part 2 measurements at steady state were made of ventilation parameters, blood gases and haemodynamics at basal ventilation, at Circuit Flushing alone and at complete ASPIDS at various combinations of RR and VT. PaCO2 was measured every 10 minutes. Dead space can not be measured during Circuit Flushing and ASPIDS. The following scheme, also depicted in Figure 2, was followed:
Figure 2.
Protocol for Part 2. At RR 20, 40 and 60 equilibration time preceded measurements denoted M, during ordinary ventilation (NONE), Circuit Flushing (Circuit Flush) and complete ASPIDS. Letters a-h correspond to instances described in the text.
a. Basal ventilation at RR20. Measurements after 30 minutes.
b. Circuit Flushing started at RR20. Measurements after 30 minutes.
c. Circuit Flushing stopped and RR increased to 40 minutes-1. Minute ventilation increased to maintain a stable CO2 elimination rate as read from the CO2 analyzer. Measurements after 40 minutes.
d. Without changing RR, Circuit Flushing started. Measurements after 30 minutes.
e. Aspiration started for complete ASPIDS. Measurements after 30 minutes.
f. Circuit Flushing and aspiration stopped and RR increased to 60 minutes-1. Minute ventilation increased to maintain stable CO2 elimination rate. Measurements after 40 minutes.
g. Procedure d repeated at RR60.
h. Procedure e repeated at RR60.
Data analysis
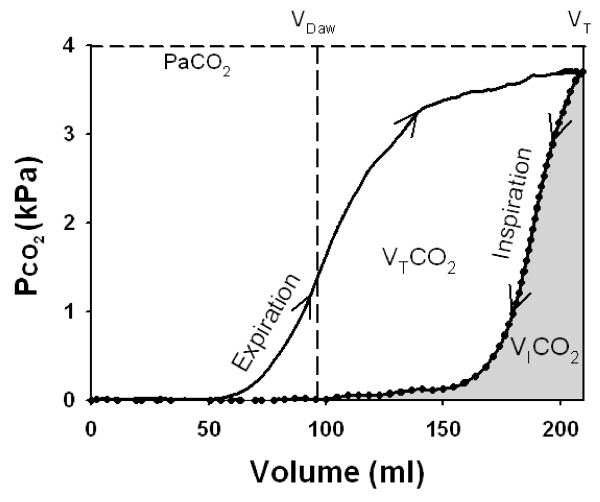
Sampled data of flow rate, airway pressure and PawCO2 were transferred to a spreadsheet (Excel 2003, Microsoft, Redmond, WA, USA). The single-breath test for CO2 was analyzed according to principles described by Beydon et al [7]. The volume of CO2 eliminated per breath (VTCO2) corresponds to the area within the loop, Figure 3. The volume of CO2 re-inspired from the Y-piece and tubing per breath (VICO2) is reflected by the area to the right of the loop. Dead space proximal to the CO2 sensor caused by VICO2(VDaw, prox) was calculated:
| (1) |
Figure 3.
SBT-CO2 of a representative animal. Partial pressure of CO2 in expired gas (solid line) and inspired gas (dotted line) plotted against volume so as to create a loop. The area within the loop corresponds to tidal elimination of CO2 (VTCO2). The area below the inspiratory limb (grey) corresponds to re-inspired volume of CO2 proximal of the CO2 sensor (VICO2). Airway dead space distal to the CO2 sensor (VDaw) is indicated (vertical interrupted line).
Pe'CO2 is the end-tidal CO2 and Pbar barometric pressure. VDaw, prox in % of VT is denoted VDaw, prox%.
Airway dead space distal to the CO2 sensor (VDaw) was determined according to an algorithm of Wolff and Brunner [13] modified to correct for a sloping alveolar plateau [10].
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Student's paired two-tailed t-test was used. Linear and logarithmic regressions were applied. P values less than 0.05 were considered significant.
Results
During the whole procedure, all animals remained stable with respect to oxygenation and arterial blood pressure. Heart rate showed a trend to increase from on average 74 ± 20 to 94 ± 22 minutes-1 (Table 1).
Table 1.
