Abstract
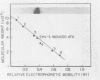
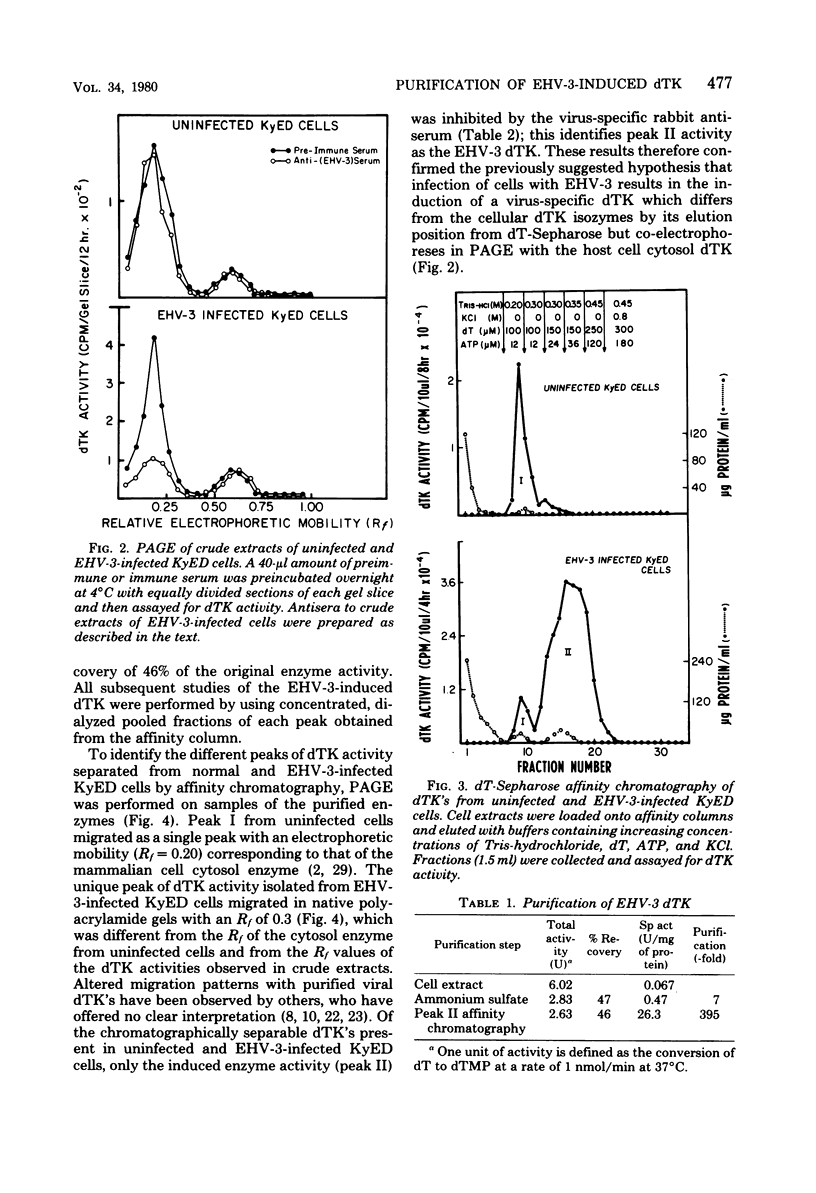
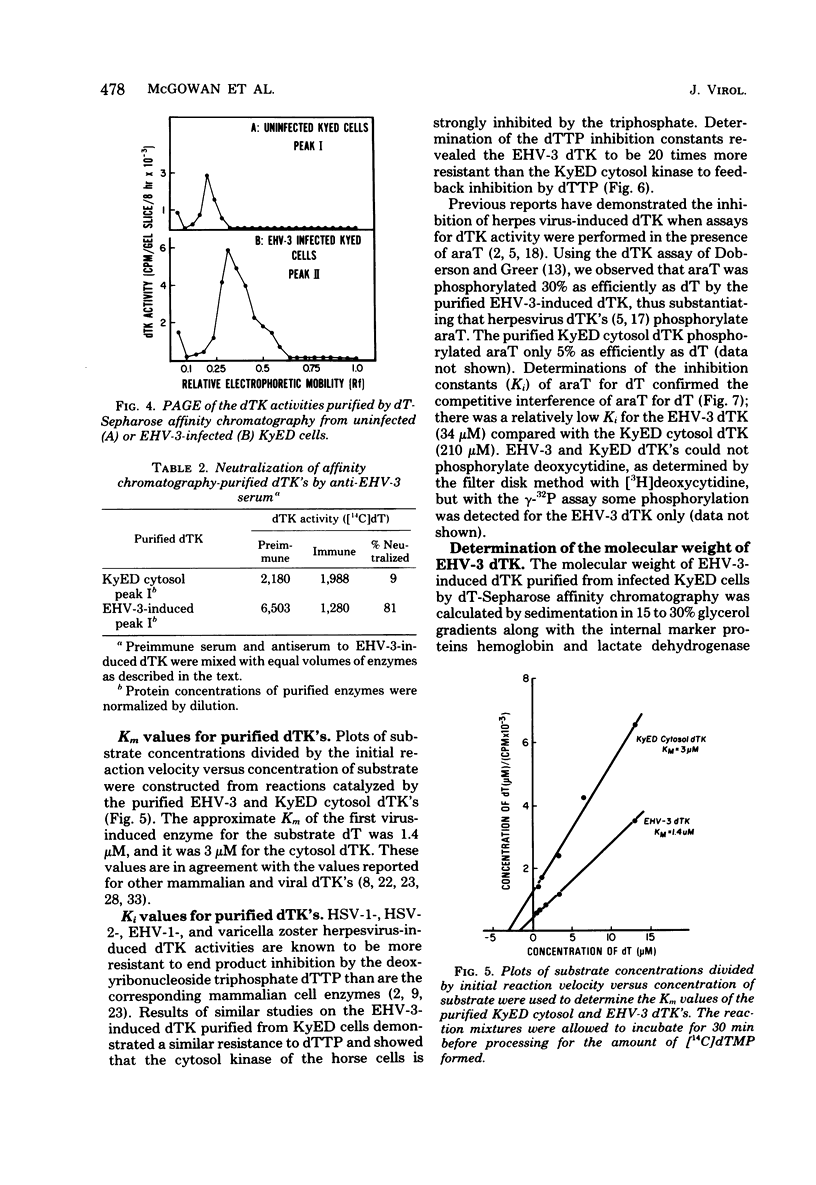
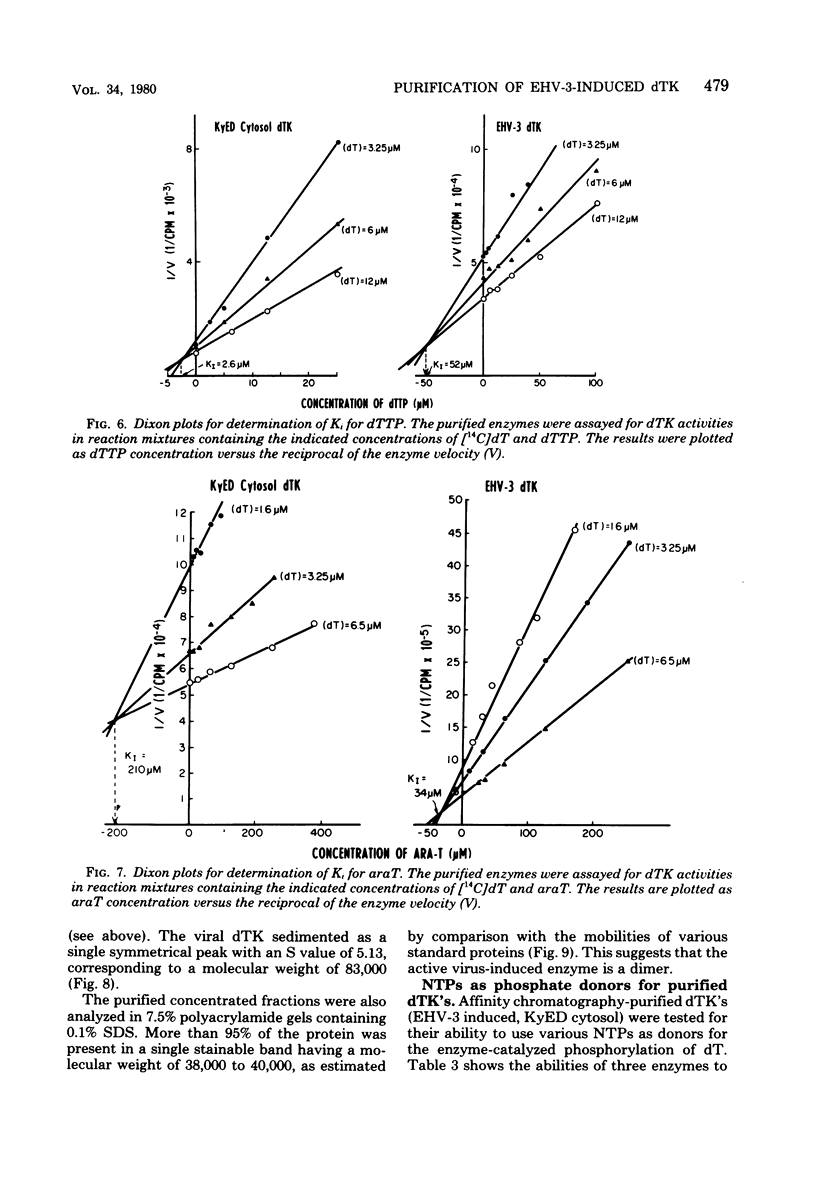
Infection of horse KyED cells with equine herpesvirus type 3 (EHV-3) resulted in a sevenfold increase in cytosol deoxythymidine kinase (dTK) activity. The EHV-3 dTK was purified from KyED cytosol dTK by affinity chromatography on deoxythymidine-Sepharose and characterized with respect to its electrophoretic mobility, molecular weight, substrate specificity, phosphate donor specificity, and immunological specificity. The purified EHV-3 dTK migrated in polyacrylamide gels with an Rf of 0.30 and sedimented in glycerol gradients with an S value of 5.13, corresponding to a molecular weight of 83,000. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis yielded a single band with a molecular weight of 38,000 to 40,000. Antiserum prepared against the EHV-3 dTK induced in KyED cells neutralized the EHV-3-induced enzyme activity but not the dTK purified from uninfected cells. EHV-3 dTK was less sensitive to feedback inhibition to dTTP and had a lower Ki for the antiviral compound 1-beta-D-arabinofuranyosylthymine and a lower Km for the substrate deoxythymidine. These results indicate that infection of cells with EHV-3 results in the induction of a new virus-coded dTK activity which meets the criteria of Jensen for an evolutionary primitive enzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., Bryans J. T. Replication of equine herpesvirus type 3: kinetics of infectious particle formation and virus nucleic acid synthesis. J Gen Virol. 1977 Mar;34(3):421–430. doi: 10.1099/0022-1317-34-3-421. [DOI] [PubMed] [Google Scholar]
