Abstract
Cockayne syndrome (CS) is a human premature aging disorder associated with severe developmental deficiencies and neurodegeneration, and phenotypically it resembles some mitochondrial DNA (mtDNA) diseases. Most patients belong to complementation group B, and the CS group B (CSB) protein plays a role in genomic maintenance and transcriptome regulation. By immunocytochemistry, mitochondrial fractionation, and Western blotting, we demonstrate that CSB localizes to mitochondria in different types of cells, with increased mitochondrial distribution following menadione-induced oxidative stress. Moreover, our results suggest that CSB plays a significant role in mitochondrial base excision repair (BER) regulation. In particular, we find reduced 8-oxo-guanine, uracil, and 5-hydroxy-uracil BER incision activities in CSB-deficient cells compared to wild-type cells. This deficiency correlates with deficient association of the BER activities with the mitochondrial inner membrane, suggesting that CSB may participate in the anchoring of the DNA repair complex. Increased mutation frequency in mtDNA of CSB-deficient cells demonstrates functional significance of the presence of CSB in the mitochondria. The results in total suggest that CSB plays a direct role in mitochondrial BER by helping recruit, stabilize, and/or retain BER proteins in repair complexes associated with the inner mitochondrial membrane, perhaps providing a novel basis for understanding the complex phenotype of this debilitating disorder.—Aamann, M. D., Sorensen, M. M., Hvitby, C., Berquist, B. R., Muftuoglu, M., Tian, J., de Souza-Pinto, N. C., Scheibye-Knudsen, M., Wilson, D. M., III, Stevnsner, T., Bohr, V. A. Cockayne syndrome group B protein promotes mitochondrial DNA stability by supporting the DNA repair association with the mitochondrial membrane.
Keywords: base excision repair, BER, CSB, oxidative stress
Cockayne syndrome (CS) is an autosomal recessive segmental premature aging syndrome, with complex and variable characteristics, including poor growth and neurological abnormalities. Other features of CS are photosensitivity, cataracts, dental caries, hearing loss, ataxia, and muscle weakness. There are 2 complementation groups for CS, CSA and CSB. Approximately 80% of CS patients carry a mutation in CSB, with the remainder carrying a mutation in CSA. Besides being sensitive toward UV irradiation (1, 2), CSB-deficient cells also exhibit increased sensitivity to γ-irradiation, hydrogen peroxide, and alkylating agents, all of which induce DNA lesions repaired by base excision repair (BER) (3, 4). Thus, in addition to playing an important role in transcription coupled nucleotide excision repair (TCR), the CSB protein contributes to, among other pathways, BER, the major system for repairing endogenously formed DNA lesions (1, 5,6,7,8,9,10,11,12,13).
Previous studies suggest that CSB plays a role in repair of oxidative DNA damage in nuclear DNA. The amount of oxidized DNA bases, such as 8-hydroxy-7,8-dihydroguanine (8-oxoG) and 7,8-dihydro-8-oxoadenine (8-oxoA), is higher in the DNA of CSB-deficient cells than in CSB-proficient cells (14). Moreover, the amount of 8-oxoG, 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) and 4,6-diamino-5-formamidopyrimidine (FapyA) is higher in brain and kidney from CSB-deficient mice than in wild-type mice (9). Evidence suggests that CSB may stimulate transcription of the 8-oxoG DNA glycosylase OGG1 gene (3, 5, 7), thereby stimulating nuclear BER indirectly. However, FapyA, which is not a canonical substrate for Ogg1 (15), accumulates in brain, liver, and kidney of CSB-deficient mice (9), suggesting a direct role of CSB in the BER process. Furthermore, PARP1 involvement in BER has been shown to be CSB dependent (16). Together, these data suggest that CSB can stimulate BER in an Ogg1-independent manner. Reported interactions of CSB with PARP1, APE1, and NEIL1 (9, 17, 18) further support a role for CSB in BER, independent of transcription regulation and Ogg1.
Some of the clinical symptoms of CS resemble those seen in several mitochondrial diseases, such as ataxia, sensorineural hearing loss, neurological dysfunction, and muscle weakness (19,20,21,22). In addition, several studies indicate that an increased load of mitochondrial DNA lesions and defective BER (both nuclear and mitochondrial) correlate with neurodegeneration and aging (23, 24). Mitochondria have very efficient BER (25), and the BER activity in mitochondria is associated with the inner mitochondrial membrane (26). Not much is known about how this association is organized, although it has been suggested that it is electrostatic in nature (26).