Effects of Circuit Flushing and ASPIDS at increasing respiratory rate
| RESPIRATORY RATE |
MV, Lit. |
VT, ml |
Pplat, cmH2O |
Cst, ml/cmH20 |
V'CO2 ml/min |
PaCO2, kPa |
pH | PaO2, kPa |
HR, b/min |
MAP, mmHg |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR20 | Baseline | 4.1 ± 0.6 | 208 ± 31 | 14 ± 2.6 | 20 ± 3 | 133 ± 10 | 5.3 ± 0.2 | 7.47 ± 0.04 | 12.3 ± 1.2 | 74 ± 20 | 84 ± 17 |
| After 30 minutes of Circuit Flushing | 4.1 ± 0.6 | 208 ± 31 | 14 ± 3.5 | 4.7 ± 0.4 * | 7.51 ± 0.04 * | 12.6 ± 1.2 | 78 ± 20 ** | 83 ± 15 | |||
| RR40 | Baseline | 4.9 ± 0.5 | 130 ± 13 | 13 ± 3.7 | 15 ± 4 | 142 ± 20 | 5.9 ± 0.4 # | 7.43 ± 0.04 | 10.7 ± 0.9 | 79 ± 15 | 81 ± 12 |
| After 30 minutes of Circuit Flushing | 4.9 ± 0.5 | 130 ± 13 | 14 ± 3.5 ** | 4.7 ± 0.3 ** | 7.51 ± 0.04 ** | 12 ± 1.5 | 81 ± 13 | 80 ± 9 | |||
| After 30 minutes of Circuit Flushing + ASPIDS | 4.9 ± 0.5 | 130 ± 13 | 13 ± 3.6 | 3.9 ± 0.2 * | 7.58 ± 0.05 ** | 12 ± 1.8 | 84 ± 11 | 79 ± 10 | |||
| RR60 | Baseline | 5.9 ± 0.5 | 101 ± 9.5 | 13 ± 2.2 | 12 ± 2 | 136 ± 16 | 6.3 ± 0.4 # | 7.40 ± 0.05 | 9.6 ± 1.3 | 84 ± 9 | 81 ± 9 |
| After 30 minutes of Circuit Flushing | 5.9 ± 0.5 | 101 ± 9.5 | 14 ± 2 | 4.6 ± 0.6 ** | 7.51 ± 0.07 * | 11.5 ± 2 * | 91 ± 20 | 83 ± 9 | |||
| After 30 minutes of Circuit Flushing + ASPIDS | 5.9 ± 0.5 | 101 ± 9.5 | 12 ± 2 ** | 3.7 ± 0.5 ** | 7.59 ± 0.07 ** | 11.2 ± 2.8 | 94 ± 22 | 79 ± 11 | |||
* P < 0.01; ** P < 0.001 (comparison were made to the preceding value). # P <0.05 (comparison between baseline at RR40 and 60 vs baseline at RR20, and between baseline at RR60 vs baseline at RR40). ASPIDS, aspiration of dead space; Cst, static compliance; HR, heart rate; MAP, mean arterial pressure; MV, minute ventilation; PaCO2, carbon dioxide arterial partial pressure; PaO2, oxygen arterial partial pressure; Pplat, plateau pressure; RR, respiratory rate; VT, tidal volume; V'CO2, carbon dioxide production.
Part 1
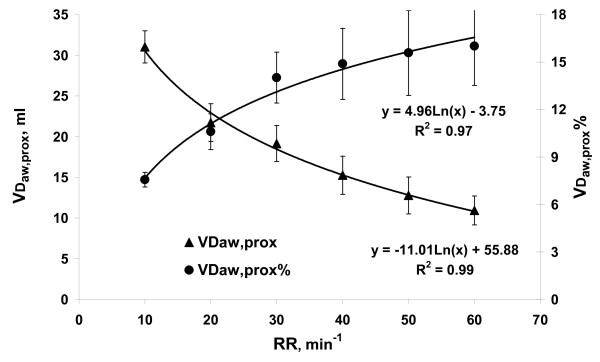
At increasing RR, VDaw, prox decreased from 31 ± 2 ml at RR10 to 11 ± 2 ml at RR60 tightly according a logarithmic equation (Figure 4). VDaw, prox % was 7.6 ± 0.5% at RR10 and increased logarithmically to 16 ± 2.5% at RR60 (Figure 4). Peak expiratory flow decreased with RR according to the equation: y = - 0.33 Ln(RR) + 0.85, (R2 = 0.99).
Figure 4.
Proximal airway dead space in ml (VDaw, prox), and in % of tidal volume (VDaw, prox%) related to respiratory rate (RR). Black lines represent the logarithmic fit.
Part 2
Table 1 shows the effects of Circuit Flushing and ASPIDS in comparison to basal ventilation at RR20 to 60. Minute ventilation and VT were maintained at all settings.
