Abstract
Decrease in fat catabolic rate on consuming a high-fat diet contributes to diet-induced obesity. This study used group 1B phospholipase A2 (Pla2g1b)-deficient mice, which are resistant to hyperglycemia, to test the hypothesis that Pla2g1b and its lipolytic product lysophospholipid suppress hepatic fat utilization and energy metabolism in promoting diet-induced obesity. The metabolic consequences of hypercaloric diet, including body weight gain, energy expenditure, and fatty acid oxidation, were compared between Pla2g1b+/+ and Pla2g1b−/− mice. The Pla2g1b−/− mice displayed normal energy balance when fed chow, but were resistant to obesity when challenged with a hypercaloric diet. Obesity resistance in Pla2g1b−/− mice is due to their ability to maintain elevated energy expenditure and core body temperature when subjected to hypercaloric diet, which was not observed in Pla2g1b+/+ mice. The Pla2g1b−/− mice also displayed increased postprandial hepatic fat utilization due to increased expression of peroxisome proliferator-activated receptor (PPAR)-α, PPAR-δ, PPAR-γ, cd36/Fat, and Ucp2, which coincided with reduced postprandial plasma lysophospholipid levels. Lysophospholipids produced by Pla2g1b hydrolysis suppress hepatic fat utilization and down-regulate energy expenditure, thereby preventing metabolically beneficial adaptation to a high-fat diet exposure in promoting diet-induced obesity and type 2 diabetes.—Labonté, E. D., Pfluger, P. T., Cash, J. G., Kuhel, D. G., Rojas, J. C., Magness, D. P., Jandacek, R. J., Tschöp, M. H., Hui, D. Y. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: the molecular link between group 1B phospholipase A2 and diet-induced obesity.
Keywords: energy expenditure, lipid absorption, peroxisome proliferator-activated receptors
The phospholipase A2 (PLA2) superfamily of proteins includes 5 major groups plus other subgroups and can be classified as either secreted small-molecular-weight PLA2, the larger cytosolic calcium-dependent PLA2, the calcium-independent PLA2, the platelet-activating factor acetylhydrolases, and the lysosomal PLA2 (1). These enzymes hydrolyze fatty acids from the sn-2 position of phospholipids to generate free fatty acids and lysophospholipids. Lipolytic products of phospholipid hydrolysis can serve as substrates for synthesis of bioactive molecules or serve directly as signaling molecules that modulate cell functions. Fatty acids liberated from phospholipids after PLA2 hydrolysis may also serve as substrates for lipogenesis and/or modulators of gene expression via activation of peroxisome proliferator-activated receptors (PPARs) (2). The other digestive products of phospholipid hydrolysis by PLA2, the lysophospholipids, also display cell signaling capabilities, primarily through interaction with G protein-coupled receptors on target cells. Since all members of the PLA2 superfamily are capable of generating free fatty acids and lysophospholipids, differences in their tissue expression pattern and cellular location suggest a unique role for each PLA2 in physiology and pathophysiology.
Group 1B PLA2 (PLA2G1B) is an enzyme abundantly expressed in the acinar cells of the pancreas and is secreted into the intestinal lumen during meal consumption. On the basis of its anatomic location and activity in hydrolyzing phospholipids (3), the importance of PLA2G1B was presumed to be the hydrolysis of phospholipids prior to the efficient absorption of lipid nutrients into the intestinal mucosa (4, 5). However, studies with Pla2g1b−/− mice showed that other phospholipases in the intestinal tract can catalyze phospholipid digestion in the absence of Pla2g1b to promote lipid absorption (6). The difference in intestinal phospholipid digestion between Pla2g1b+/+ and Pla2g1b−/− mice is the lipolytic products formed after hydrolysis, with significantly less phospholipid absorbed as lysophospholipids in the Pla2g1b−/− mice (7). The reduction in lysophospholipid absorption results in increased hepatic insulin sensitivity, and the Pla2g1b−/− mice are protected from diabetes induced by consuming a high-fat/high carbohydrate diet (7, 8).
Our previous studies noted that the Pla2g1b−/− mice are also protected from diet-induced obesity (8). However, the mechanism by which Pla2g1b−/− mice are resistant to diet-induced obesity has not been delineated. Whether their obesity resistance phenotype is secondary to the increased hepatic insulin sensitivity or results from other metabolic consequences has not been explored. The current study was undertaken to identify the mechanism by which Pla2g1b deficiency protects against diet-induced obesity. In view of recent genome-wide scans identifying the linkage between the PLA2G1B gene and human obesity (9), understanding the mechanism by which PLA2G1B contributes to diet-induced obesity has direct clinical relevance, with results aiding the design of novel therapeutics to combat obesity and obesity-related metabolic diseases.
MATERIALS AND METHODS
Animals
Wild-type Pla2g1b+/+ and congenic Pla2g1b−/− mice in C57BL/6J background were fed either a basal diet (D12328; Research Diets, New Brunswick, NJ, USA) or a hypercaloric diet (D12331; Research Diets) composed of 58.5% fat, 25% sucrose, and 16.5% protein. Weight gain was monitored weekly, and body composition was determined by 1H magnetic resonance spectroscopy (EchoMRI-100; EchoMedical Systems, Houston, TX, USA). Food intake (in grams) by individual mice was recorded daily for 5 d. Preprandial and postprandial body core temperatures were recorded utilizing a rectal thermometer. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Chronic fat absorption measurements
Fat absorption in mice was determined by the sucrose polybehenate method, essentially as described previously (10, 11). Fecal samples were collected from individually housed mice fed for 3 d a test diet containing 58% fat (1:1 mix of lard and coconut oil), 27% sucrose, 15% protein (nonfat powdered milk), and trace amounts of sucrose polybehenate. Lipids were extracted from the fecal samples and quantified by gas chromatography after saponification and methylation. Fat absorption was calculated by the difference between food and excretion content, utilizing behenate as the nonabsorbed internal standard.