Previous studies suggest that CSB also plays a role in BER in mitochondrial DNA (mtDNA). CSB-deficient human cells and liver tissue from CSB-deficient mice have lower mitochondrial 8-oxoG incision capacity and decreased mitochondrial capacity to remove Fpg -sensitive sites from the mtDNA than control cells (3). More recently it has been shown that liver mtDNA from CSB-deficient mice have more FapyA lesions than control mtDNA (9), indicating possible involvement of CSB in mtDNA repair. A recent study found that the organization of the mitochondrial oxidative phosphorylation complexes into super-complexes were compromised in CSB-deficient mouse cells. These cells were also sensitive toward inhibitors of mitochondrial complexes (27), indicating a general role of CSB in mitochondrial maintenance.
Here we investigate the role of CSB in mitochondrial BER. We show that the CSB protein is located in mitochondria in different cell types and at increased levels after menadione-induced oxidative stress. Yeast 2 hybrid screening detected CSB interactions with mitochondrial proteins. CSB deficiency resulted in a decreased incision activity of mitochondrial extracts for oxidative DNA base lesions, and the association of BER-related incision activity to the mitochondrial inner membrane was affected by CSB. Moreover, a CSB defect led to a higher mutation frequency in mtDNA. These data taken together indicate a biological role for CSB in mitochondrial genome maintenance, likely through regulation of BER.
MATERIALS AND METHODS
Cell culture
The CS1AN cell line is an SV40 transformed human fibroblast cell line, belonging to CS complementation group B (28). Stable transfectant CS1AN cells lines, with empty pcDNA3.1 vector or vector expressing wild-type CSB (previously described by Selzer et al.; ref. 29) were grown in a humidified 5% CO2 atmosphere in minimal essential medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 1% penicillin/streptomycin, and 400 μg/ml geneticin. Cells were harvested at ∼90% confluence, washed twice in phosphate buffered saline (PBS), and either used for mitochondrial purification immediately or kept at −80°C for later mitochondrial isolation. CS1AN with vector (CSB-deficient) and wild-type (wt) CSB were treated identically.
HeLa cells were grown in a humidified 5% CO2 atmosphere in Dulbecco’s modified Eagle medium (DMEM) with 10% FBS and 1% penicillin/streptomycin. For mitochondrial purification, cells were grown to ∼90% confluence, washed twice in PBS, treated, washed, and used immediately for mitochondrial purification. When treated with 200 μM menadione, the cells were incubated with the drug (in DMEM) for 1 h at 37°C, washed twice with PBS, and collected for mitochondrial isolation. For mock, DMEM without drug was used for 1-h treatment.
For CSB knockdown, HeLa cells were transfected with shRNA targeting human CSB (catalog no. TR313176; OriGene, Rockville, MD, USA) or nontargeting (control) shRNA (OriGene) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. For a stable knockdown, cells were maintained in DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin with 2 μg/ml puromycin. Individual clones were selected and tested for CSB knockdown by Western blot.
Immunocytochemistry of HeLa cells
HeLa cells were plated on 4-well Lab-Tek Chamber Slides (Nunc, Roskilde, Denmark) and grown for 24 h. After washing 2 times with PBS, either DMEM or DMEM with 200 μM menadione (Sigma-Aldrich, St. Louis, MO, USA), for mock and menadione treatment, respectively, was added, and cells were incubated for 1 h at 37°C. After treatment, cells were washed twice in PBS and fixed for 15 min at room temperature in 3.7% formaldehyde, followed by 3 washes with PBS. Permeabilization was done using 0.25% TritonX-100 for 10 min on ice, followed by 3 washings in PBS. Unspecific binding was blocked by incubation with 5% FBS overnight at 4°C. The cells were then incubated with primary antibody against CSB (rabbit anti-CSB 1:750, sc-25370) and TFAM (goat anti-mtTFA 1:500, sc-19050), both from Santa Cruz Biotechnology (Santa Cruz, CA, USA) in 5% FBS, followed by 3 washings with PBS. Alexa Fluor donkey-anti-goat 488 and donkey-anti-rabbit 594 secondary antibodies diluted in 5% FBS (both 1:500) were added and incubated for 40 min at room temperature. Finally, cells were washed ≥6 times in PBS and covered with VectaShield hard set mounting medium with 4′6-diamidino-2-phenylindol (DAPI) from Vector Laboratories (Burlingame, CA, USA) to preserve the sample and to stain the nucleus. All buffers used were based on PBS. Antibodies were checked for cross-reaction and bleed-through between the two channels by using one primary and both secondary antibodies. Neither cross-reaction nor bleed-through was detected. Background from the secondary antibody was checked using no primary and both secondary antibodies. Pictures were acquired on a Nikon Eclipse TE-2000e confocal microscope (Nikon, Tokyo, Japan) using the ×60 objective lens, 0.2-μm z stacks throughout the cells using Volocity software (Improvision, Waltham, MA, USA). Quantification was performed using Volocity colocalization software using the same threshold settings for both mock and menadione-treated samples. For the images, low-level intensity signals were removed, and the contrast was enhanced using Volocity software.