- Allen G. P., McGowan J. J., Bryans J. T., Randall C. C., Gentry G. A. Induction of deoxythymidine kinase activity in several mammalian cell lines after infection with six different strains of equine herpesvirus type 1 (EHV-1). Virology. 1978 Oct 15;90(2):351–359. doi: 10.1016/0042-6822(78)90319-7. [DOI] [PubMed] [Google Scholar]
- Allen G. P., McGowan J. J., Randall C. C., Mancini W., Cheng Y. C., Gentry G. A. Purification and characterization of deoxythymidine kinase (dTK) induced in dTK- 3T3 mouse cells by equine herpesvirus type 1 (EHV-1). Virology. 1979 Jan 30;92(2):367–374. doi: 10.1016/0042-6822(79)90141-7. [DOI] [PubMed] [Google Scholar]
- Anderson C. M., Zucker F. H., Steitz T. A. Space-filling models of kinase clefts and conformation changes. Science. 1979 Apr 27;204(4391):375–380. doi: 10.1126/science.220706. [DOI] [PubMed] [Google Scholar]
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti S., Graham F. L. Characterization of human TK- cell lines transformed to a TK+ phenotype by herpes simplex virus type 2 DNA. J Gen Virol. 1979 Jan;42(1):149–157. doi: 10.1099/0022-1317-42-1-149. [DOI] [PubMed] [Google Scholar]
- Barnett J. M., McGowan J. J., Gentry G. A. Arabinosylthymine: inhibitor of splenic lymphocyte macromolecular synthesis in vitro. Infect Immun. 1979 Oct;26(1):294–297. doi: 10.1128/iai.26.1.294-297.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C. A rational approach to the development of antiviral chemotherapy: alternative substrates of herpes simplex virus Type 1 (HSV-1) and Type 2 (HSV-2) thymidine kinase (TK). Ann N Y Acad Sci. 1977 Mar 4;284:594–598. doi: 10.1111/j.1749-6632.1977.tb21992.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C. Deoxythymidine kinase induced in the HELA TK- cells by herpes simplex virus type I and type II. Substrate specificity and kinetic behavior. Biochim Biophys Acta. 1976 Dec 8;452(2):370–381. doi: 10.1016/0005-2744(76)90186-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Cheng Y. C., Tsou T. Y., Hackstadt T., Mallavia L. P. Induction of thymidine kinase and DNase in varicella-zoster virus-infected cells and kinetic properties of the virus-induced thymidine kinase. J Virol. 