Blood chemistry
Animals fed the indicated diets were deprived of food for 12 h; then a bolus of olive oil (8 ml/kg body wt) was administered by oral gavage. Blood was collected via the tail vein at hourly intervals. Plasma β-hydroxybutyrate and nonesterified fatty acid levels were determined using colorimetric assay kits (Wako Chemicals, Richmond, VA, USA). The concentration of plasma lysophospholipids was determined by a modification of the enzymatic assay procedure of Kishimoto et al. (12), as described previously (7). Blood glucose was determined with an Accu-Chek Active Glucometer (Roche Applied Science, Indianapolis, IN, USA). Plasma insulin levels were measured with the Ultra Sensitive Rat Insulin ELISA kit (Crystal Chem, Chicago, IL, USA) using rat insulin as the standard.
Hepatic fatty acid oxidation
Livers were excised from hypercaloric-diet-fed Pla2g1b+/+ and Pla2g1b−/− mice 2 h after ingesting a bolus olive oil meal as described above. In selected experiments, the animals also received an intraperitoneal injection of saline with or without lysophosphatidylcholine (32 mg/kg body wt) 5 min prior to administration of the lipid meal (7). The livers were rinsed in Krebs-Ringer buffer solution and diced into small fragments. The diced liver fragments (100 mg) were incubated for 30 min at 37°C in 3 ml of oxygenated Krebs-Ringer buffer solution containing 100 μM [14C]oleate (3 μCi/mM) complexed with BSA at a molar ratio of 5:1. The β-oxidation of [14C]oleate was determined on the basis of the amount of 14CO2 released and trapped in a filter paper inside the sealed incubation flask, as described previously (13).
RNA extraction and real-time PCR
Total RNA was isolated from frozen tissues with the TRI Reagent system (Molecular Research Center, Cincinnati, OH, USA), treated with Turbo DNase (Applied Biosystems/Ambion, Austin, TX, USA), and repurified utilizing the RNeasy mini kit (Qiagen, Inc., Valencia, CA, USA). One microgram of RNA was used to generate cDNA with oligo (dT) and random hexamer primers using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). Quantitative real-time PCR was performed on an iCycler IQ thermocycler (Bio-Rad Laboratories) using the IQ SYBR Supermix system (Bio-Rad Laboratories) with primers as indicated in Table 1.
TABLE 1.
Primers and reaction conditions used for real-time RT-PCR analyses of RNA
| mRNA | Sense primer | Antisense primer |
|---|---|---|
| Cd36/Fat | CTGTTATTGGTGCAGTCCTGGC | TATGTGGTGCAGCTGCTACAGC |
| Cpt1a | AACCCAGTGCCTTAACGATG | GAACTGGTGGCCAATGAGAT |
| Cpt1b | CAAGTTCAGAGACGAACGCC | TCAAGAGCTGTTCTCCGAACTG |
| Hmgcs2 | GGTGTCCCGTCTAATGGAGA | ACACCCAGGATTCACAGAGG |
| UCP1 | CTGGGCTTAACGGGTCCTC | CTGGGCTAGGTAGTGCCAGTG |
| UCP2 | GACCTCCCTTGCCACTTCAC | GAAGGCATGAACCCCTTGTAG |
| UCP3 | CCGATACATGAACGCTCCC | AAGCTCCCAGACGCAGAAAG |
| Cyclophilin | TCATGTGCCAGGGTGGTGAC | CCATTCAGTCTTGGCAGTGC |
| 18S RNA | TTCCGATAACGAACGAGACTCT | TGGCTGAACGCCACTTGTC |
| PPAR-α | GCAGCTCGTACAGGTCATCA | CTCTTCATCCCCAAGCGTAG |
| PPAR-δ | CTCTTCATCGCGGCCATCATT | TCTGCCATCTTCTGCAGCAGC |
| PPAR-γ | ATGCCAAAAATATCCCTGGTTTC | GGAGGCCAGCATGGTGTAGA |
Energy balance measurements
Cohorts of Pla2g1b+/+ and Pla2g1b−/− mice (n=16) fed a basal diet followed by 12 wk of hypercaloric diet were acclimatized to the respiratory chambers of the indirect calorimeter for 2 d. Subsequently, energy expenditure (EE), food intake, and locomotive activity were measured simultaneously over 24-h periods for 4 consecutive days using a customized 32-cage indirect calorimetry system combined with drinking and feeding monitors and fitted with a TSE ActiMot system (TSE Systems, Midland, MI, USA), as described previously (14). The volumes of oxygen (Vo2, ml/kg/h) consumed and carbon dioxide produced (Vco2, ml/kg/h) were measured to calculate EE (kcal/kg/h, EE=3.815×10−3 × Vo2 + 1.232×10−3 × Vco2), and the data were normalized to the metabolically active mass of the animals (15,16,17,18). The locomotive activities were monitored with the custom addition of a multidimensional light beams and sensors to the TSE indirect calorimeter.
Cold adaptation studies
Pla2g1b+/+ mice (n=10) and Pla2g1b−/− mice (n=11) fed a hypercaloric diet for 18 wk were placed in a 4°C environment. Body core temperature was monitored and recorded before and every 2 h using a rectal thermometer for 30 h. Animals deemed hypothermic (body core temperature ≤31°C) were recorded and removed from the test and placed in heating pads for recovery. Hypothermic rates between the two groups were statistically compared by Gehan-Breslow analysis.
Statistical analysis
All results are expressed as means ± se with the sample size listed. For direct comparison of 2 means, an unpaired 2-tailed Student’s t test assuming equal variance was performed. All statistical analyses, including the Pearson correlation, were conducted using SigmaPlot 9.01 (Systat Software, San Jose, CA, USA); values of P < 0.05 were considered significant.