Compared to baseline ventilation, Circuit Flushing reduced PaCO2 by 10, 20 and 26% at RR20, RR40 and RR60, respectively. ASPIDS reduced PaCO2 by 33% at RR40 and 41% at RR60, Table 2. Accordingly, the reduction in PaCO2 achieved by Circuit Flushing alone was at RR40 60% of the total ASPIDS effect and 63% at RR60.
Table 2.
Change in PaCO2 in % of baseline value at each RR
| RR, min-1 | 20 | 40 | 60 |
|---|---|---|---|
| Change in PaCO2 in % of baseline value at each RR | |||
| Circuit Flushing | -10.3 ± 4, P = 0.005 | -20 ± 3, P = 0.0002 | -26 ± 7, P = 0.001 |
| ASPIDS | - | -33 ± 5, P = 0.0004 | -41 ± 6, P = 0.0002 |
ASPIDS, aspiration of dead space; PaCO2, carbon dioxide arterial partial pressure; RR, respiratory rate.
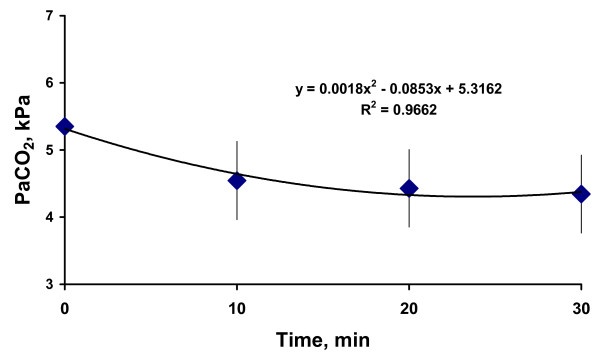
During Circuit Flushing and ASPIDS period PaCO2 decreased fast during the first 10 minutes and later at a slower rate in accordance with the equation: y = 0.0018x2-0.085x + 5.3 (R2 = 0.97) (Figure 5).
Figure 5.
Average of PaCO2 evolution during Circuit Flushing and ASPIDS periods.
Discussion
The study was performed in healthy pigs to allow a detailed analysis over several hours without problems related to patient care and physiological stability. The study relates to events in ventilator tubing, y-piece and tracheal tube, which are relatively independent on the physiology of the subject studied. The principle results should be valid also in a clinical context. To what extent the dead space reduction achieved with ASPIDS and Circuit Flushing is of clinical value can only be judged from clinical studies.
In previous experiments with ASPIDS at health [4,6] and in animals and patients with acute respiratory failure [5,14] VT and airway pressures were reduced while normocapnia was maintained. The present study is the first in which ASPIDS and also Circuit Flushing was shown to modify PaCO2. This is also the first comprehensive analysis of how a wide range of VT - RR combinations affect airway dead space resulting from re-inspiration of CO2 from Y-piece and adjacent tubing. In confirmation of the hypothesis it was shown that ASPIDS and Circuit Flushing are particularly efficient at high RR. It was shown for the first time that Circuit Flushing significantly may enhance CO2 elimination and reduce PaCO2 through its effects on VDaw, prox. This aspect may be important for future development because Circuit Flushing can very easily be implemented as further discussed below.
In Part 1 it was shown that VDaw, prox in ml decreased at higher RR and correspondingly lower VT, in line with previous observations [10]. This reflects that VDaw, prox reflects admixture of CO2 to the inspiratory ventilator line during expiration and re-inspiration of CO2 from both ventilator lines during inspiration. These phenomena are related to diffusion, turbulence, Venturi, and Coandă effects around the Y-piece [7,8,10]. At higher RR, less time is available for these phenomena, while expiratory flow rate that promotes gas mixing in tubing around the y-piece is lower, as shown. Thereby, VDaw, prox becomes lower at high RR. However, VDaw, prox% increased two-fold over the interval RR10 to RR60 in spite of that VDaw, prox in ml fell to one third. These data (Figure 4) show that the importance of CO2 re-inspiration from ventilator lines and Y-pieces increases at a high RR which is essential for understanding the results in Part 2.
Fletcher et al. suggested the use of non-return valves in the Y-piece to avoid re-breathing [8]. Safety issues might be a reason why such valves have not been introduced. At present the need for ventilation at low VT and high RR asks for a safe solution of the significant re-breathing problem.
In Part 2, a period of 30 to 40 minutes was allowed for steady state establishment on the basis of previous data [15]. Longer periods would increase risks of significant changes in physiological status of the animals. Data in Figure 5 confirm that a steady state was achieved.
