Abstract
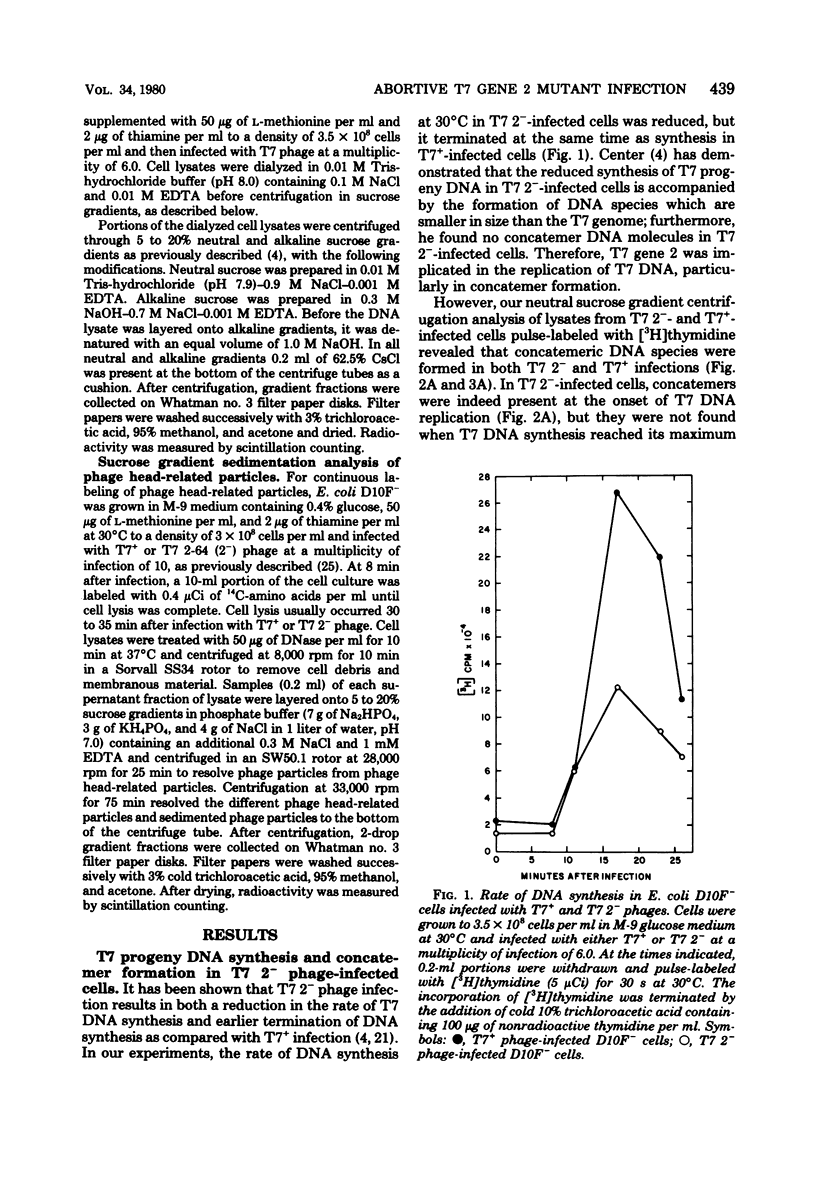
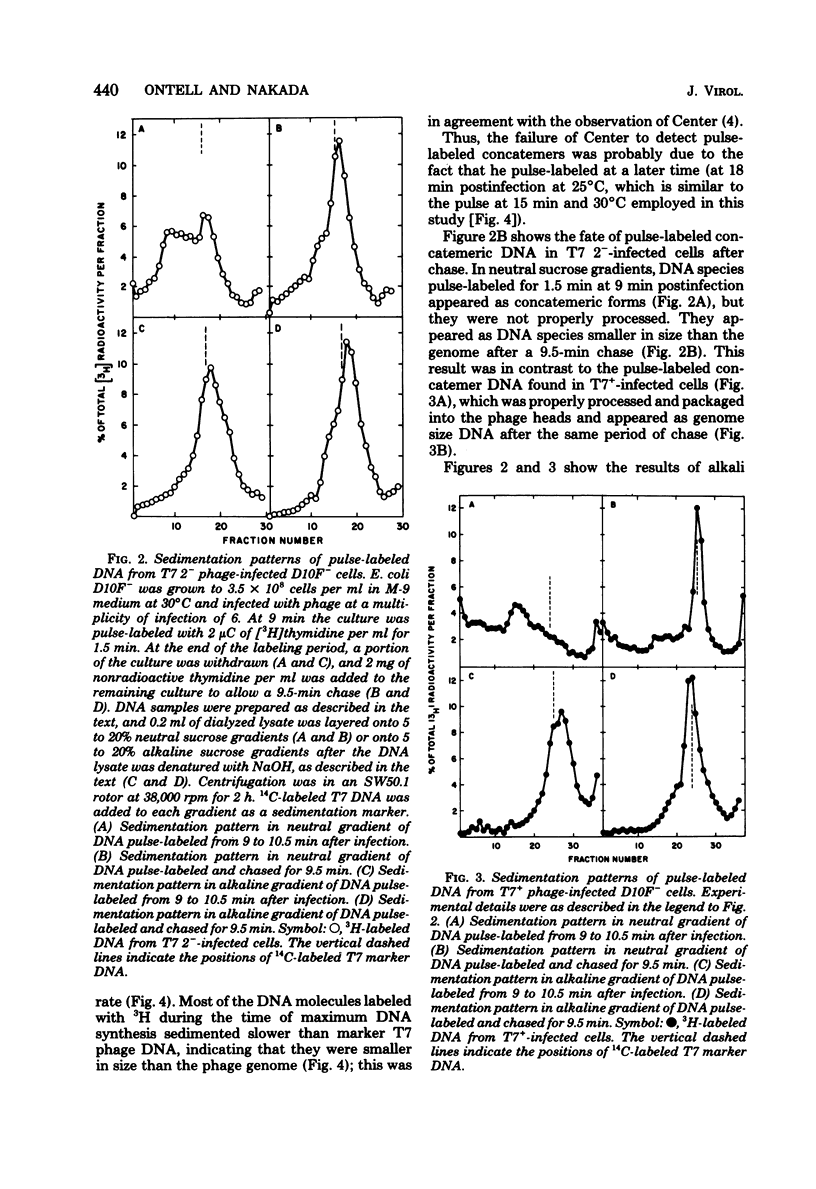
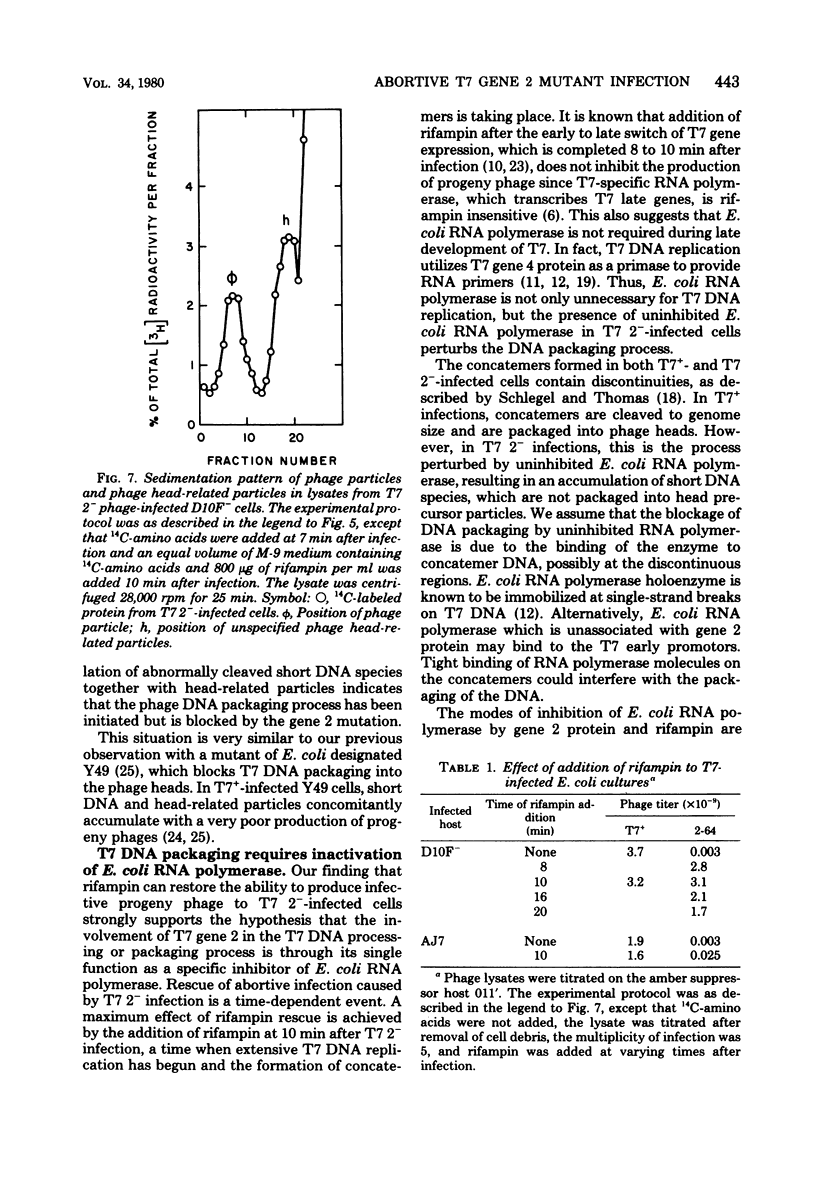
Infection of Escherichia coli with T7 gene 2 mutant phage was abortive; concatemeric phage DNA was synthesized but was not packaged into the phage head, resulting in an accumulation of DNA species shorter in size than the phage genome, concomitant with an accumulation of phage head-related structures. Appearance of concatemeric T7 DNA in gene 2 mutant phage infection during onset of T7 DNA replication indicates that the product of gene 2 was required for proper processing or packaging of concatemer DNA rather than for the synthesis of T7 progeny DNA or concatemer formation. This abortive infection by gene 2 mutant phage could be rescued by rifampin. If rifampin was added at the onset of T7 DNA replication, concatemeric DNA molecules were properly packaged into phage heads, as evidenced by the production of infectious progeny phage. Since the gene 2 product acts as a specific inhibitor of E. coli RNA polymerase by preventing the enzyme from binding T7 DNA, uninhibited E. coli RNA polymerase in gene 2 mutant phage-infected cells interacts with concatemeric T7 DNA and perturbs proper DNA processing unless another inhibitor of the enzyme (rifampin) was added. Therefore, the involvement of gene 2 protein in T7 DNA processing may be due to its single function as the specific inhibitor of the host E. coli RNA polymerase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd D. H., Zillig W. Reference mutations for the beta subunit of RNA polymerase. Mol Gen Genet. 1974 Jun 27;130(4):315–320. doi: 10.1007/BF00333870. [DOI] [PubMed] [Google Scholar]