RESULTS
Pla2g1b inactivation protects against obesity induced by high-calorie diet
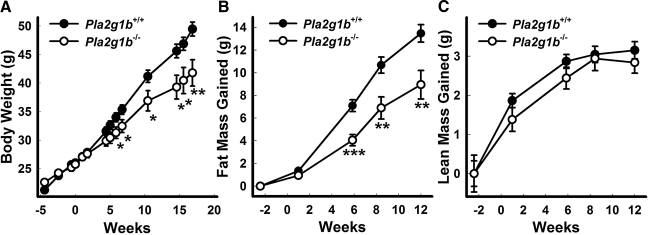
The current study compared the susceptibility of Pla2g1b+/+ and Pla2g1b−/− mice to obesity in response to a hypercaloric diet rich in both fat and carbohydrate. Body weights were found to be significantly higher in Pla2g1b+/+ mice compared to Pla2g1b−/− mice after 5 wk of this dietary challenge. The weight difference was sustained throughout the remaining 12-wk study period (Fig. 1A) and persisted for as long as 12 mo (data not shown). Whereas the fat mass was similar between Pla2g1b+/+ and Pla2g1b−/− mice when fed the basal low-fat diet, the Pla2g1b−/− mice accrued much less fat when fed the hypercaloric diet compared to Pla2g1b+/+ mice (Fig. 1B). The difference in their body weight gain can be attributed to the gain in fat content, as lean mass was similar between Pla2g1b+/+ and Pla2g1b−/− mice fed either the basal or hypercaloric diet (Fig. 1C).
Figure 1.
Diet-induced weight gain and increased adiposity is attenuated in Pla2g1b−/− mice. Male mice 15 wk of age, previously fed a basal diet, were given a hypercaloric diet at the time designated wk 0. A) Body weights were monitored in Pla2g1b+/+ and Pla2g1b−/− mice. B, C) Fat mass (B) and lean mass (C) were also periodically determined by body composition analysis utilizing magnetic resonance spectroscopy. Data represent means ± se from 12–15 mice/group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. Pla2g1b+/+.
Normal intestinal uptake and lipid nutrient absorption in Pla2g1b−/− mice
We have previously shown that basal-diet-fed Pla2g1b+/+ and Pla2g1b−/− mice, after ingesting an oral lipid meal containing [3H]triolein, accumulated radiolabel in their plasma to a similar extent (8). In contrast, a modest reduction in apparent fat absorption was observed 4 h after administering the [3H]triolein-containing lipid-rich meal in Pla2g1b−/− mice compared to Pla2g1b+/+ mice when the animals were maintained on a Western type diet (8). We deduced from this observation that the relatively minor delay in postprandial hyperlipidemia in Pla2g1b−/− mice could not explain the extent of protection against diet-induced obesity in these animals. The current study measured total fat absorption in a more chronic setting, utilizing the nonabsorbable lipid sucrose polybehenate to normalize the recovery of fecal fat excretion (10, 11), and confirmed that total dietary fat absorption was similar between hypercaloric-diet-fed Pla2g1b+/+ and Pla2g1b−/− mice (95.3±1.1 vs. 94.9±0.6%, respectively). Finally, measurements of daily caloric intake or feeding behavior also showed no difference between age-matched Pla2g1b+/+ and Pla2g1b−/− mice when fed the hypercaloric diet, which was consistent with our previously reported results (7, 8).
Increased fatty acid oxidation in Pla2g1b−/− mice
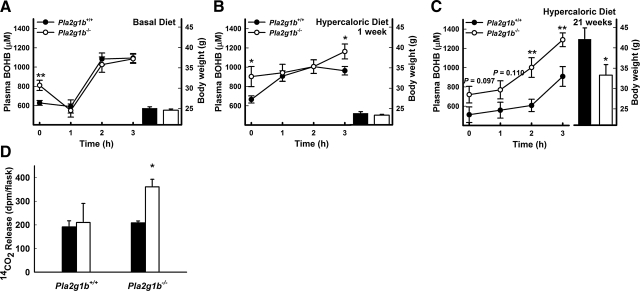
The difference in body weight gain despite their similarities in dietary fat absorption suggested a different metabolic fate of the dietary fat in Pla2g1b+/+ and Pla2g1b−/− mice. To test this possibility, Pla2g1b+/+ and Pla2g1b−/− mice were fed either the basal diet or the hypercaloric diet. The animals were then deprived of food overnight, and plasma was collected either before or 3 h after administering a lipid bolus of olive oil. Hepatic fatty oxidation, initially assessed by measuring plasma β-hydroxybutyrate levels, was found to be higher in food-deprived Pla2g1b−/− mice compared to Pla2g1b+/+ mice when either fed the basal diet or fed the hypercaloric diet for 1 or 21 wk (Fig. 2A–C), thus suggesting an elevated fatty acid catabolic rate in the Pla2g1b−/− mice. The difference in plasma β-hydroxybutyrate levels between basal-diet-fed Pla2g1b+/+ and Pla2g1b−/− mice was no longer observed during the postprandial period after refeeding the animals with a bolus lipid meal (Fig. 2A), indicating that exogenously supplied fatty acids were utilized as an energy source by Pla2g1b+/+ and Pla2g1b−/− mice to a similar extent. However, differences in β-hydroxybutyrate levels between Pla2g1b+/+ and Pla2g1b−/− mice remained apparent after a lipid-rich meal when the animals were maintained on the hypercaloric diet, and these differences were exaggerated after prolonged consumption of the hypercaloric diet for 21 wk (Fig. 2C). Plasma free fatty acids were also shown to have an exaggerated postprandial difference after 21 wk of hypercaloric diet consumption (Supplemental Fig. 1).
Figure 2.
Increased hepatic fatty acid oxidation in Pla2g1b−/− mice. A–C) Three separate cohorts of male Pla2g1b−/− and Pla2g1b+/+ mice at 10–12 wk of age were measured for plasma β-hydroxybutyrate (BOHB) under preprandial and postprandial conditions after consuming basal diet (A), after 1 wk of hypercaloric diet (B), or after 21 wk of hypercaloric diet (C). Body weights of mice at time of experiment are at right of each postprandial plasma BOHB analysis. D) Ex vivo fatty acid oxidation as measured by liberation of 14CO2 from [14C]oleate by 100 mg of liver from hypercaloric-diet-fed Pla2g1b+/+ or Pla2g1b−/− mice under food deprivation (solid bars) or 2 h after bolus fat meal (open bars). All data represent means ± se (n=9 mice/group). *P ≤ 0.05, **P ≤ 0.01vs. Pla2g1b+/+.
