Abstract
Impairment in blood-to-brain transport of leptin is a major cause as well as consequence of obesity. Leptin crosses the blood-brain barrier by transcytosis rather than undergoing intracellular degradation. Results from previous studies have indicated that the membrane juxtapositional cytoplasmic sequence of the leptin receptor ObR is responsible for leptin transport. To identify the specific structural domains, we generated a series of ObR truncates with different lengths of the intracellular sequence, overexpressed them in 3 types of mammalian cells including cerebral endothelia, and quantified leptin binding and endocytosis. All mutant ObRs were able to bind and mediate the internalization of leptin. Surprisingly, ObR860, a construct with no cytoplasmic sequence, could act like the classical ObRa transporter in internalizing leptin. There were some cell type-dependent variations in the intracellular trafficking of Alexa-labeled leptin when mediated by ObR860 or ObRa because of differential involvement of membrane microdomains, as shown by use of the clathrin inhibitor chlorpromazine and the dynamin inhibitor Dynasore. The clathrin- and dynamin-mediated endocytosis of leptin contrasts with the lack of effect of the caveolae inhibitors nystatin and filipin. Thus, leptin-induced internalization of the ligand-receptor complex can occur without specific sorting signals in the cytoplasmic region of ObR. This novel finding may have significant implications for leptin transport.—Tu, H., Hsuchou, H., Kastin, A. J., Wu, X., Pan, W. Unique leptin trafficking by a tailless receptor.
Keywords: ObR, endocytosis motif
Leptin is a 16-kDa protein produced mainly by adipocytes in adulthood. It induces feeding suppression and neuroprotection in the CNS, stimulates musculoskeletal development, and modulates immune function. Its specific receptors are type I glycoproteins structurally related to class I cytokine receptors, and they encompass several isoforms generated by alternative splicing of the RNA transcript (1, 2). All ObRs share an identical N-terminal extracellular domain. With the exception of the soluble ObRe (aa 22–806 in mice) that inhibits leptin transport (3), the isoforms also use a common transmembrane domain (aa 840–860) and a membrane juxtapositional 29 aa of the cytoplasmic domain. ObRb (aa 22–1162) has the longest cytoplasmic tail, activates signal transducer and activator of transcription 3 (STAT3), and is abundantly expressed in the hypothalamus. The shorter transmembrane receptors include ObRa (aa 22–894), ObRc (aa 22–892), ObRd (aa 22–900, in mice), and ObRf (in rats). Like ObRb, these receptors also have a proline-rich Box 1 sequence of 9 aa (aa 869–877) required for activation of Janus kinase 2 (JAK2). ObRa is abundantly present in cerebral microvessels and thus plays a major role in mediating leptin transport across the blood-brain barrier (BBB) (4,5,6,7).
Resistance to leptin is the major cause of obesity in humans and in many rodent obesity models. Considerable evidence indicates that this resistance is mainly because of impairment in the blood to brain transport of leptin. Obese humans who have a 300% elevation in serum levels of leptin have only a 30% elevation in cerebrospinal fluid levels of leptin (8, 9). Obese rodents do not respond to peripherally administered leptin by reduction of food intake; however, centrally administered leptin remains effective (10,11,12). Although the presence of a specific transport system for leptin to cross the BBB was reported as early as 1996 (4), the molecular basis of leptin transport is not yet fully understood.
Recently, we showed that all ObRs can effectively transport leptin with similar rates of exocytosis in intact form, given equal amounts of cell surface expression (13). It has also been reported that the endocytosis of leptin is mediated by clathrin-coated pits (14,15,16,17). However, the motifs involved in the interactions of ObRs with adaptor protein-2 (AP-2) complexes have not yet been identified. Studies on Hela cells transiently transfected with ObRa or ObRb indicate that the constitutive endocytosis of the receptor isoforms is ligand independent but differs for each, suggesting a role of the cytoplasmic domain in receptor endocytosis. There are two ubiquitylation sites in the cytoplasmic tail of ObRa (K877 and K889). Mutation of these lysine residues abolishes clathrin-mediated endocytosis and lysosomal degradation of ObRa. This led to the speculation that a monoubiquitin conjugate acts as the internalization motif for clathrin-dependent endocytosis of ObRa (15, 18).
By contrast, studies in Chinese hamster ovary (CHO) or COS cells transiently transfected with ObRa or its mutants show that neither deletion of the last 5 aa at the C-terminus of ObRa, nor mutation of the JAK Box 1 motif (aa 869–877), affect 125I-leptin binding or endocytosis. Nonetheless, deletion of 26 aa at the C terminus has been reported to induce a 40–60% reduction of 125I-leptin endocytosis without changes in surface binding (16). This led to the speculation that there is a potential internalization motif located between aa 8 and 29 of the intracellular domain of ObRa. Since the truncated ObR can still mediate ∼50% of leptin endocytosis, the result also suggests that other motifs, possibly located in the extreme membrane proximal region of ObRa, may play a role in internalization of leptin and ObR. To address this point, we generated a series of ObR truncates and studied the endocytosis of both 125I- and Alexa555-labeled leptin in transient transfected cells. We also tested the effects of different classes of endocytosis inhibitors to determine the pathways involved in leptin endocytosis.
MATERIALS AND METHODS
Plasmid construction
To generate truncated ObRs by PCR, mouse ObRb plasmid DNA (kindly provided by Dr. Christian Bjorbaek, Harvard Medical School, Boston, MA, USA) was used as the template. The forward and reverse primers contained XbaI and HindIII restriction enzyme sites, respectively. The sequences for the shared forward primer and distinctive reverse primers are listed in Table 1. A stop codon TAA was inserted into the primer at the end of the ObR sequence. High Fidelity Accu Prime TaqDNA polymerase (Invitrogen, Carlsbad, CA, USA) was used for PCR amplification. PCR products were cloned into the pcDNA3.1(−) vector (Invitrogen). The plasmids were verified by restriction enzyme digestion and DNA automated sequencing. After transient transfection of human embryonic kidney (HEK)293 cells, receptor protein expression was confirmed by Western blot and immunofluorescent staining with a goat polyclonal antibody against the shared N terminus of ObR (R&D Systems, Minneapolis, MN, USA).