ASPIDS clears the tubing of CO2 down to the tip of the tracheal tube, while Circuit Flushing only clears tubes to and into the y-piece. Therefore, as expected, the effect on PaCO2 of ASPIDS was more important than that of Circuit Flushing. Still, the effect of Circuit Flushing was about 60% of the full ASPIDS effect. This reflects that the y-piece was connected directly to the tracheal tube, thereby minimising the apparatus dead space that is cleared of CO2 only by ASPIDS. While ASPIDS optimally reduces re-inspiration of CO2 from ventilator lines, Circuit Flushing is an easier technique to implement. No extra tube or channel is needed in the tracheal tube and no system for aspiration. As many modern ventilators have a computer controlled inspiratory pneumatic system, Circuit Flushing can be achieved by programming this system to perform Circuit Flushing without any extra tubes, valves or other hardware.
In a recent Editorial Frutos-Vivar et al. suggested that in ARDS 'the ideal ventilation would be that one that does not damage respiratory muscles or lung parenchyma' and 'that individual tailoring may be necessary' [16]. Lung parenchyma is damaged by barotrauma, related to high airway pressure, and by shearing forces at tidal lung collapse and re-opening. Limitation of airway pressure to prevent barotrauma while applying a PEEP high enough to keep the lung open, calls for low or even very low VT. One must consider that a particular dead space reduction allows more than an equal reduction in VT, because it also paves the way for an extra increase in RR and a secondary reduction in VT. This can be understood by considering a system in which dead space would approach zero. Then, VT can be reduced toward zero by approaching infinite RR. PEEP and peak pressure would be similar and lung protection from damaging forces could be truly optimized. The more efficient elimination of CO2 using Circuit Flushing and ASPIDS at RR40 and RR60 would in a clinical setting allow a significant reduction in VT and serve as one step in the direction of lung protection. It is realized that tailoring means much more. In an animal ARDS model, Uttman et al. recently studied how VT might be reduced by tailoring ventilation to actual lung mechanics and dead space [17]. VT could be modestly reduced from 7.2 to 6.6 ml/kg when RR was increased from 40 to 60 minutes-1 and other ventilation parameters optimized. By using ASPIDS, VT could be further reduced to 4.0 ml/kg at RR of 80 minutes-1. It is realized that application of very high respiratory rates is associated with high requirements of tuning ventilation to circumstances. It is associated with significant difficulties with respect to monitoring. Dead space reduction is only a part of a complex strategy. With all respect for the difficulties, it is time to perform clinical studies in which true tailoring of ventilation to physiology is adapted to clinical circumstances and then to apply such techniques in controlled studies.
Conclusions
In conclusion, re-breathing of CO2 rich gas present in the circuit line, although not clinically relevant at health and at low respiratory rates, should be considered when high frequencies are used. Circuit Flushing and ASPIDS were confirmed to be safe and efficient techniques to reduce tubing dead space, re-breathing of CO2 and, accordingly, PaCO2. Our results merit further studies in clinical settings and in different categories of critically ill patients.
Key messages
• Re-breathing of CO2, although not clinically relevant at health and at low RR, should be considered at high RR.
• Minimizing circuit dead space, Circuit Flushing explains 60% of the full Aspiration of dead space.
• Circuit Flushing and Aspiration of dead space are safe and efficient techniques to reduce tubing dead space, re-breathing of CO2 and, PaCO2.
Abbreviations
ASPIDS: aspiration of dead space; LTVV: low tidal volume ventilation; MV: minute ventilation; PawCO2: airway partial pressure of CO2 at the proximal end of the tracheal tube; PEEP: positive end-expiratory pressure; RR: respiratory rate; TGI: tracheal gas insufflation; VDaw, prox: proximal airway dead space; VDaw: airway dead space; VICO2: CO2 re-inspired from Y-piece and tubing per breath; VT: tidal volume; VTCO2: volume of CO2 eliminated per breath.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EDR designed the study, carried out the experiments, analysed row data and drafted the manuscript. LU carried out the experiments and analysed row data. BJ participated in the study design, coordinated the study, and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Edoardo De Robertis, Email: ederober@unina.it.
Leif Uttman, Email: leif.uttman@med.lu.se.
Björn Jonson, Email: bjorn.jonson@med.lu.se.
Acknowledgements
We thank the International Programs Office of the University of Napoli Federico II and the Heart-Lung foundation, Sweden for financial support.