It is important to note that differences in preprandial and postprandial β-hydroxybutyrate levels were observed between Pla2g1b+/+ and Pla2g1b−/− mice after 1 wk of consuming the hypercaloric diet, a time point prior to any observed difference in body weight and adiposity (Fig. 2B). These observations suggested that more fatty acids from the diet were oxidized and less were available for storage in the Pla2g1b−/− mice compared to Pla2g1b+/+ mice, which may account for the decreased adiposity and body weight gain in the Pla2g1b−/− mice after prolonged consumption of the hypercaloric diet. To directly test the possibility of increased fatty acid oxidation in hypercaloric-diet-fed Pla2g1b−/− mice compared to that in Pla2g1b+/+ mice, liver slices were prepared from these animals under both preprandial and postprandial conditions and incubated ex vivo with [14C]oleate. Fatty acid oxidation was monitored based on the amount of 14CO2 released. Results showed no difference in fatty acid oxidation in liver of Pla2g1b+/+ mice under preprandial and postprandial conditions. Fatty acid oxidation was also similar between Pla2g1b+/+ and Pla2g1b−/− mice under preprandial conditions. However, significantly elevated fatty acid oxidation was observed in liver slices of Pla2g1b−/− mice after administering the lipid-rich meal (Fig. 2D).
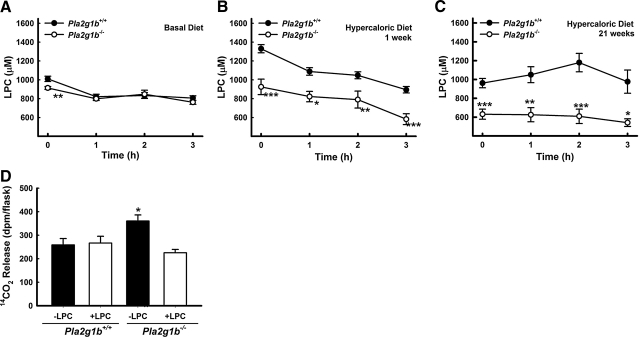
Our previous studies have shown that postprandial lysophospholipid levels were lower in Pla2g1b−/− mice compared to that in Pla2g1b+/+ mice (7). Therefore, the current study also measured preprandial and postprandial plasma lysophospholipid levels in Pla2g1b+/+ and Pla2g1b−/− mice fed either a basal diet or the hypercaloric diet for 1 or 21 wk. Preprandial lysophospholipid levels were significantly lower in plasma of Pla2g1b−/− mice compared to Pla2g1b+/+ mice under basal dietary conditions (Fig. 3A). The differences in preprandial lysophospholipid levels between Pla2g1b+/+ and Pla2g1b−/− mice were exaggerated after consuming the hypercaloric diet for 1 or 21 wk (Fig. 3B, C). Postprandial lysophospholipid levels were similar between basal-diet-fed Pla2g1b+/+ and Pla2g1b−/− mice 3 h after ingesting a bolus lipid meal, but the differences in plasma lysophospholipid levels between hypercaloric-diet-fed Pla2g1b+/+ and Pla2g1b−/− mice persisted throughout the postprandial period after a lipid-rich meal (Fig. 3B, C). Interestingly, changes in plasma lysophospholipid levels in Pla2g1b+/+ and Pla2g1b−/− mice mirrored preprandial and postprandial changes in plasma β-hydroxybutyrate levels in these animals, suggesting that the product of Pla2g1b hydrolysis may be responsible for their differences in fatty acid oxidation in response to a lipid-rich meal. This possibility was confirmed by results showing that administration of lysophosphatidylcholine (LPC) to Pla2g1b−/− mice prior to fat feeding decreased hepatic fatty acid oxidation to levels observed in Pla2g1b+/+ mice (Fig. 3D). Administration of LPC to Pla2g1b+/+ mice had no effect on hepatic fatty acid oxidation, indicating that postprandial digestion of the bile-derived phospholipid and absorption of the lysophospholipid was sufficient to abolish meal-induced hepatic fatty acid oxidation in Pla2g1b+/+ mice.
Figure 3.
Decreased postprandial plasma lysophosphatidylcholine (LPC) is responsible for elevated fatty acid oxidation in Pla2g1b−/− mice. Plasma obtained from the same cohorts of Pla2g1b+/+ and Pla2g1b−/− mice used for studies in Fig. 2 was used for colorimetric assay of LPC concentration. A–C) Plasma was collected from preprandial and postprandial mice fed basal diet (A), 1 wk of hypercaloric diet (B), or 21 wk of hypercaloric diet (C). Data represent means ± se (n=9 mice/group). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. Pla2g1b+/+. D) Ex vivo hepatic fatty acid oxidation as measured by liberation of 14CO2 from [14C]oleate. Livers were obtained from hypercaloric-diet-fed Pla2g1b+/+ or Pla2g1b−/− mice 2 h after bolus olive oil meal (8 ml/kg body wt) with (open bars) or without (solid bars) intraperitoneal injection of 32 mg/kg LPC 5 min prior to oral lipid load. Data represent means ± se (n=9 Pla2g1b+/+ mice; 21 Pla2g1b−/− mice). *P ≤ 0.05 vs. saline-injected Pla2g1b+/+.