TABLE 1.
PCR primers for subcloning of truncated ObRs
| Name | Sequence |
|---|---|
| Forward primer | 5′-TGTCTAGAGCCACCATGATGTGTCAGAAATTCTATGTGG |
| ObR860 reverse | 5′-ACGAAGCTTTTAAATTAACAGTGTTCCGAGCAGTAG |
| ObR865 reverse | 5′-ACGAAGCTTTTACATTCTCTGGTGTGAAATTAACAG |
| ObR875 reverse | 5′-ACGAAGCTTTTAGTTTGGAACATCGTCCCAAAACA |
| ObR889 reverse | 5′-ACGAAGCTTTTACTTTTGGAAATTCAGTCCTTGTG |
| ObR895 reverse | 5′-ACGAAGCTTTTACTCAAATGTTTCAGGCTTTTGGAA |
| ObR907 reverse | 5′-ACGAAGCTTTTAACCAAATATCACTGATTCTGCATG |
| ObR1137 reverse | 5′-TACAAAGTTCTCACCAGAGGTCCACGAAGCTTTTA |
Cell culture and transfection
HEK293 cells and CHO cells were obtained from American Type Culture Collection (Manassas, VA, USA). They were grown in DMEM supplemented with 10% FBS and antibiotics at 37°C with 5% CO2. RBE4 cells of rat cerebral microvessel endothelial cell origin were kindly provided by Dr. Pierre-Olivier Couraud (Institut Cochin, INSERM, Paris, France), and maintained in αMEM and F10 with 10% FBS, geneticin, penicillin, streptomycin, and antimycotics. For transient transfection with Lipofectamine 2000 (Invitrogen), cells were grown in 6- or 12-well plates overnight in antibody-free medium to achieve ∼90–95% confluency. Two micrograms of plasmid DNA/well for 6-well plates or 0.8 μg/well for 12-well plates was used. The cells were studied 24 h after transfection. In all studies, a group transfected with the pcDNA3.1 empty vector was included as a negative control.
Luciferase reporter assays
HEK293 cells grown in 6-well plates were cotransfected with ObR DNA and the STAT1-luc reporter pGAS (Invitrogen) by use of Lipofectamine 2000 reagent. At 24 h after transfection, cells were cultured in serum-free medium for an additional 24 h, and then treated with leptin (50 nM) for 6 h. Cellular proteins were extracted by cell lysis buffer (Promega, Madison, WI, USA) and analyzed for luciferase activity according to the manufacturer’s instructions. The relative luminescent units indicating STAT1 activity were normalized to the amount of the respective total cellular protein.
Fluorescent labeling of leptin
Alexa Fluor 555 or 568 protein labeling kits (Molecular Probes, Eugene, OR, USA) were used to label carrier-free leptin (R&D Systems). One milligram of mouse recombinant leptin was added to the reaction vial and incubated for 1 h at room temperature. The reaction mixture was loaded unto a Bio-Gel P10 Gel column (Bio-Rad Laboratories, Hercules, CA, USA) and eluted with PBS. The elution was monitored under a UV lamp. Forty fractions were collected. The protein concentrations of Alexa555- or Alexa568-labeled leptin were determined with a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA).
Iodination of leptin
Ten micrograms of mouse recombinant leptin was radioactively labeled by incubation with chloramine-T (0.25 mg/ml of final concentration) and 125I-Na (0.5 mCi) at room temperature for 1 min. The reaction was stopped by the addition of sodium metabisulfite (0.7 mg/ml of final concentration). 125I-leptin was purified on Sephadex G-10 columns, yielding a specific activity of ∼40–50 Ci/g. The acid precipitability of 125I-leptin was >96% for all experiments.
Western blot analysis
To test the level of ObR protein expression, 80 μg of protein extracted from ObR-transfected HEK293 cells was loaded onto 7% polyacrylamide gels and electrophoresed for 2 h. The proteins were transferred to nitrocellulose membrane (Bio-Rad Laboratories), and probed with a primary goat antibody against the shared extracellular domain of mouse leptin receptor (R&D Systems). After subsequent incubation with an HRP-conjugated secondary antibody (Pierce Biotechnology), the signal was developed with an enhanced chemiluminescent (ECL) plus Western blotting Detection System (GE Healthcare, Piscataway, NJ, USA). To test the STAT3 signaling pathway activated by leptin, HEK293 cells transfected with ObR DNA for 24 h were serum starved for 24 h and treated with mouse recombinant leptin (50 nM; Sigma, St. Louis, MO, USA) for 30 min. The lysates were resolved by electrophoresis, transferred to nitrocellulose, and probed with anti-phospho-STAT3 Tyr705 (Millipore, Billerica, MA, USA). Proteins were visualized with a sheep anti-mouse secondary antibody conjugated to HRP (GE Healthcare, New York, NY, USA) and the ECL plus system.
Surface binding and endocytosis of 125I-leptin
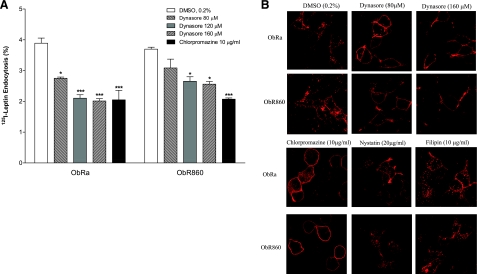
To determine the percentage surface binding, 125I-leptin (7×105 cpm/ml) was incubated with the cells on ice for 1 h. Specific binding was determined from the amount removed by acid wash with cold strip buffer (0.2 N acetic acid and 0.1 M NaCl, pH 3.5), after subtraction of the background shown in the control group in the presence of 200-fold excess of leptin. The cellular uptake assays were performed as described previously (3, 19,20,21,22). Briefly, the cells were preequilibrated in transport buffer (basic medium containing 25 mM HEPES and 0.5% albumin) at 37°C for 30 min before assay with the radioactively labeled tracer. Then the cells were incubated with 125I-leptin for 30 min at 37°C. The remaining cell surface binding was collected by acid wash, and the internalized radioactivity was determined after cell lysis and scraping. Nonspecific endocytosis was determined by inclusion of a control group with 200-fold excess of unlabeled leptin, and this background was subtracted. To determine the membrane microdomains involved in the endocytosis, we used chlorpromazine (10 μg/ml) to inhibit the assembly of clathrin-coated pits, nystatin, or filipin (both 20 μg/ml) to inhibit caveolae, and 3 doses of Dynasore (80, 120, and 160 μM) to inhibit Dynamin. These reagents (all from Sigma) were dissolved in DMSO; a control group of 0.2% DMSO was also included in the studies.