- Brunovskis I., Summers W. C. The process of infection with coliphage 17. VI. A phage gene controlling shutoff of host RNA synthesis. Virology. 1972 Nov;50(2):322–327. doi: 10.1016/0042-6822(72)90383-2. [DOI] [PubMed] [Google Scholar]
- Brunovskis I., Summers W. C. The process of infection with coliphage T7. V. Shutoff of host RNA synthesis by an early phage function. Virology. 1971 Jul;45(1):224–231. doi: 10.1016/0042-6822(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Center M. S. Role of gene 2 in bacteriophage T7 DNA synthesis. J Virol. 1975 Jul;16(1):94–100. doi: 10.1128/jvi.16.1.94-100.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S., Studier F. W., Richardson C. C. The structural gene for a T7 endonuclease essential for phage DNA synthesis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):242–248. doi: 10.1073/pnas.65.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Hayward R. S., Scaife J. G. Systematic nomenclature for the RNA polymerase genes of prokaryotes. Nature. 1976 Apr 15;260(5552):646–648. doi: 10.1038/260646a0. [DOI] [PubMed] [Google Scholar]
- Hesselbach B. A., Nakada D. "Host shutoff" function of bacteriophage T7: involvement of T7 gene 2 and gene 0.7 in the inactivation of Escherichia coli RNA polymerase. J Virol. 1977 Dec;24(3):736–745. doi: 10.1128/jvi.24.3.736-745.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbach B. A., Nakada D. I protein: bacteriophage T7-coded inhibitor of Escherichia coli RNA polymerase. J Virol. 