Immunocytochemistry of primary human fibroblast cells
Primary human fibroblasts (GM00969; Coriell, Camden, NJ, USA) were cultured in Amniomax-II (Invitrogen) and plated on 4-chambered slides (Lab-Tek). The cells were exposed to 200 μM Menadione (Sigma) (in Amniomax-II) for 1 h at 37°C. Cells were fixed with 3.7% paraformaldehyde in PBS for 15 min at room temperature and washed twice with PBS, then permeabilized with 0.25% Triton X-100 in PBS for 10 min on ice. After blocking in PBS containing 5% BSA for 1 h at 37°C, slides were incubated 1 h at 37°C with primary antibodies, mouse polyclonal anti-TFAM (1:500; Abnova, Taipei, Taiwan), and rabbit polyclonal anti-CSB (1:500; Santa Cruz); all antibodies were diluted in blocking solution. Cells were then rinsed twice with PBS and incubated with Alexa Fluor 568-conjugated goat anti-mouse and Alexa Fluor 647-conjugated donkey anti-rabbit (Invitrogen A21124 and A31573, respectively; 1:500) secondary antibodies for 1 h at 37°C. After washing 5 times with PBS, cells were mounted with Prolong-Gold Antifade mounting medium with DAPI (Invitrogen). Images were captured by a Nikon Eclipse TE2000e confocal microscope as described above.
Yeast 2-hybrid screen
The yeast 2-hybrid screen using CSB as bait was carried out by Dualsystems Biotech (Zurich, Switzerland). The bait construct was made by subcloning a cDNA encoding aa 1-1493 of CSB into the vector pLexA-DIR (Dualsystems Biotech). The bait construct was transformed into strain NMY32 (MATa, his3Δ200, trp1-901, leu2-3,112, (lexAop)8-ADE2, LYS2::(lexAop)4-HIS3, URA3::(lexAop)8-lacZ Gal4). Correct expression of the bait was verified by Western blotting of cell extracts using a mouse monoclonal antibody directed against the LexA domain (Dualsystems Biotech). The absence of self-activation was verified by cotransformation of the bait together with a control prey and selection on minimal medium lacking tryptophan, leucine, and histidine. For the yeast 2-hybrid screen, the bait was cotransformed with a normalized human universal cDNA library into NMY32. Positive transformants were tested for β-galactosidase activity using a PXG β-galactosidase assay (Dualsystems Biotech). Those isolates that were HIS+ and lacZ+ were considered to be true positives.
Mitochondrial isolation from cell culture
CS1AN and HeLa cells were washed, harvested, and resuspended in MSHE buffer (210 mM mannitol; 70 mM sucrose; 10 mM HEPES, pH 7.4; 1 mM EGTA; 2 mM EDTA; 0.15 mM spermine; 0.75 mM spermidine; and 5 mM DTT) with protease inhibitors added just prior to use, and homogenized with a Potter-Elvehjem glass/glass homogenizer. Cells were subjected to mild treatment with digitonin prior to homogenization to ensure permeabilization of the plasma membrane. The homogenate was centrifuged at 500 g for 12 min, and the supernatant was transferred to a new tube and centrifuged at 10,000 g. The pellet from the first centrifugation was homogenized again, centrifuged at 500 g, and the supernatant was spun at 10,000 g. The pellets from the two 10,000 g centrifugations were then combined (crude mitochondrial extract) and resuspended in 1:1 Percoll/2× MSHE and layered onto a 1:1 Percoll:2× MSHE gradient and centrifuged at 50,000 g for 1 h. The mitochondrial layer was collected, washed in 1× MSHE, and resuspended in MSHE, snap-frozen, and stored at −80°C. For HeLa cells, a small fraction of the nuclear-containing pellet (pellet after homogenization and centrifugation at 500 g) was diluted in MSHE buffer and sonicated at 20 pulses at 2 W with cooling on ice in between, for later use in Western blots.