Fluorescent trafficking, immunocytochemistry, and confocal microscopic analysis
The cells were grown on collagen-coated coverslips and transfected with ObR plasmid DNA by Lipofectamine 2000 reagent. At 24 h after transfection, the cells were pretreated in DMEM/HEPES for 30 min and incubated with Alexa Fluor-labeled leptin (5 ng/ml) for the desired time points. For blocking experiments, Dynasore, chlorpromazine, filipin, or nystatin (as specified above) were added into DMEM/HEPES buffer during the pretreatment and uptake procedures. The cells were then fixed with 3% paraformaldehyde at 4°C for 20 min, permeabilized with 0.1% Triton X-100 at room temperature for 10 min, and blocked with 5% bovine serum albumin for 30 min. The cells were stained with the goat polyclonal antibody against ObR and Alexa Fluor-conjugated secondary antibody for 1 h, with thorough washes in between. A negative control, in which cells were incubated with secondary antibody only, did not show fluorescent staining representing ObR immunoreactivity. The coverslips were mounted and sealed for fluorescent microscopic observation. The colocalization of leptin and ObR was examined with a Zeiss LSM 510 META confocal microscope(Carl Zeiss, Oberkochen, Germany).
Studies on RBE4 and CHO cells to verify the findings in HEK293 cells
For radioactive tracer endocytosis assays, RBE4 or CHO cells grown to 90% confluency in 12-well plates were transfected with pcDNA, ObRa, or ObR860 by use of Lipofectamine 2000 (0.8 μg/well) in serum and antibiotic-free medium. Endocytosis of 125I-leptin was performed 24 h later. The cells were pretreated with inhibitors of endocytosis or DMSO vehicle for 30 min, then incubated with 125I-leptin (9×105 cpm/ml) with or without the inhibitors for another 30 min at 37°C. Surface binding and endocytosis were determined in the same way as for HEK293 cells.
For fluorescent leptin uptake assays, transfection of RBE4 cells by Lipofectamine 2000 was performed in cell suspension, rather than on attached cells, to enhance the transfection efficiency. The cells were pretreated with inhibitors (120 μM Dynasore or 20 μg/ml nystatin) or DMSO (0.2%) for 1 h, followed by incubation with Alexa568-leptin (10 ng/ml) for 20 min along with inhibitors. The cells were fixed with paraformaldehyde and immunostained for ObR as described above.
Statistical analysis
Values are expressed as means ± se. One-way ANOVA and Tukey’s post hoc test were performed to determine the differences among groups. Linear regression analysis was performed by use of the least squares method.
RESULTS
Generation and expression of truncated ObRs
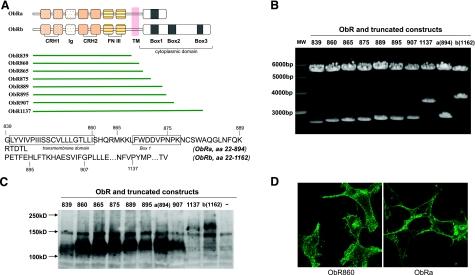
On the basis of the sequences of mouse ObRa and ObRb, we designed strategies to subclone truncated ObRs (Fig. 1A). ObR860 has both extracellular and transmembrane domains, but no cytoplasmic tail at the C terminus. ObR865 terminates before Box 1, ObR875 lies within Box 1, ObR889 encompasses the last shared amino acids between ObRa and ObRb, ObR895 is an ObRb truncate that is 1 aa longer than ObRa, ObR907 terminates in Box 2 with deletion of the downstream trileucine sequence that might be an essential endocytosis signal, and ObR1137 terminates before the third tyrosine residue of ObRb within Box 3 that is known to play an important role in STAT3 activation. These 7 plasmids were generated by PCR cloning methods in order to identify the endocytosis motif within the cytoplasmic region of ObR. All of the constructs were verified by restriction enzyme digestion (Fig. 1B) and automated sequencing.
Figure 1.
Scenario for construction and characterization of mutant leptin receptors. A) Schematic drawing of the deletion constructs directed to different functional domains of ObRa and ObRb. Both receptor isoforms share the signal peptide (aa 1–21), an extracellular domain (aa 22–839), the single transmembrane domain (aa 840–860), and the first 29 aa of the cytoplasmic tail until aa 889. Deletion constructs with the last aa labeled in the protein sequence are shown. B) Truncated ObR constructs were generated by PCR cloning and cloned into a pcDNA3.1(−) vector at XbaI and HindIII restriction enzyme sites. Correct sizes of insertions were verified by restriction enzyme digestion following agarose gel electrophoresis. C) Truncated ObR constructs were expressed in HEK293 cells. Protein lysates 24 h after transient transfection showed efficient protein expression at the predicted molecular weight. Last lane is a negative control transfected with pcDNA3.2 vector. D) Immunocytochemistry of ObR in HEK293 cells transfected by ObR860 (tailless receptor) and ObRa. Both constructs showed cell surface expression of ObR.
The expression of ObRs in the whole lysate of HEK293 cells was measured by Western blot analysis 24 h after transfection (Fig. 1C). The positive controls were HEK293 cells transfected with ObRa or ObRb, which showed respective signals at the molecular size of 130 and 180 kDa, respectively. HEK293 cells transfected with the pcDNA empty vector did not show signals at either position, thus serving as a negative control (Fig. 1C, last lane). All ObR truncates produced signals immunopositive for ObR. Compared with ObRb and ObR1137, the shorter ObR forms had a relative higher level of expression. The subcellular location of ObRs after overexpression was further confirmed by immunostaining of ObR (Fig. 1D). Cells overexpressing ObR860 or ObRa both showed strong ObR immunofluorescent at the cell surface, as well as in the cytoplasm.