1977 Dec;24(3):746–760. doi: 10.1128/jvi.24.3.746-760.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbach B. A., Nakada D. Inactive complex formation between E. coli RNA polymerase and inhibitor protein purified from T7 phage infected cells. Nature. 1975 Nov 27;258(5533):354–357. doi: 10.1038/258354a0. [DOI] [PubMed] [Google Scholar]
- Hesselbach B. A., Yamada Y., Nakada D. Isolation of an inhibitor protein of E. coli RNA polymerase from T7 phage infected cell. Nature. 1974 Nov 1;252(5478):71–74. doi: 10.1038/252071b0. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro. Purification and properties of the gene 4 protein of bacteriophage T7. J Biol Chem. 1975 Jul 25;250(14):5523–5529. [PubMed] [Google Scholar]
- Hinkle D. C., Ring J., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. 3. Tight binding of RNA polymerase holoenzyme to single-strand breaks in T7 DNA. J Mol Biol. 1972 Sep 28;70(2):197–207. doi: 10.1016/0022-2836(72)90533-5. [DOI] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Gene 6 exonuclease of bacteriophage T7. I. Purification and properties of the enzyme. J Biol Chem. 1972 Jan 10;247(1):305–310. [PubMed] [Google Scholar]
- LeClerc J. E., Richardson C. C. Gene 2 protein of bacteriophage T7: purification and requirement for packaging of T7 DNA in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4852–4856. doi: 10.1073/pnas.76.10.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Seiffert D. Studies on bacteriophage T7 DNA synthesis in vitro. I. Resolution of the T7 replication system into its components. Mol Gen Genet. 