1979 Jul;31(1):172–177. doi: 10.1128/jvi.31.1.172-177.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S. Comparative biochemistry and drug design for infectious disease. Science. 1979 Sep 7;205(4410):964–971. doi: 10.1126/science.382357. [DOI] [PubMed] [Google Scholar]
- Dobersen M. J., Greer S. An assay for pyrimidine deoxyribonucleoside kinase using gamma-32P-labeled ATP. Anal Biochem. 1975 Aug;67(2):602–610. doi: 10.1016/0003-2697(75)90335-8. [DOI] [PubMed] [Google Scholar]
- Dobersen M. J., Greer S. Herpes simplex virus type 2 induced pyrimidine nucleoside kinase: enzymatic basis for the selective antiherpetic effect of 5-halogenated analogues of deoxycytidine. Biochemistry. 1978 Mar 7;17(5):920–928. doi: 10.1021/bi00598a028. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus-specific polypeptides studied by polyacrylamide gel electrophoresis of immune precipitates. J Gen Virol. 1974 Feb;22(2):171–185. doi: 10.1099/0022-1317-22-2-171. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- Kemp M. C., Perdue M. L., Rogers H. W., O'Callaghan D. J., Randall C. C. Structural polypeptides of the hamster strain of equine herpes virus type 1: products associated with purification. Virology. 1974 Oct;61(2):361–375. doi: 10.1016/0042-6822(74)90274-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Jorgensen G. N., Liav A., Zaslavsky V. Purification of vaccinia virus-induced thymidine kinase activity from [35S]methionine-labeled cells. Virology. 1977 Apr;77(2):661–676. doi: 10.1016/0042-6822(77)90490-1. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Thymidine kinase isozymes of normal and virus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):703–715. doi: 10.1101/sqb.1974.039.01.084. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Viral-induced thymidine kinase isozymes. Prog Med Virol. 1975;21:13–34. [PubMed] [Google Scholar]
