Abstract
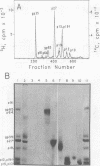
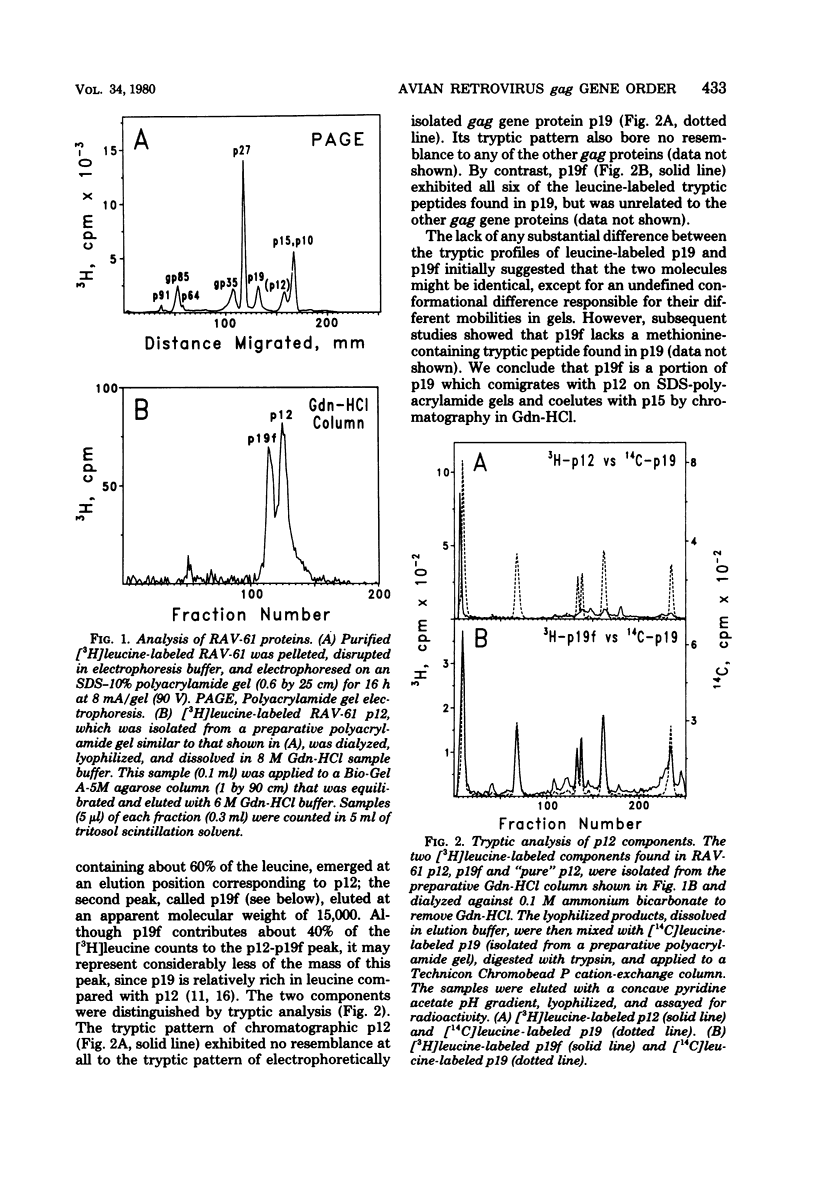
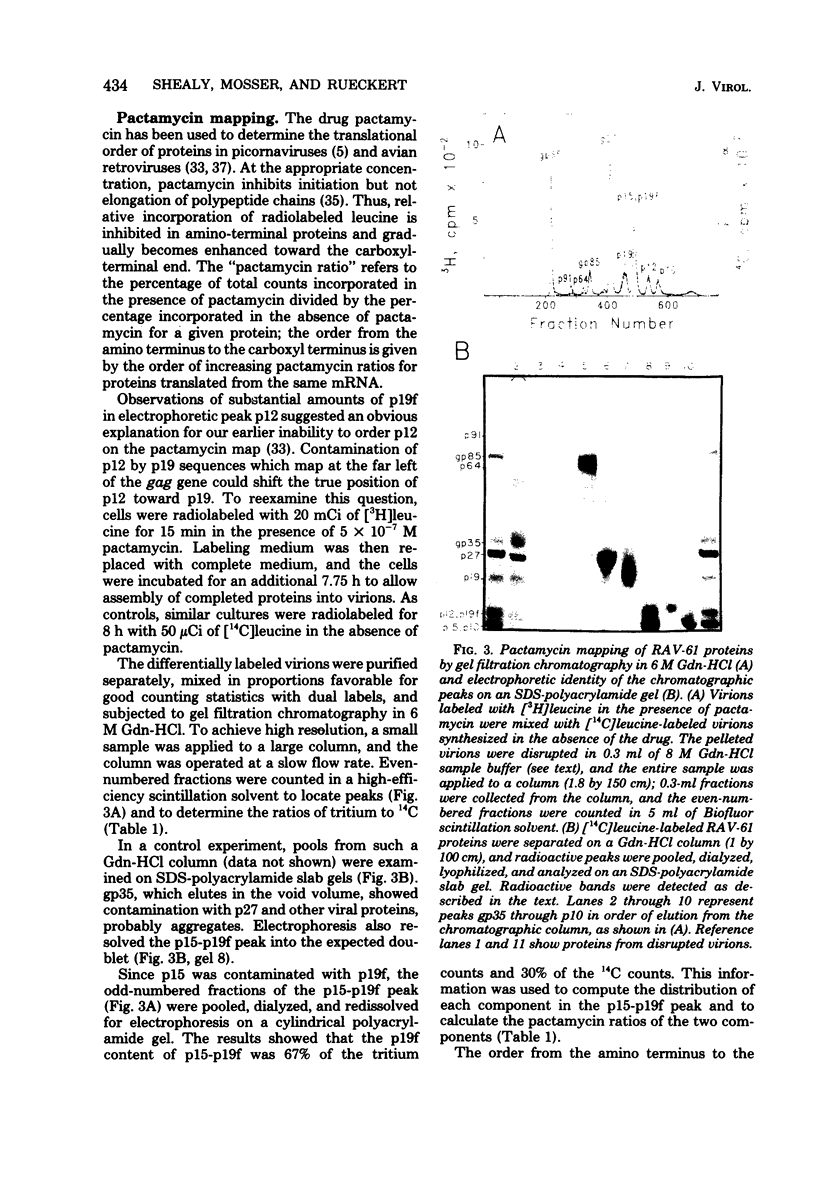
Virions of Rous-associated virus type 61 contain a previously unrecognized p19-related protein, called p19f, which comigrates with gag protein p12 during electrophoresis in sodium dodecyl sulfate-polyacrylamide gels but can be separated by gel filtration chromatography in 6 M guanidine hydrochloride. It is shown that the existence of p19f accounts for the earlier inability to order p27 and p12 by the pactamycin mapping procedure. Remapping with pactamycin by using methods which take this new protein into account yielded a gag gene order of NH2-p219-p27-p12-p15-COOH. It also confirmed earlier positions for the env and pol genes and placed unclassified protein p10 near a translational initiation site. The pactamycin-derived mapping position of p12 differs from reports based on tryptic analysis. An analysis of procedural shortcomings emphasizes the need for more definitive determinations of the avian gag gene order.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Stephenson J. R., Aaronson S. A. gag Gene of mammalian type-C RNA tumour viruses. Nature. 1976 Aug 12;262(5569):554–559. doi: 10.1038/262554a0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Ishizaki R., Hüper G., Vanaman T. C., Smith R. E. Immunological properties of avian oncornavirus polypeptides. Virology. 1975 Apr;64(2):349–357. doi: 10.1016/0042-6822(75)90111-7. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Gene order of encephalomyocarditis virus as determined by studies with pactamycin. J Virol. 1972 May;9(5):823–828. doi: 10.1128/jvi.9.5.823-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- England J. M., Bolognesi D. P., Dietzschold B., Halpern M. S. Evidence that a precursor glycoprotein is cleaved to yield the major glycoprotein of avian tumor virus. J Virol. 1977 Feb;21(2):810–814. doi: 10.1128/jvi.21.2.810-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P., Nowinski R. C., Tress E., Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. III. Avian viral proteins with group-specific antigenicity. Virology. 