Characterization of STAT signal transduction mediated by truncated ObRs
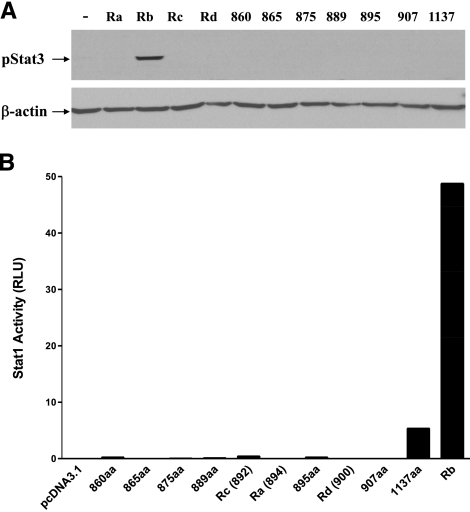
STAT3 transactivation assays were used to further validate the signaling function of the truncated ObRs. Twenty-four hours after transient transfection of ObR, HEK293 cells underwent serum starvation and leptin treatment. Phosphorylated STAT3 (pSTAT3) was measured by Western blot with a specific anti-phospho-STAT3 Tyr705 monoclonal antibody. The long form of the leptin receptor, ObRb, induced pSTAT3 after 30 min of leptin treatment. pSTAT3 was not detectable in groups of cells overexpressing shorter isoforms of ObR, including ObR1137 (Fig. 2A). This is consistent with the report that the tyrosine residue at aa1138 of ObRb is essential for recruitment of STAT3 (23, 24).
Figure 2.
Test of intracellular leptin signaling mediated by mutant leptin receptors. A) Among all ObRs, only ObRb mediated STAT3 activation 15 min after leptin treatment. Housekeeping gene β-actin was probed as a loading control. B) Among ObR constructs, ObR1137 induced a 268-fold increase of STAT1 transcriptional activation, shown by luciferase reporter activity. This contrasts with less activation by ObR860, ObR889, ObR895, and ObRc, and a 2439-fold increase mediated by ObRb.
To test the STAT1 signaling pathway, pGAS-luciferase-STAT1 reporter was cotransfected with one of the ObR constructs. After 6 h of leptin treatment, ObRb-transfected cells showed a 50-fold increase of luciferase activity. ObR1137-overexpressing cells showed a mild increase in STAT1 luciferase activity on leptin stimulation, while the other truncated ObRs failed to do so (Fig. 2B).
Truncated ObR-mediated cell surface binding and endocytosis of 125I-leptin in HEK293 cells
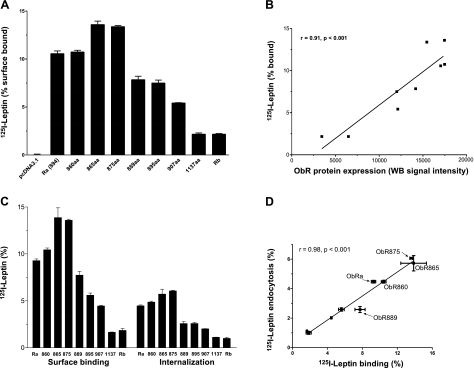
Since the truncated ObRs showed abundant protein expression 24 h after transient transfection in HEK293 cells, this time point was chosen for binding and endocytosis assays of membrane-bound ObRs. ObR865 and ObR875 had the highest percent 125I-leptin binding after 1 h of incubation at 4°C, followed by ObR860 and ObRa, whereas the longer truncates showed relatively low binding capacity (Fig. 3A). Since all the constructs share the same extracellular sequence, the different binding capacity may be caused by the different levels of ObR expression. However, 125I-leptin binding had a linear correlation with the level of ObR protein expression, the latter quantified by densitometry analysis of Western blot results with ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA; Fig. 3B). Moreover, in endocytosis assays performed at 37°C, the percentage of 125I-leptin endocytosis correlated with the remaining cell surface binding at the end of the study (Fig. 3C, D). In particular, the tailless ObR860 acted like the other transmembrane ObR constructs with short cytoplasmic tails in mediating leptin binding and endocytosis. These results indicate that deletion of the cytoplasmic sequence of ObR did not abolish leptin endocytosis.
Figure 3.
Binding and endocytosis of 125I-leptin in HEK293 cells overexpressing mutant ObR constructs. A) Cell surface binding of 125I-leptin at 4°C was increased in all groups transfected with truncated ObRs, as well as ObRa or ObRb. Higher binding was seen in ObR865 and ObR875 than in all other groups. ObRa and ObR860 had the second highest percentage of leptin binding, whereas ObR1137 and ObRb had the least increase in comparison with the pcDNA vector control. B) Percentage of leptin binding correlated linearly with the level of ObR expression in groups of cells (r=0.91, P<0.001). C) Cell surface binding and endocytosis of 125I-leptin after incubation at 37°C for 30 min. D) Percentage of leptin endocytosis correlated linearly with that of residual cell surface binding (r=0.98, P<0.0001).
Endocytosis of Alexa555-leptin in HEK293 cells occurred regardless of whether mediated by the tailless ObR860 or ObRa
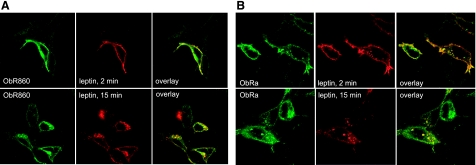
Endocytosis of Alexa555-leptin was performed 24 h after HEK293 cells were transfected with ObR860 or ObRa. The cells were incubated with Alexa555-leptin for 2 or 15 min, washed, fixed with paraformaldehyde, and immunostained for ObR. Confocal microscopic analysis showed that Alexa555-leptin was colocalized with ObR860 at both time points. At 2 min, most of the Alexa555-leptin remained at the cell surface. At 15 min, Alexa555-leptin and ObR860 were colocalized intracellularly (Fig. 4A). ObRa-overexpressing cells were studied in parallel. Similar to that seen in ObR860-overexpressing cells, Alexa555-leptin largely remained at the cell surface at 2 min and colocalized with ObRa. At 15 min, Alexa555-leptin and ObRa remained colocalized, but resided intracellularly (Fig. 4B). The similar time course and trafficking patterns of leptin mediated by either ObR860 or ObRa further confirmed that the length of the ObR cytoplasmic tail did not have much effect on leptin endocytosis. This result also suggests that both ObR860 and ObRa were internalized by similar mechanisms.