1975 Dec 1;141(3):213–232. doi: 10.1007/BF00341801. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Thomas C. A., Jr Some special structural features of intracellular bacteriophage T7 concatemers. J Mol Biol. 1972 Jul 21;68(2):319–345. doi: 10.1016/0022-2836(72)90216-1. [DOI] [PubMed] [Google Scholar]
- Strome S., Young E. T. Translational control of the expression of bacteriophage T7 gene 0.3. J Mol Biol. 1978 Oct 15;125(1):75–93. doi: 10.1016/0022-2836(78)90255-3. [DOI] [PubMed] [Google Scholar]
- Strätling W., Knippers R. Function and purification of gene 4 protein of phage T7. Nature. 1973 Sep 28;245(5422):195–197. doi: 10.1038/245195a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Silnutzer J., Nakada D. Accumulation of bacteriophage T7 head-related particles in an Escherichia coli mutant. J Virol. 1979 Jul;31(1):209–219. doi: 10.1128/jvi.31.1.209-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Silnutzer J., Nakada D. Mutant of Escherichia coli which blocks T7 bacteriophage assembly: accumulation of short T7 DNA. J Mol Biol. 1978 May 5;121(1):95–111. doi: 10.1016/0022-2836(78)90264-4. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Whitaker P. A., Nakada D. Chemical stability of bacteriophage T7 early mRNA. J Virol. 1975 Dec;16(6):1683–1687. doi: 10.1128/jvi.16.6.1683-1687.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Whitaker P. A., Nakada D. Functional instability of T7 early mRNA. Nature. 1974 Mar 22;248(446):335–338. doi: 10.1038/248335a0. [DOI] [PubMed] [Google Scholar]