Protein concentration of the nuclear pellets and the purified mitochondria were determined by the Bradford method (Bio-Rad Protein Assay, Bio-Rad Laboratories, Hercules, CA, USA) using BSA as a standard and stored at −80°C until use. For use in AP endonuclease experiments, small modifications to the purification protocol were introduced: a Kontes glas/Teflon homogenizer was used for homogenization, and digitonin treatment was omitted. Following homogenization and centrifugations, the crude mitochondrial pellet was washed in MSHE buffer and centrifuged at 8500 g for 10 min. The pellet was resuspended in MSHE buffer and subjected to a final centrifugation at 9500 g for 10 min to pellet the washed mitochondria. The mitochondria were resuspended in 1:1 Percoll/2× MSHE and layered onto a 1:1 Percoll:2× MSHE gradient and purified as described above.
All manipulations were carried out on ice, and centrifugation was carried out at 4°C.
Mitochondrial isolation from muscle tissue
Isolation of mitochondria from skeletal muscle was performed as described previously (30) with the following modifications: after homogenization, nuclear fractions and cellular debris were precipitated at 1000 g for 10 min at 4°C. The supernatant was transferred, and mitochondria were precipitated in 2 consecutive spins (5400 g, 10 min, 4°C; and 6700 g, 10 min, 4°C). Further isolation of mitochondrial matrix was performed by resuspending the mitochondrial pellet in lysis buffer (10 mM tris-HCl, pH 7.8; 400 mM KCl; 1 mM EDTA; 1 mM DTT; 20% glycerol; 0.1% Nonidet P-40; 0.25 mM PMSF; and 1× protease inhibitor cocktail from Roche) following a 1.5 h incubation on ice. The lysate was sonicated 5 times for 5 s at 5 W on ice, and DNA and lipid membranes were precipitated in a 130,000 g spin at 4°C for 1 h. The supernatant was dialyzed overnight against the dialysis buffer (25 mM HEPES, 100 mM KCl, 1 mM EDTA, 17% glycerol, and 12 mM MgCl2). Finally, the lysate was spun at 16,000 g for 10 min at 4°C to precipitate any nonsoluble fraction, and the supernatant containing purified matrix enzymes was stored at −80°C for later use.
Mitochondrial subfractionation
Mitochondrial fractionation was carried out essentially as described by Stuart et al. (26). After diluting the isolated mitochondria in MSHE buffer at 10 μg/μl, the mitochondria were subjected to four 5-s sonication bursts at 5 W, with 1 min between each burst. An aliquot of this homogenate was kept on ice [whole mitochondria (WM) extract], and the rest was ultracentrifuged at 130,000 g for 1 h, resulting in the separation of soluble fraction 1 from membrane-bound fraction 1. The volumes of both fractions were adjusted to the starting volume with MSHE buffer, as depicted in Fig. 5A. WM, membrane-bound fraction 1, and soluble fraction 1 were then given one more burst of sonication before storing at −80°C for later use. All manipulations were carried out on ice, and centrifugation was carried out at 4°C.
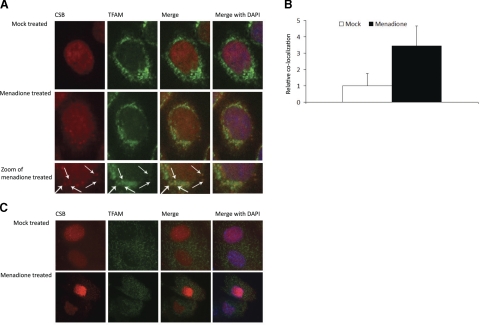
Figure 1.