Figure 4.
Comparison of ObR860 and ObRa in mediating leptin trafficking in HEK293 cells. A) Most ObR860 immunoreactivity (green) was located at cell surface after leptin stimulation for 2 min, at which time Alexa555-leptin (red) showed complete colocalization with the receptor. At 15 min, Alexa555-leptin remained colocalized with ObR860, but mainly intracellularly. B) Immunoreactivity of ObRa was present both at cell surface and in cytosplasmic vesicles 2 min after leptin treatment. At this time, Alexa555-leptin (red) showed complete colocalization with ObRa (green). Fifteen minutes later, most of the Alexa555-leptin was colocalized with ObRa in intracellular vesicles.
Both ObRa and ObR860 undergo clathrin-mediated endocytosis in HEK293 cells
In the presence of the dynamin inhibitor Dynasore (25, 26), ObRa-mediated endocytosis of 125I-leptin was significantly reduced (29.3–47.3%). Although the low dose of Dynasore (80 μM) did not significantly affect ObR860-mediated endocytosis of 125I-leptin, higher doses (120 and 160 μM) were effective. In parallel, chlorpromazine (10 μg/ml), a cationic amphiphilic drug that induces redistribution of the AP-2 component of clathrin-coated pits and thereby inhibits clathrin-mediated endocytosis (27), also caused ∼55% reduction of 125I-leptin endocytosis, mediated by either ObRa or ObR860 (Fig. 5A). The inhibitory effect of the Dynasore and chlorpromazine on fluorescent leptin trafficking was seen in cells overexpressing either ObRa or ObR860. In comparison with the control group treated with the diluent DMSO, Dynasore and chlorpromazine effectively blocked leptin endocytosis and led to its cell surface accumulation.
Figure 5.
Test of membrane microdomains involved in leptin-induced internalization of the ligand-receptor complex in HEK293 cells. A) Effects of Dynasore and chlorpromazine on 125I-leptin endocytosis. Dynasore inhibited leptin endocytosis in both ObRa- and ObR860-overexpressing cells in a dose-dependent manner. *P < 0.05; ***P < 0.005. B) Cellular distribution of Alexa555-leptin was similar in HEK293 cells overexpressing either ObRa or ObR860. In DMSO vehicle-treated cells, 20 min after initiation of endocytosis, leptin fluorescence was seen at both cell surface and vesicular compartments in cytoplasm. High dose of Dynasore (160 μM) blocked internalization in both ObRa- and ObR860-overexpressing cells. Chlorpromazine also blocked internalization of leptin mediated by ObR860 but not by ObRa. Lower dose of Dynasore (80 μM), nystatin, or filipin did not alter trafficking pattern of leptin.
To further test the possibility that leptin undergoes caveolae-dependent internalization pathway, the inhibitors for caveolin-mediated endocytosis-filipin and nystatin (20 μg/ml)—were also used in the endocytosis assay. Neither inhibitor showed a significant effect (Fig. 5B). This result indicates that lipid rafts may not be involved in the endycytosis of leptin in HEK293 cells.
Verification of the finding that ObR860 mediates efficient leptin endocytosis in additional cell lines
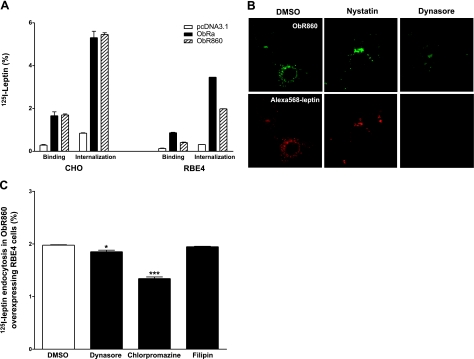
Three plasmid groups and two cell lines were studied simultaneously to determine the percentage of 125I-leptin endocytosis at 37°C for 30 min (n=2/group). CHO cells showed a similar pattern as HEK293 cells; i.e., ObR860 and ObRa mediated leptin uptake with equal efficiency (Fig. 6A). In RBE4 cells, the endocytosis of 125I-leptin was increased ∼6-fold in ObR860-overexpressed cells compared with the control group transfected with pcDNA3.1 vector. The amplitude of increase was lower than that in the ObRa-expressed cells, a difference probably explained by the lower surface expression level of ObR860 in RBE4 cells. If normalized by the percentage of surface binding, ObR860 and ObRa showed similar efficiency in mediating leptin endocytosis. The ability of ObR860 to internalize leptin and enable its intracellular trafficking in RBE4 cells was further confirmed by confocal microscopic analysis of fluorescently labeled leptin. At 20 min after the initiation of endocytosis, Alexa568-leptin was colocalized with ObR860 in perinuclear cytoplasmic compartments and showed a vesicular pattern of distribution (Fig. 6B).
Figure 6.
Endocytosis of leptin in CHO and RBE4 cell lines. A) Binding and endocytosis of 125I-leptin in CHO and RBE4 cells overexpressing ObRa and ObR860 after incubation at 37°C for 30 min. Both ObRa and ObR860 induced a significant (P<0.01) increase in residual cell surface binding and endocytosis of 125I-leptin. B) Endocytosis of Alexa-568 leptin in RBE4 cells overexpressing ObR860. Alexa-568 leptin (red) was effectively internalized into cells expressing ObR860 (green) after incubation at 37°C for 20 min. Dynasore almost completely abolished intracellular fluorescence of leptin, whereas nystatin had no apparent effects. C) Endocytosis of 125I-leptin in RBE4 cells overexpressing ObR860 after treatment with different inhibitors. 125I-leptin endocytosis was effectively inhibited by chlorpromazine and Dynasore, whereas nystatin had no effect. *P < 0.05; ***P < 0.005.