1975 Apr;64(2):358–366. doi: 10.1016/0042-6822(75)90112-9. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Gilson W., Gilson R., Rueckert R. R. An automatic high-precision acrylamide gel fractionator. Anal Biochem. 1972 Jun;47(2):321–328. doi: 10.1016/0003-2697(72)90125-x. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Lewandowski L. J. Isolation of the major viral glycoprotein and a putative precursor from cells transformed by avian sarcoma viruses. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2342–2346. doi: 10.1073/pnas.71.6.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A. C., Green R. W., Bolognesi D. P., Vanaman T. C. Comparative chemical properties of avian oncornavirus polypeptides. Virology. 1975 Apr;64(2):339–348. doi: 10.1016/0042-6822(75)90110-5. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline leukemia virus: biochemical and immunological characterization of gag gene-coded structural proteins. J Virol. 1977 Sep;23(3):599–607. doi: 10.1128/jvi.23.3.599-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline sarcoma virus-coded polyprotein: enzymatic cleavage by a type C virus-coded structural protein. J Virol. 1979 Feb;29(2):649–656. doi: 10.1128/jvi.29.2.649-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. The generation of the two envelope glycoproteins of Rous sarcoma virus from a common precursor polypeptide. Virology. 1978 Mar;85(1):63–74. doi: 10.1016/0042-6822(78)90411-7. [DOI] [PubMed] [Google Scholar]
- Lai M. M. Phosphoproteins of Rous sarcoma viruses. Virology. 1976 Oct 15;74(2):287–301. doi: 10.1016/0042-6822(76)90336-6. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Mosser A. G., Montelaro R. C., Rueckert R. R. Proteins of Rous-associated virus type 61: polypeptide stoichiometry and evidence that glycoprotein gp35 is not a cleavage product of gp85. J Virol. 1977 Jul;23(1):10–19. doi: 10.1128/jvi.23.1.10-19.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser A. G. Polypeptide composition of spleen necrosis virus, a reticuloendotheliosis virus. J Virol. 