Colocalization of CSB with TFAM in menadione treated cells. A) HeLa cells either mock treated or treated with 200 μM menadione were stained for endogenous CSB and TFAM. Colocalization in the merged fields appears as yellow foci (arrows). B) Colocalization was quantified using Volocity software. Solid bar, fold increase in colocalization of CSB to TFAM after menadione treatment; open bar, mock treatment. Results from one representative experiment are shown. C) Primary human skin fibroblast cells either mock treated or treated with 200 μM menadione were stained for endogenous CSB and TFAM. Colocalization in merged fields appears as yellow foci.
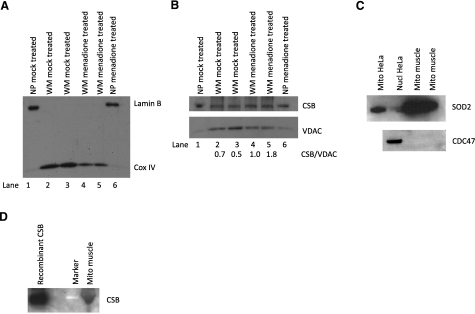
Figure 2.
Presence of CSB in whole mitochondrial extract. A, B) Mitochondria were isolated from HeLa cells either mock treated or treated with 200 μM menadione; see Materials and Methods. A) Fifteen micrograms purified whole mitochondria (WM) or nuclear pellet (NP) from both mock and menadione-treated cells were used for Western blot using Lamin B as nuclear marker and mitochondrial protein Cox IV. B) Fifteen micrograms of both NP and WM were used for Western blot, which was cut at 100 kDa; bottom part was probed for structural mitochondrial protein VDAC and top part for CSB. Two separate samples for each treatment (lanes 2, 3 and 4, 5, respectively) of whole mitochondria were loaded on the gel. Relative band intensities of CSB/VDAC, shown below the Western blot, were quantified using ImageQuant TL. C, D) Mitochondria were isolated from primary human muscle cells. C) Top panel: 40-μg nuclear and mitochondrial samples from HeLa cells and 40-μg mitochondrial extract from muscles were used for Western blot against SOD-2. Bottom panel: 60-μg mitochondrial extract from muscles was used for Western blot against CDC47 to test for nuclear contamination with 60-μg nuclear samples from HeLa cells as positive control. D) Purified CSB and muscle mitochondria (50 μg) were used for Western blot against CSB.
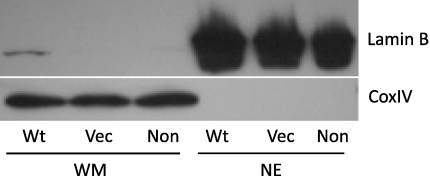
Figure 3.
Purity of mitochondria extract from CS1AN cells. Mitochondria from SV-40 transformed CS1AN cells either nontransfected or stably transfected with either empty vector (vec) or wild-type CSB expression vector (wt) were purified as described in Materials and Methods. Whole mitochondrial extract (WM; 40 μg) or nuclear extract (NE; 25 μg) was resolved on a 12% Tris-glycine gel and transferred to a PVDF membrane. Membrane was probed with antibodies against nuclear protein Lamin B (Novocastra) and mitochondrial protein CoxIV (Santa Cruz), and film was overexposed for the Lamin B signal.
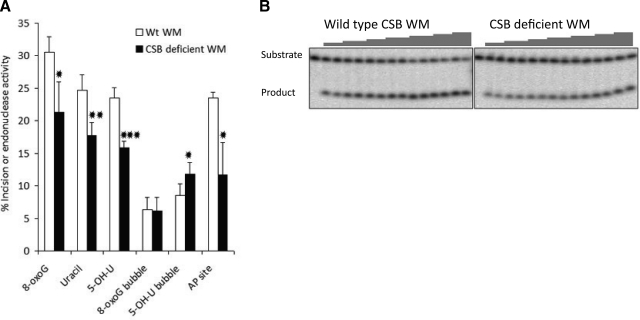
Figure 4.