In RBE4 cells overexpressing ObR860, leptin endocytosis could be inhibited by both chlorpromazine (10 μg/ml) and Dynasore (120 μM), whereas nystatin had no effect (Fig. 6C). These results again suggest the involvement of clathrin-coated pits and dynamin in ObR860-mediated internalization of leptin. The lack of effect of nystatin ruled out a crucial role of caveolae in this process. In the Alexa568-leptin trafficking study, the fluorescent intensity was reduced by pretreatment and cotreatment of the cells with Dynasore, but remained unchanged by pretreatment or cotreatment with nystatin. These results further indicate that ObR860-mediated leptin endocytosis was mainly mediated by dynamin and its associated clathrin-coated pits, but not by caveolae.
DISCUSSION
In this study, we unexpectedly identified a novel phenomenon in leptin endocytois by showing that it can be mediated by a tailless mutant receptor that does not have any intracellular domains to trigger signaling events. This was observed in three different cell lines by both classical radiotracer endocytosis assays and fluorescent trafficking studies. The results provide novel information about the interactions of leptin receptors with membrane microdomains during the course of leptin endocytosis.
It is well known that receptor-mediated endocytosis may be largely dependent on specific internalization signals within the cytoplasmic domain (28). Most signals consist of short, linear sequences of amino acid residues such as tyrosine-based motifs (NPXY or YXXØ) and dileucine-based motifs ([DE]XXXL[LI] or DXXLL). These motifs can be recognized by clathrin-associated adaptor proteins and lead to the recruitment of membrane proteins into clathrin-coated vesicles. In addition to peptide motifs, ubiquitination of cytosolic lysine residues also serves as a signal for sorting at various stages of the endosomal-lysosomal system (29).
The initial goal of this study was to test the relative contribution of the cytoplasmic region of the leptin receptor in ligand-induced endocytosis. Previously, Belouzard et al. (15)reported that ObRa is ubiquitylated at lysine residues K877 and K889 in the cytoplasmic tail, and that the monoubiquitin conjugates act as internalization motifs for clathrin-dependent endocytosis of leptin receptors in transiently transfected Hela cells. In a CHO-overexpressing system, Uotani et al. (16) showed that deletion of the last 5 residues of ObRa did not affect receptor-mediated internalization of 125I-leptin. However, deletion of the 26-aa cytoplasmic tail reduced internalization to ∼60% at 5 min and 40% at 10 min as compared with ObRa. This mutant ObR is truncated immediately before Box 1 and leaves only a short 8-aa cytoplasmic tail. Reduction of the endocytosis of 125I-leptin led to the speculation of an endocytosis motif located in aa 868–889 of ObRa, although this region does not contain known tyrosine- or dileucine-based sorting signals (16). Nonetheless, in cells overexpressing truncated ObRa without the 26 aa at the C terminus, internalization still occurred at a significant rate, although reduced ∼50% in comparison with the full-length ObR (aa 894). This suggests that other motifs, possibly located in the membrane juxtapositional region of cytoplasmic ObRa, may also play a role in internalization of leptin and ObR. Thus, we constructed a series of ObR truncates to test the alternative endocytosis motifs.
The construction of truncated receptors was based on known signaling functions of the cytoplasmic region of ObRb and motifs potentially involved in internalization of the receptor. Construction of ObR860 (truncated immediately after the transmembrane domain) enabled the testing of the overall function of the cytoplasmic tail. ObR865 (including the membrane domain proximal to Box 1), and ObR875 (within Box 1) enabled examination of the function of Box 1 and the possible sorting sequence proposed by Uotani et al. (16). ObR889 was used to test whether ObRa and ObRb have different sorting signals, as aa 889 is the last amino acid shared by both isoforms. Within the unique sequence for ObRb, mutation of ObR895 reduced ObRb to almost the same length as ObRa (894 aa), and ObR907 was useful in determination of whether the trileucine sequence downstream plays an important role in receptor trafficking. Lastly, ObR1137 was truncated immediately before aa 1138, which is a tyrosine residue important for STAT3 activation (30,31,32,33).
These truncated ObR receptors were all successfully expressed in mammalian cells, and showed differential effects in activating STAT1 and STAT3 as expected. However, the levels of cell surface expression varied among the truncated receptors. This is probably related to differential turnover of the transcripts and proteins, as a similar difference has been observed for ObRa and ObRb and is difficult to reconcile by use of stable cell lines (16). Nonetheless, the expression levels of most constructs were comparable and adequate to assess whether different domains affect leptin binding and uptake.
ObR865- and ObR875-overexpressing cells showed the highest levels of leptin binding and endocytosis. This is seemingly supportive of the putative endocytosis motif located in aa 868–889 of ObRa; however there are two discrepancies. First, ObR865 was as efficient as ObR875. Second, the amount of leptin endocytosis affects the level of mutant ObR expression in the cells, and this is variable, as shown by Western blot analysis and cell surface 125I-leptin binding assays conducted at 4°C in parallel study groups. When normalized for equal amounts of cell surface expression, the internalization of 125I-leptin by any of the truncated ObRs in HEK293 cells did not differ significantly from each other or from that of ObRa and ObRb. ObRa and ObRb have been shown to have similar binding affinity in multiple cell lines after either transient or stable overexpression (16, 34,35,36). This further supports the notion that differential efficacy of leptin binding and endocytosis may merely be a function of variable levels of cell surface expression of the truncated receptors.
The most important finding of this study is the ability of the tailless ObR860 to bind leptin and mediate its efficient endocytosis. Besides the 125I-leptin binding and endocytosis studies, immunocytochemistry also showed identical internalization and trafficking patterns of Alexa555-leptin in HEK293 cells overexpressing either ObR860 or ObRa. The endocytosed Alexa555-leptin was colocalized with its mediating receptor. Since ObR860-overexpressing cells showed similar kinetics in the intracellular trafficking of Alexa555-leptin as did ObRa-overexpressing cells, we can conclude that the cytoplasmic tail of ObR is not necessary for leptin endocytosis along with its receptors. The discrepancy with the previous observation that the cytoplasmic tail aa 868–889 plays an important role in ObR internalization (15, 16) may be explained by a different focus and methodology, since our study addresses ligand endocytosis and trafficking rather than constitutive ligand-independent ObR recycling.