Changes in hepatic gene expression induced by Pla2g1b ablation
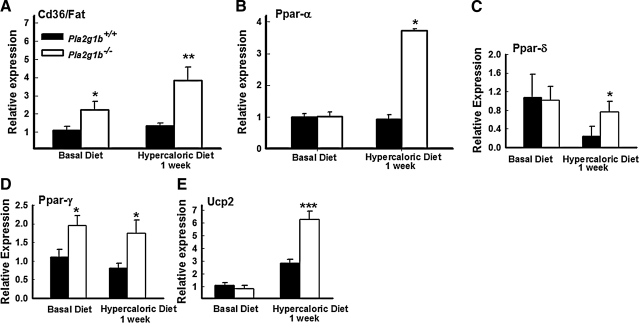
In an effort to understand the cause of increased hepatic fatty acid oxidation in the Pla2g1b−/− mice, livers of postprandial Pla2g1b+/+ and Pla2g1b−/− mice were collected after consuming the basal diet or after 1 wk of hypercaloric diet consumption, and expression of genes reflecting hepatic fatty acid handling was analyzed by quantitative real-time PCR (qPCR). Increased Cd36/Fat mRNA was detected in Pla2g1b−/− mice after 1 wk of hypercaloric diet consumption, but no change was observed in Pla2g1b+/+ mice at this early stage of hypercaloric diet feeding (Fig. 4A). In contrast, expression of genes responsible for mitochondrial fatty acid uptake and oxidation such as Cpt1a, Cpt1b, and Hmgcs2 were not significantly different between Pla2g1b+/+ and Pla2g1b−/− mouse livers (data not shown). Interestingly, hepatic expression of PPAR-α was not different between Pla2g1b+/+ and Pla2g1b−/− mice under basal dietary conditions, but was elevated in Pla2g1b−/− mice after hypercaloric diet consumption (Fig. 4B). Hepatic expression of PPAR-δ was suppressed in Pla2g1b+/+ mice but not in Pla2g1b−/− mice after consuming the hypercaloric diet (Fig. 4C). Expression of PPAR-γ was also elevated in the liver of Pla2g1b−/− mice compared to Pla2g1b+/+ mice under both dietary conditions (Fig. 4D). Therefore, the increase in hepatic fatty acid oxidation in Pla2g1b−/− mice is likely due to preferential peroxisomal oxidation of dietary fatty acids in these animals. These data are consistent with the recent report that PPAR-δ activation increases fatty acid oxidation but does not increase mitochondrial gene expression and function (19). There were no observable differences in expression levels of fatty acid metabolism and uncoupling protein genes in white and brown adipose tissues or in skeletal muscle. However, uncoupling protein 2 (Ucp2) mRNA levels were found to be increased to a higher level in the liver of Pla2g1b−/− mice after only 1 wk of hypercaloric diet consumption (Fig. 4E), which is consistent with the elevation of PPAR-γ activity (20). Taken together, these data suggest that Pla2g1b−/− mice responded to hypercaloric diet with increased postprandial fatty acid flux to the liver for β-oxidation in the peroxisomes. The difference in fatty acid utilization and metabolic rate in the liver of Pla2g1b+/+ and Pla2g1b−/− mice accounts for their difference in susceptibility to diet-induced obesity.
Figure 4.
Changes in expression of genes involved in hepatic fatty acid flux. Gene expression of Cd36/Fat (A), PPAR-α (B), PPAR-δ (C), PPAR-γ (D), and Ucp2 (E) was assessed by qPCR and normalized to cyclophilin mRNA. Livers from animals fed basal diet or hypercaloric diet for 1 wk were collected at the end of their dark cycle and extracted for total RNA. Data are means ± se (n=6–12). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. Pla2g1b+/+.
Difference in energy expenditure between Pla2g1b+/+ and Pla2g1b−/− mice
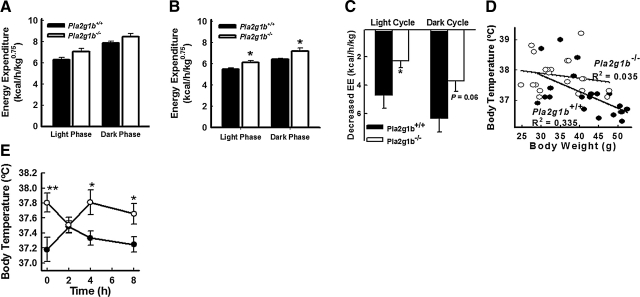
Indirect calorimetry measurements of energy metabolism documented similar oxygen consumption and energy expenditure between age-matched Pla2g1b+/+ and Pla2g1b−/− mice when fed the basal diet (Fig. 5A and Supplemental Fig. 2), but significantly higher in Pla2g1b−/− mice compared to Pla2g1b+/+ mice after feeding the animals a hypercaloric diet (Fig. 5B and Supplemental Fig. 2). The higher energy expenditure displayed by the Pla2g1b−/− mice was observed during both the dormant light phase and the more active dark phase (Fig. 5B and Supplemental Fig. 2). No difference in stationary or ambulatory motor activity was detected between Pla2g1b+/+ and Pla2g1b−/− mice (data not shown), thus indicating that the observed energy expenditure difference results from changes in metabolic thermogenesis rather than spontaneous physical activity. Comparison of the data collected from mice fed basal vs. hypercaloric diets revealed that, whereas energy expenditure was significantly repressed in overnourished Pla2g1b+/+ mice, the hypercaloric diet did not reduce energy expenditure in the Pla2g1b−/− mice to the same extent (Fig. 5C).
Figure 5.
Pla2g1b−/− mice are resistant to the decrease energy expenditure and body temperature associated with diet-induced obesity. Indirect calorimetry was used to measure hourly O2 consumption and CO2 output by each mouse over a period of 3 d (high-fat diet) or 4 d (basal diet) (n=12). A, B) Cumulative energy expenditure over the 3-d period by Pla2g1b+/+ (solid bars) and Pla2g1b−/− mice (open bars) fed either basal low-fat diet (A) or high-fat/high-carbohydrate diet (B). Data are normalized to metabolically active mass of the animals. C) Reduction of energy expenditure compared to original chow-fed animals. D) Correlation between body weight and preprandial core body temperature of Pla2g1b−/− (open circles) and Pla2g1b+/+ mice (solid circles) fed hypercaloric diet for 8 and 20 wk (n=10–11/genotype/diet period). Linear regression is displayed for each group; Pearson analysis demonstrates correlation for Pla2g1b+/+ mice (P=0.006) but not Pla2g1b−/− mice (P=0.417). E) Core body temperature of the same groups of mice deprived of food overnight for 12 h (t=0) and after feeding. Data represent means ± se. *P ≤ 0.05, **P ≤ 0.01 vs. Pla2g1b+/+.