1975 May;15(5):1088–1095. doi: 10.1128/jvi.15.5.1088-1095.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Tryptic peptide analyses of polypeptides generated by premature termination of cell-free protein synthesis allow a determination of the Rauscher leukemia virus gag gene order. J Virol. 1978 Dec;28(3):929–935. doi: 10.1128/jvi.28.3.929-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Karess R. E., Anderson S. M., Hanafusa H. Tryptic peptide analysis of avian oncovirus gag and pol gene products. J Virol. 1979 Oct;32(1):102–113. doi: 10.1128/jvi.32.1.102-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. K., Stephenson J. R. Intracistronic mapping of the murine type C viral gag gene by use of conditional lethal replication mutants. Virology. 1977 Sep;81(2):328–340. doi: 10.1016/0042-6822(77)90149-0. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977 Jan;10(1):91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Shaikh R., Linial M., Brown S., Sen A., Eisenman R. Recombinant avian oncoviruses. II. Alterations in the gag proteins and evidence for intragenic recombination. Virology. 1979 Jan 30;92(2):463–481. doi: 10.1016/0042-6822(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Shealy D. J., Rueckert R. R. Proteins of Rous-associated virus 61, an avian retrovirus: common precursor for glycoproteins gp85 and gp35 and use of pactamycin to map translational order of proteins in the gag, pol, and env genes. J Virol. 1978 May;26(2):380–388. doi: 10.1128/jvi.26.2.380-388.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Kew O., Pallansch M., Rueckert R., Kaesberg P. Cell-free synthesis and processing of the proteins of poliovirus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5807–5811. doi: 10.1073/pnas.75.12.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Blair M. L., Yanowitz I. S., Goldberg I. H. Inhibition of synthesis of new globin chains in reticulocyte lysates by pactamycin. Biochemistry. 1971 Nov;10(23):4198–4206. doi: 10.1021/bi00799a007. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]