To show whether the findings are cell-line dependent, we validated the HEK293 cell studies by use of two additional cell lines. Polarized brain endothelial cells are probably the best model to study the molecular mechanisms underlying leptin trafficking, since blood-borne leptin has to negotiate the blood-brain barrier to reach the brain (7, 37, 38). However, the low transfection and expression efficiencies of these cells pose a challenge in their use to identify the endocytosis motif. Therefore, in addition to the RBE4 cerebral microvessel endothelial cells that were used as an in vitro model of the BBB, we also tested CHO cells that have low endogenous ObR and equally high transfection efficiency as HEK293 cells. The results from both RBE4 and CHO cells confirmed the main findings in HEK293 cells. The levels of ObR expression in RBE4 cells were less robust, and ObR860 seemed to have a lower level of expression resulting in a smaller increase in leptin endocytosis. Regardless, ObR860-mediated leptin endocytosis was inhibited by Dynasore and chlorpromazine, indicating that it involves dynamin and clathrin-coated pits similar to that seen in HEK293 cells.
The major internalization pathway for membrane receptors is clathrin-mediated, although some receptors use lipid rafts and caveolae-dependent routes. We used chemical inhibitors to identify the pathways that ObRa and tailless ObR860 may utilize. Dynamin is a multidomain protein essential for clathrin-coated vesicle formation in endocytosis, and Dynasore is a noncompetitive inhibitor that acts on the GTPase domain of dynamin (25, 26). In the presence of Dynasore, there was membrane retention of Alexa Fluor-labeled leptin in cells overexpressing either ObR860 or ObRa, and there was reduced endocytosis of 125I-leptin. Similar results were seen in the presence of chlorpromazine, an inhibitor of clathrin-coated pit formation inducing a redistribution of the AP-2 component of the clathrin-coated pit to endosomes (22, 27, 39, 40). The results illustrate that both ObRa and ObR860 were internalized, at least partially, by clathrin-coated vesicles in a dynamin-dependent manner. The lack of an inhibitory effect of nystatin and filipin suggests that caveolae did not play a major role in leptin endocytosis in these cells.
The effective endocytosis of Alexa568-leptin by RBE4 cells was also shown by confocal colocalization of the ligand and the mutant receptor. It should be noted that the intracellular distribution of leptin and ObR860 in RBE4 cells was not entirely the same as that in HEK293 cells. Different cell types have variable levels of endogenous receptor expression, and the fate of the receptors after ligand stimulation can also be affected by such variables as the ratio of specific adaptor proteins and the lipid composition of the membrane. This may, in part, determine the trafficking pathway that the receptors use. In addition, the type of post-translational modifications of the receptor can also have major effects on ligand-mediated signaling.
How ObR retains its ability to interact with clathrin-coated pits despite the absence of the cytoplasmic domain is not yet clear. It is possible that ObR860 is associated with other proteins that contain endocytosis signals. Although the search for these associated molecules is beyond the scope of the current study, results from viral endocytosis have shown that the chemokine receptor CCR5 can serve as coreceptor for human immunodeficiency viruses to enter cells (41). Leptin itself has been shown to interact with receptors for corticotropin-releasing hormone and facilitate the transport of urocortin across the blood-brain barrier (42,43,44,45). Studies in Koletsky rats devoid of ObRa showed that some leptin can still be transported across the blood-brain barrier, suggesting that there might be alternative leptin transporters distinct from the canonical leptin receptor (6, 46).
Overall, the interactions of the tailless receptor with dynamin and clathrin broaden our view that the extracellular or transmembrane domains may also be important in interacting with clathrin-coated pits, either directly or indirectly by association with other proteins. It is also noteworthy that the inhibitors chlorpromazine and Dynasore only caused a 30–50% reduction of 125I-leptin endocytosis, even in HEK293 cells. This suggests that although ObR uses the clathrin-mediated endocytic pathway, much leptin enters cells through a nonclathrin, noncaveolae pathway. The membrane receptors with little or no cytoplasmic tails may also recycle in the cell with the bulk of membrane flow and thus mediate endocytosis of the ligand.
In summary, the cytoplasmic domains of ObRs determine the JAK/STAT signaling pathway but have little effect on leptin-stimulated receptor internalization in transiently transfected HEK293, CHO, and RBE4 cells. This novel finding adds another level of complexity to understanding the processes involved in leptin transport.
Acknowledgments
This study was supported by U.S. National Institutes of Health grants (DK54880 to A.J.K.; NS62291 and NS46528 to W.P.). The ObR plasmids were kindly provided by Dr. Christian Bjorbaek (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA). The RBE4 cells were kindly provided by Dr. Pierre-Olivier Couraud (Institut Cochin, INSERM, Paris, France).