The difference between Pla2g1b+/+ and Pla2g1b−/− mice in their response to hypercaloric-diet-induced repression of energy expenditure was reflected by the negative correlation between preprandial core body temperature and body weight observed in the Pla2g1b+/+ mice but not apparent in the Pla2g1b−/− mice (Fig. 5D). These results are consistent with the conclusion of a higher metabolic rate with elevated hepatic fatty acid oxidation in Pla2g1b−/− mice as compared to the Pla2g1b+/+ mice. Interestingly, the Pla2g1b+/+ and Pla2g1b−/− mice displayed similar core body temperature 2 h after refeeding with a hypercaloric meal (Fig. 5E). Body temperature of Pla2g1b+/+ mice was found to be elevated after refeeding, presumably due to the thermic effect of food, i.e., the energetic cost of food processing, storage, and use (21). However, the increase in body temperature was rapidly lowered to the preprandial level after food consumption in the Pla2g1b+/+ mice. In contrast, body temperature of Pla2g1b−/− mice was sustained throughout the nonfeeding and refeeding period (Fig. 5E). The latter observation suggested a continuous elevated rate of metabolism in the Pla2g1b−/− mice. In addition, Pla2g1b−/− mice fed the hypercaloric diet were not as tolerant to cold exposure as Pla2g1b+/+ mice (Supplemental Fig. 3A). This is seemingly due to an elevated metabolism with elevated hepatic fatty acid oxidation; thereby, less energy resources are available for adaptive heat generation. Indeed, despite being leaner, the Pla2g1b−/− mice lost a greater mass and percentage of body weight during this regimen (Supplemental Fig. 3B). Interestingly, Western blot analysis determined that levels of uncoupling protein 1, a key component in adaptive thermogenesis, were similar in the brown adipose tissues of these animals (Supplemental Fig. 3C).
Hepatic gluconeogenesis
Our previous studies have shown improved glucose tolerance and insulin sensitivity in Pla2g1b−/− mice compared with Pla2g1b+/+ mice in response to a high-fat diet (7, 8). In view of our results showing that obesity resistance in Pla2g1b−/− mice is due primarily to differences in fatty acid uptake and utilization by the liver, we compared postprandial insulin levels and hepatic gluconeogenesis in Pla2g1b+/+ and Pla2g1b−/− mice in response to a lipid/glucose mixed meal. Mice that were maintained on the hypercaloric diet were deprived of food for 12 h and then fed a bolus lipid/glucose mixed meal containing 50% glucose (2 g/kg body wt), 2.6 mM phosphatidylcholine, and 13.33 mM triolein. Plasma insulin levels were found to be similar between Pla2g1b+/+ and Pla2g1b−/− mice after meal feeding (Supplemental Fig 4A). When the animals were injected intraperitoneally with sodium pyruvate (2 g/kg) 2 h after meal feeding, no difference in plasma glucose levels were observed between the 2 groups of mice over a 3-h period (Supplemental Fig. 4B). The latter observations indicated no difference in gluconeogenesis between Pla2g1b+/+ and Pla2g1b−/− mice in response to the lipid/glucose mixed meal. Taken together, these results indicate that obesity resistance of Pla2g1b−/− mice is not due to difference in postprandial insulin secretion or hepatic insulin sensitivity, but rather a direct effect of increased fatty acid oxidation and utilization by the liver of these animals. Moreover, the improved glucose tolerance and insulin sensitivity of the Pla2g1b−/− mice in response to chronic high-fat diet consumption (7, 8) was most likely secondary to their resistance to obesity.
DISCUSSION
Although PLA2G1B has long been thought to be important for phospholipid digestion and absorption (22, 23), Pla2g1b−/− mice have been previously demonstrated to have normal phospholipid absorption due to the compensatory phospholipase B activity present at intestinal brush-border membranes (24, 25). The difference between PLA2G1B and phospholipase B is that the latter enzyme does not produce lysophospholipids. The 5-fold reduction of absorbed lysophospholipid levels in Pla2g1b−/− mice was shown to have far-reaching consequences, including resistance to diet-induced hyperglycemia (7). The current study further demonstrated that the decreased absorption of the biologically active lysophospholipids, which are normally almost completely targeted to the liver via portal circulation (26), also results in a hepatically driven resistance to diet-induced obesity.
Resistance to diet-induced obesity in Pla2g1b−/− mice is not due to alterations in fat absorption, but rather is a result of metabolic recalibration. In contrast to the decrease in energy expenditure common in humans and animal subjects with prolonged periods of overnutrition (27,28,29,30), which is predictive of weight gain (31), Pla2g1b−/− mice maintain elevated levels of energy expenditure in response to hypercaloric-diet feeding. Interestingly, despite the increase in fatty acid oxidation in Pla2g1b−/− mice compared to that observed in Pla2g1b+/+ mice, respiratory quotients were similar between the two groups of animals (Supplemental Fig. 2C). These observations suggested carbohydrate nutrient utilization was also elevated in the Pla2g1b−/− mice. These interpretations are consistent with our previous observations of elevated glucose uptake and metabolism in liver, heart, and skeletal muscle of Pla2g1b−/− mice (7). In the current study, we showed increased hepatic fatty acid oxidation in these animals. Thus, the simultaneous derepression of both fatty acid and glucose utilization is likely to account for the increased energy expenditure and resistance to diet-induced hyperglycemia and obesity phenotypes in the Pla2g1b−/− mice.
Note that ketone body accumulation is commonly observed in diabetic individuals. Hence, the elevated preprandial plasma β-hydroxybutyrate levels observed in Pla2g1b−/− mice may appear at first glance to contradict the previous report of diabetes-resistant phenotype of the Pla2g1b−/− mice. This apparent dichotomy can be explained by the fact that the increased accumulation of β-hydroxybutyrate and other ketone bodies in these animals is caused by the increased rate of hepatic β-oxidation of fat instead of hepatic and extrahepatic glucose intolerance in diabetics. Higher preprandial plasma ketone bodies in Pla2g1b−/− mice fed only a basal diet suggest that hepatic fat oxidation occurs earlier or at a greater rate as the animal’s metabolism switches to starvation mode. However, when considering a postprandial state, it is not until fed a high-fat diet that the ketogenic lipid bolus enhances fat oxidation in Pla2g1b−/− mice compared to Pla2g1b+/+ mice. Further, this can be detected after only 1 wk of hypercaloric diet, prior to diet-induced obesity. Thus, a decrease in plasma lysophospholipid levels precedes the recalibration of fat oxidation in Pla2g1b−/− mice and allows for the elevated energy expenditure. This suggests that lysophospholipid, especially in a postprandial state, is a rheostat in the regulation of fat oxidation in response to fat uptake.