- Kowal E. P., Markus G. Affinity chromatography of thymidine kinase from a rat colon adenocarcinoma. Prep Biochem. 1976;6(5):369–385. doi: 10.1080/00327487608061625. [DOI] [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- Leung W. C., Dubbs D. R., Trkula D., Kit S. Mitochondrial and herpesvirus-specific deoxypyrimidine kinases. J Virol. 1975 Sep;16(3):486–497. doi: 10.1128/jvi.16.3.486-497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Matsukage A., Ono K., Ohashi A., Takahashi T., Nakayama C., Saneyoshi M. Inhibitory effect of 1-beta-D-arabinofuranosylthymine 5'-triphosphate and 1-beta-D-arabinofuranosylcytosine 5'-triphosphate on DNA polymerases from murine cells and oncornavirus. Cancer Res. 1978 Sep;38(9):3076–3079. [PubMed] [Google Scholar]
- McGowan J. J., Allen G. P., Gentry G. A. Purification and biochemical characterization of deoxythymidine kinase of deoxythymidine kinase-deficient mouse 3T3 cells biochemically transformed by equine herpesvirus type 1. Infect Immun. 1979 Aug;25(2):610–614. doi: 10.1128/iai.25.2.610-614.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Wildy P. Deoxypyrimidine kinases of herpes simplex viruses types 1 and 2: comparison of serological and structural properties. J Gen Virol. 1975 Feb;26(2):159–170. doi: 10.1099/0022-1317-26-2-159. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., van Ketel R., Becker-Bloemkolk M. J. A sensitive method for the detection of herpes simplex virus type 2 specific thymidine kinase. Intervirology. 1977;8(3):186–192. doi: 10.1159/000148894. [DOI] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]