We thank Gert-Inge Jönsson for the construction of the ASPIDS device, Elisabet Åström and Lisbet Niklason for valuable assistance during experiments and in data analysis.
References
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Jonson B, Similowski T, Levy P, Viires N, Pariente R. Expiratory flushing of airways: a method to reduce deadspace ventilation. Eur Respir J. 1990;3:1202–1205. [PubMed] [Google Scholar]
- Marini JJ. Tracheal gas insufflation: a useful adjunct to ventilation? Thorax. 1994;49:735–737. doi: 10.1136/thx.49.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E, Sigurdsson S, Drefeldt B, Jonson B. Aspiration of airway dead space. A new method to enhance CO2 elimination. Am J Respir Crit Care Med. 1999;159:728–732. doi: 10.1164/ajrccm.159.3.9712140. [DOI] [PubMed] [Google Scholar]
- De Robertis E, Servillo G, Jonson B, Tufano R. Aspiration of dead space allows normocapnic ventilation at low tidal volumes in man. Intensive Care Med. 1999;25:674–679. doi: 10.1007/s001340050929. [DOI] [PubMed] [Google Scholar]
- De Robertis E, Servillo G, Tufano R, Jonson B. Aspiration of dead space allows isocapnic low tidal volume ventilation in acute lung injury. Relationships to gas exchange and mechanics. Intensive Care Med. 2001;27:1496–1503. doi: 10.1007/s001340101046. [DOI] [PubMed] [Google Scholar]
- Beydon L, Uttman L, Rawal R, Jonson B. Effects of positive end-expiratory pressure on dead space and its partitions in acute lung injury. Intensive Care Med. 2002;28:1239–1245. doi: 10.1007/s00134-002-1419-y. [DOI] [PubMed] [Google Scholar]
- Fletcher R, Werner O, Nordstrom L, Jonson B. Sources of error and their correction in the measurement of carbon dioxide elimination using the Siemens-Elema CO2 Analyzer. Br J Anaesth. 1983;55:177–185. doi: 10.1093/bja/55.2.177. [DOI] [PubMed] [Google Scholar]
- Svantesson C, Drefeldt B, Sigurdsson S, Larsson A, Brochard L, Jonson B. A single computer-controlled mechanical insufflation allows determination of the pressure-volume relationship of the respiratory system. J Clin Monit Comput. 1999;15:9–16. doi: 10.1023/A:1009916905078. [DOI] [PubMed] [Google Scholar]
- Åström E, Uttman L, Niklason L, Aboab J, Brochard L, Jonson B. Pattern of inspiratory gas delivery affects CO2 elimination in health and after acute lung injury. Intensive Care Med. 2008;34:377–384. doi: 10.1007/s00134-007-0840-7. [DOI] [PubMed] [Google Scholar]
- Devaquet J, Jonson B, Niklason L, Si Larbi AG, Uttman L, Aboab J, Brochard L. Effects of inspiratory pause on CO2 elimination and arterial PCO2 in acute lung injury. J Appl Physiol. 2008;105:1944–1949. doi: 10.1152/japplphysiol.90682.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboab J, Niklason L, Uttman L, Kouatchet A, Brochard L, Jonson B. CO2 elimination at varying inspiratory pause in acute lung injury. Clin Physiol Funct Imaging. 2007;27:2–6. doi: 10.1111/j.1475-097X.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- Wolff G, Brunner JX. Series dead space volume assessed as the mean value of a distribution function. Int J Clin Monit Comput. 1984;1:177–181. doi: 10.1007/BF01872769. [DOI] [PubMed] [Google Scholar]
- Uhlig S, Ranieri M, Slutsky AS. Biotrauma hypothesis of ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;169:314–315. doi: 10.1164/ajrccm.169.2.950. [DOI] [PubMed] [Google Scholar]
- Taskar V, John J, Larsson A, Wetterberg T, Jonson B. Dynamics of carbon dioxide elimination following ventilator resetting. Chest. 1995;108:196–202. doi: 10.1378/chest.108.1.196. [DOI] [PubMed] [Google Scholar]
- Frutos-Vivar F, Ferguson ND, Esteban A. Mechanical ventilation: quo vadis? Intensive Care Med. 2009;35:775–778. doi: 10.1007/s00134-009-1450-3. [DOI] [PubMed] [Google Scholar]
- Uttman L, Ögren H, Niklason L, Drefeldt B, Jonson B. Computer simulation allows goal-oriented mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2007;11:R36. doi: 10.1186/cc5719. [DOI] [PMC free article] [PubMed] [Google Scholar]