The most abundant lysophospholipid in plasma, LPC, is known to be proinflammatory and is elevated in patients with diseases associated with dyslipidemia (32, 33). High serum levels of many lysophospholipids, especially monostearoyl-glycerophosphocholine, correlate positively with early-stage obesity in a study of obesity-discordant monozygotic twins (34). Animal studies have also demonstrated elevated systemic monostearoyl-glycerophosphocholine with chronic feeding of a high-fat diet and obesity (35). Recent population studies identifying the PLA2G1B gene as a locus for obesity susceptibility (9) suggest that different amounts of lysophospholipids produced by PLA2G1B may be responsible for this relationship. The current study demonstrates decreased postprandial lysophospholipid levels and resistance to diet-induced obesity in Pla2g1b−/− mice and provides strong support for this hypothesis.
Lysophosphatidylcholine is known to interfere with PPAR activation in hepatic-derived dendritic cells (36). In the current study, we showed elevated expression of PPAR-α, PPAR-δ, PPAR-γ, Cd36/Fat, and Ucp2 in liver of Pla2g1b−/− mice. These liver-specific changes in gene expression are most likely due to the reduced level of LPC absorbed through the intestine and transported directly to the liver via the portal circulation. It is important to note that the elevated gene expression in liver of Pla2g1b−/− mice was observed prior to differences in obesity between Pla2g1b+/+ and Pla2g1b−/− mice. Therefore, the increased fatty acid uptake and utilization by the liver result in significantly less fatty acid availability to extrahepatic tissues, such as skeletal muscle and adipose tissues, thereby contributing directly to the reduced obesity of Pla2g1b−/− mice in response to the hypercaloric diet. As obesity develops in humans, elevated LPC levels precede the lipotoxic phenomena (34) hallmarked by the overflow of saturated fatty acids and dysregulation of the PPAR regulatory system (37).
The LPC-induced dysregulation of PPAR activation by fatty acids is evident by our data showing similar hepatic fatty acid oxidation rates in Pla2g1b+/+ and Pla2g1b−/− mice under food-deprivation conditions (Fig. 2D) despite their significant differences in plasma LPC levels (Fig. 3B). The differences in hepatic fatty acid oxidation between Pla2g1b+/+ and Pla2g1b−/− mice were only observed after feeding a lipid bolus meal (Fig. 2D). These results indicate that dietary fat transported to the liver may act similarly as newly synthesized fatty acids in activating PPAR to promote hepatic fatty acid oxidation (38) in the absence of LPC. However, under the typical situation of fat absorption where fatty acids and LPC are both transported to the liver, fatty acid oxidation is suppressed as a result of concomitant LPC absorption. The inhibition of LPC generation via Pla2g1b inactivation reduces LPC transport to the liver (7), thus overcoming the inhibitory effects on fatty acid-induced fatty acid oxidation. Taken together, this may indicate that LPC could play a major regulatory role in PPAR-directed gene expression via the repression of PPAR activation. The resistance to diet-induced obesity exhibited by Pla2g1b−/− mice fed hypercaloric diet may be due to the derepression of hepatic PPAR activity. Thus, pharmacologic inhibition of Pla2g1b activity during fat absorption may be a viable strategy to suppress diet-induced obesity and diabetes (39).
Supplementary Material
Acknowledgments
The authors thank Eliza Wynn and Beth Coburn for their superb technical assistance. The authors also thank Josh Basford, Dr. Dean Gilham, Dr. Diego Perez-Tilve, and Dr. Susanna Hofmann for their helpful discussions and advice. This work was supported by U.S. National Institutes of Health grant RO1 DK69967 (to D.Y.H.) and a Postdoctoral Fellowship (0525340B) from the Ohio Affiliates of the American Heart Association to E.D.L. Energy expenditure and fat absorption measurements were performed in the Cincinnati Mouse Metabolic Phenotype Center, supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK59630).
References
- Schaloske R H, Dennis E A. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–925. [PubMed] [Google Scholar]
- Carey M C, Small D M, Bliss C M. Lipid digestion and absorption. Annu Rev Physiol. 1983;45:651–677. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
- Mackay K, Starr J R, Lawn R M, Ellsworth J L. Phosphatidylcholine hydrolysis is required for pancreatic cholesterol esterase- and phospholipase A2-facilitated cholesterol uptake into intestinal Caco-2 cells. J Biol Chem. 1997;272:13380–13389. doi: 10.1074/jbc.272.20.13380. [DOI] [PubMed] [Google Scholar]
- Young S C, Hui D Y. Pancreatic lipase-colipase mediated triglyceride hydrolysis is required for cholesterol transport from lipid emulsions to intestinal cells. Biochem J. 1999;339:615–620. [PMC free article] [PubMed] [Google Scholar]
- Richmond B L, Boileau A C, Zheng S, Huggins K W, Granholm N A, Tso P, Hui D Y. Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A2-deficient mice. Gastroenterology. 2001;120:1193–1202. doi: 10.1053/gast.2001.23254. [DOI] [PubMed] [Google Scholar]
- Labonté E D, Kirby R J, Schildmeyer N M, Cannon A M, Huggins K W, Hui D Y. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes. 2006;55:935–941. doi: 10.2337/diabetes.55.04.06.db05-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins K W, Boileau A C, Hui D Y. Protection against diet-induced obesity and obesity-related insulin resistance in Group 1B PLA2-deficient mice. Am J Physiol Endocrinol Metab. 2002;283:E994–E1001. doi: 10.1152/ajpendo.00110.2002. [DOI] [PubMed] [Google Scholar]
- Wilson S G, Adam G, Langdown M, Reneland R, Braun A, Andrew T, Surdulescu G L, Norberg M, Dudbridge F, Reed P W, Sambrook P N, Kleyn P W, Spector T D. Linkage and potential association of obesity-related phenotypes with two genes on chromosome 12q24 in a female dizygous twin cohort. Eur J Hum Genet. 2006;14:340–348. doi: 10.1038/sj.ejhg.5201551. [DOI] [PubMed] [Google Scholar]