References
- Lee G H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J M. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Klausner R D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Tu H, Kastin A J, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214:301–305. doi: 10.1002/jcp.21195. [DOI] [PubMed] [Google Scholar]
- Banks W A, Kastin A J, Huang W, Jaspan J B, Maness L M. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist J K, Michl P, Ahima R S, van Bueren A, McCall A L, Flier J S. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–3491. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- Kastin A J, Pan W, Maness L M, Koletsky R J, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–1453. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Tu H, Kastin A J. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2008;149:877–885. doi: 10.1210/en.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J F, Kolaczynski J W, Nyce M R, Ohannesian J P, Opentanova I, Goldman W H, Lynn R B, Zhang P L, Sinha M K, Considine R V. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- Schwartz M W, Peskind E, Raskind M, Boyko E J, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz D D, Hileman S M, Bjorbaek C, Flier J S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas J L, Boozer C, Blair-West J, Fidahusein N, Denton D A, Friedman J M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heek M, Compton D S, France C F, Tedesco R P, Fawzi A B, Graziano M P, Sybertz E J, Strader C D, Davis H R., Jr Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Pan W, Feucht L, Kastin A J. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa-ObRd. J Cell Physiol. 2007;212:215–222. doi: 10.1002/jcp.21020. [DOI] [PubMed] [Google Scholar]
- Barr V A, Lane K, Taylor S I. Subcellular localization and internalization of the four human leptin receptor isoforms. J Biol Chem. 1999;274:21416–21424. doi: 10.1074/jbc.274.30.21416. [DOI] [PubMed] [Google Scholar]
- Belouzard S, Rouille Y. Ubiquitylation of leptin receptor OB-Ra regulates its clathrin-mediated endocytosis. EMBO J. 2006;25:932–942. doi: 10.1038/sj.emboj.7600989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uotani S, Bjorbaek C, Tornoe J, Flier J S. Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes. 1999;48:279–286. doi: 10.2337/diabetes.48.2.279. [DOI] [PubMed] [Google Scholar]
- Wilcke M, Walum E. Characterization of leptin intracellular trafficking. Eur J Histochem. 2000;44:325–334. [PubMed] [Google Scholar]
- Belouzard S, Delcroix D, Rouille Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J Biol Chem. 2004;279:28499–28508. doi: 10.1074/jbc.M400508200. [DOI] [PubMed] [Google Scholar]
- Pan W, Tu H, Kastin A J. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27:911–916. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Pan W, Tu H, Yu C, Hsuchou H, Yang Y, Kastin A J. Differential role of TNF receptors in cellular trafficking of intact TNF. Cell Physiol Biochem. 2007;20:559–568. doi: 10.1159/000107539. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu Y, Cain C M, Nyberg F, Couraud P O, Kastin A J. Permeation of growth hormone across the blood-brain barrier. Endocrinology. 2005;146:4898–4904. doi: 10.1210/en.2005-0587. [DOI] [PubMed] [Google Scholar]
- Tu H, Kastin A J, Pan W. Corticotropin-releasing hormone receptor (CRHR)1 and CRHR2 are both trafficking and signaling receptors for urocortin. Mol Endocrinol. 2007;21:700–711. doi: 10.1210/me.2005-0503. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Uotani S, da Silva B, Flier J S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- Li C, Friedman J M. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc Natl Acad Sci U S A. 1999;96:9677–9682. doi: 10.1073/pnas.96.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Macia E, Pelish H E. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 2008;438:77–93. doi: 10.1016/S0076-6879(07)38006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Subtil A, Hemar A, Dautry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J Cell Sci. 1994;107:3461–3468. doi: 10.1242/jcs.107.12.3461. [DOI] [PubMed] [Google Scholar]
- Benmerah A, Lamaze C. Clathrin-coated pits: vive la difference? Traffic. 2007;8:970–982. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Bonifacino J S, Traub L M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Ahima R S, Osei S Y. Leptin signaling. Physiol Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Harrold J A, Williams G. Melanocortin-4 receptors, beta-MSH and leptin: key elements in the satiety pathway. Peptides. 2006;27:365–371. doi: 10.1016/j.peptides.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Hegyi K, Fulop K, Kovacs K, Toth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004;28:159–169. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Myers M G., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- Rosenblum C I, Tota M, Cully D, Smith T, Collum R, Qureshi S, Hess J F, Phillips M S, Hey P J, Vongs A, Fong T M, Xu L, Chen H Y, Smith R G, Schindler C, Van der Ploeg L H. Functional STAT 1 and 3 signaling by the leptin receptor (OB-R); reduced expression of the rat fatty leptin receptor in transfected cells. Endocrinology. 1996;137:5178–5181. doi: 10.1210/endo.137.11.8895396. [DOI] [PubMed] [Google Scholar]
- Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, Muir C, Sanker S, Moriarty A, Moore K J, Smutko J S, Mays G G, Wool E A, Monroe C A, Tepper R I. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kuropatwinski K K, White D W, Hawley T S, Hawley R G, Tartaglia L A, Baumann H. Leptin receptor action in hepatic cells. J Biol Chem. 1997;272:16216–16223. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- Kastin A J, Pan W. Intranasal leptin: blood-brain barrier bypass (BBBB) for obesity? Endocrinology. 2006;147:2086–2087. doi: 10.1210/en.2006-0208. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin A J. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hamme E, Dewerchin H L, Cornelissen E, Verhasselt B, Nauwynck H J. Clathrin- and caveolae-independent entry of feline infectious peritonitis virus in monocytes depends on dynamin. J Gen Virol. 2008;89:2147–2156. doi: 10.1099/vir.0.2008/001602-0. [DOI] [PubMed] [Google Scholar]
- Wang L H, Rothberg K G, Anderson R G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret N, Pelchen-Matthews A, Mack M, Proudfoot A E, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin A J, Akerstrom V, Pan W. Activation of urocortin transport into brain by leptin. Peptides. 2000;21:1811–1817. doi: 10.1016/s0196-9781(00)00349-1. [DOI] [PubMed] [Google Scholar]
- Kastin A J, Pan W, Akerstrom V, Hackler L, Wang C, Kotz C M. Novel peptide-peptide cooperation may transform feeding behavior. Peptides. 2002;23:2189–2196. doi: 10.1016/s0196-9781(02)00247-4. [DOI] [PubMed] [Google Scholar]
- Sweep F, Rijnkels C, Hermus A. Activation of the hypothalamus-pituitary-adrenal axis by cytokines. Acta Endocrinol (Copenh) 1991;125:84–91. [PubMed] [Google Scholar]
- Tu H, Kastin A J, Bjorbaek C, Pan W. Urocortin trafficking in cerebral microvessel endothelial cells. J Mol Neurosci. 2007;31:171–181. doi: 10.1385/jmn/31:02:171. [DOI] [PubMed] [Google Scholar]
- Banks W A, Niehoff M L, Martin D, Farrell C L. Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res. 2002;950:130–136. doi: 10.1016/s0006-8993(02)03013-5. [DOI] [PubMed] [Google Scholar]