- Gilham D, Labonté E D, Rojas J C, Jandacek R J, Howles P N, Hui D Y. Carboxyl ester lipase deficiency exacerbates dietary lipid absorption abnormalities and resistance to diet-induced obesity in pancreatic triglyceride lipase knockout mice. J Biol Chem. 2007;282:24642–24649. doi: 10.1074/jbc.M702530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandacek R J, Heubi J E, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 2004;127:139–144. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Soda Y, Matsuyama Y, Mizuno K. An enzymatic assay for lysophosphatidylcholine concentration in human serum and plasma. Clin Biochem. 2002;35:411–416. doi: 10.1016/s0009-9120(02)00327-2. [DOI] [PubMed] [Google Scholar]
- Feingold K R, Wang Y, Moser A, Shigenaga J K, Grunfeld C. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J Lipid Res. 2008;49:2179–2187. doi: 10.1194/jlr.M800233-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S M, Zhou L, Perez-Tilve D, Greer T, Grant E, Wancata l, Thomas A, Pfluger P T, Basford J E, Gilham D, Herz J, Tschöp M H, Hui D Y. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J Clin Invest. 2007;117:3271–3282. doi: 10.1172/JCI31929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber M, Rogers T A. Energy metabolism. Annu Rev Physiol. 1961;23:5–36. doi: 10.1146/annurev.ph.23.030161.000311. [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Leon L R, Marcus-Samuels B, Mason M M, Castle A L, Refetoff S, Vinson C, Reitman M L. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 1999;96:14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G B, Brown J H, Enquist B J. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–1679. doi: 10.1126/science.284.5420.1677. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Gutierrez J A, Solenberg P J, Pfluger P T, Czyzyk T A, Willency J A, Schurmann A, Joost H-G, Jandacek R J, Hale J E, Heiman M L, Tschop M H. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner S, Nguyen-Tran V, Bare O, Huang X, Spiegelman B, Wu Z. PPARδ agonism activates fatty acid oxidation via PGC-1α but does not increase mitochondrial gene expression and function. J Biol Chem. 2009;284:18624–18633. doi: 10.1074/jbc.M109.008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F, Iglesias R, Giralt M. PPARs in the control of uncoupling proteins gene expression. PPAR Res. 2007;2007:74364. doi: 10.1155/2007/74364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonge L, Bray G A. The thermic effect of food and obesity: a critical review. Obes Res. 1997;5:622–631. doi: 10.1002/j.1550-8528.1997.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Dennis E A. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- Dijkstra B W, Renetseder R, Kalk K H, Hol W G, Drenth J. Structure of porcine pancreatic phospholipase A2 at 2.6 Å resolution and comparison with bovine phospholipase A2. J Mol Biol. 1983;168:163–179. doi: 10.1016/s0022-2836(83)80328-3. [DOI] [PubMed] [Google Scholar]
- Pind S, Kuksis A. Further characterization of a novel phospholipase B (phospholipase A2-lysophospholipase) from intestinal brush border membranes. Biochem Cell Biol. 1991;69:346–357. doi: 10.1139/o91-054. [DOI] [PubMed] [Google Scholar]
- Tojo H, Ichida T, Okamoto M. Purification and characterization of a catalytic domain of rat intestinal phospholipase B/lipase associated with brush border membranes. J Biol Chem. 1998;273:2214–2221. doi: 10.1074/jbc.273.4.2214. [DOI] [PubMed] [Google Scholar]
- Ikeda I, Imaizumi K, Sugano M. Absorption and transport of base moieties of phosphatidylcholine and phosphatidylethanolamine in rats. Biochim Biophys Acta. 1987;921:245–253. [PubMed] [Google Scholar]
- Abbott W G, Howard B V, Ruotolo G, Ravussin E. Energy expenditure in humans: effects of dietary fat and carbohydrate. Am J Physiol. 1990;258:E347–E351. doi: 10.1152/ajpendo.1990.258.2.E347. [DOI] [PubMed] [Google Scholar]
- Hurni M, Burnand B, Pittet P, Jequier E. Metabolic effects of a mixed and a high carbohydrate low-fat diet in man, measured over 24 h in a respiration chamber. Br J Nutr. 1982;47:33–43. doi: 10.1079/bjn19820006. [DOI] [PubMed] [Google Scholar]
- Lean M E, James W P. Metabolic effects of isoenergetic nutrient exchange over 24 hours in relation to obesity in women. Int J Obes. 1988;12:15–27. [PubMed] [Google Scholar]
- Schwartz R S, Ravussin E, Massari M, O'Connell M, Robbins D C. The thermic effect of carbohydrate versus fat feeding in man. Metabolism. 1985;34:285–293. doi: 10.1016/0026-0495(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Lillioja S, Knowler W C, Christin L, Freymond D, Abbott W G, Boyce V, Howard B V, Bogardus C. Reduced rate of energy expenditure as a risk factor for body weight gain. N Engl J Med. 1988;318:467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- Lusis A J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabini R A, Galassi R, Fumelli P, Dousset N, Solera M L, Valdiguie P, Curatola G, Ferretti G, Taus M, Mazzanti L. Reduced Na+-K+ ATPase activity and plasma lysophosphatidylcholine concentrations in diabetic patients. Diabetes. 1994;43:915–919. doi: 10.2337/diab.43.7.915. [DOI] [PubMed] [Google Scholar]
- Pietilainen K H, Sysi-Aho M, Rissanen A, Seppanen-Laakso T, Yki-Jarvinen H, Kaprio J, Oresic M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects—a monozygotic twin study. PLoS ONE. 2007;2:e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili O, Versari D, Sattler K J, Olson M L, Mannheim D, McConnell J P, Chade A R, Lerman L O, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- Coutant F, Agaugue S, Perrin-Cocon L, Andre P, Lotteau V. Sensing environmental lipids by dendritic cell modulates its function. J Immunol. 2004;172:54–60. doi: 10.4049/jimmunol.172.1.54. [DOI] [PubMed] [Google Scholar]
- Unger R H, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 2001;15:312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- Chakravarthy M V, Pan Z, Zhu Y, Tordjman K, Schneider J G, Coleman T, Turk J, Semenkovich C F. “New” hepatic fat activates PPAR-α to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hui D Y, Cope M J, Labonte E D, Chang H-T, Shao J, Goka E, Abousalham A, Charmot D, Buysse J. The phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. Br J Pharmacol. 2009;157:1263–1269. doi: 10.1111/j.1476-5381.2009.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.