Decreased repair activity in CSB-deficient mitochondrial extract. Incision of whole mitochondrial extract (WM) from CS1AN cells stably transfected with either empty vector (CSB-deficient) or CSB wild-type expression vector (wt). A) Incision represented as percentage cleaved substrate containing 8-oxoG, uracil, or 5-OH-U in a double-stranded oligodeoxynucleotide; 8-oxoG or 5-OH-U in an 11-nt bubble; or THF, an AP site analog, in a double-stranded oligodeoxynucleotide (Table 1). Results are means ± sd for 3–4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. wt. B) Representative gel picture for THF incision assay using WM from CSB wild-type cells and CSB-deficient cells, with increasing amounts of mitochondrial extracts from left to right.
Figure 5.
Design of mitochondrial fractionation procedure. A) Fractionation of whole mitochondria into membrane-bound fraction and soluble fraction, used for experiments shown in Fig. 6. B) Additional subfractionation of membrane-bound fraction 1. Membrane-bound fraction was subjected to Nonidet P-40 treatment, followed by increasing salt concentrations, followed by centrifugation, resulting in soluble fraction 2 to 6, all originating from the initial membrane-bound fraction 1. Soluble fractions were used for experiments shown in Fig. 7.
Some of the membrane-bound fraction 1 was further fractionated, as outlined in Fig. 5B; membrane-bound fraction 1 diluted to 5 μg/μl in MSHE buffer was incubated with 0.1% Nonidet P-40 for 20 min at 4°C, and then centrifuged for 30 min at 130,000 g. The volumes of the resulting soluble fraction 2 and membrane-bound fraction 2 were adjusted to the starting volume with MSHE buffer containing 0.1% Nonidet P-40. Then 25 mM NaCl was added to the membrane-bound fraction 2 and incubated for 20 min, before another round of centrifugation. This cycle was repeated with the subsequent membrane-bound fractions 3, 4, and 5 being incubated with 50, 100, and 150 mM NaCl, respectively. This experimental setup hence generated soluble fractions 2 to 6 containing proteins, originally from membrane-bound fraction 1, solubilized with Nonidet P-40, 25 mM NaCl, 50 mM NaCl, 100 mM NaCl, and 150 mM NaCl, respectively.
Incision activities
Uracil incision was measured by incubation of 0.5 μg WM, or an equal volume of the indicated mitochondrial subfractions, with 100 fmol 32P end-labeled uracil-containing DNA substrate (Table 1) 30 min at 37°C, in 10 μl reaction buffer, containing 70 mM HEPES-KOH (pH 7.5), 5 mM EDTA, 1 mM DTT, 10% glycerol, and 75 mM NaCl. The 8-oxoG incision activity was determined by incubation of 10 μg of WM or subfractions thereof for 4 h at 37°C with 100 fmol 32P end-labeled 8-oxoG containing DNA substrate (Table 1) in 10 μl reactions, under the same buffer conditions as for uracil incision. Incision of 5-hydroxy-uracil (5-OH-U) was determined by incubation of 5 μg WM, or subfractions thereof, with 100 fmol DNA substrate, containing 5-OH-U (Table 1) for 30 min at 37°C, under the same buffer conditions as for 8-oxoG and uracil incision.
TABLE 1.
Oligodeoxynucleotides used for incision and AP-endonuclease assays
| Name | Sequence |
|---|---|
| 8-OxoG | 5′-ATATACCGCG(OG)CCGGCCGATCAAGCTTATT-3′ |
| 3′-TATATGGCGC C GGCCGGCTAGTTCGAATAA-5′ | |
| Uracil | 5′-ATATACCGCGGUCGGCCGATCAAGCTTATT-3′ |
| 3′-TATATGGCGCCGGCCGGCTAGTTCGAATAA-5′ | |
| 5-OH-U | 5′-ATATACCGCGG(OH-U)CGGCCGATCAAGCTTATT-3′ |
| 3′-TATATGGCGCC G GCCGGCTAGTTCGAATAA-5′ | |
| 5-OH-U bubble | 5′-GCTTAGCTTGGAATCGTATCATGTA(OU)ACTCGTGTGCCGTGTAGACCGTGCC-3′ |
| 3′-CGAATCGAACCTTAGCATAGGCACCCGACAAACACGGCACATCTGGCACGG-5′ | |
| 8-oxoG bubble | 5′-GCTTAGCTTGGAATCGTATCATGTA(OG)ACTCGTGTGCCGTGTAGACCGTGCC-3′ |
| 3′-CGAATCGAACCTTAGCATAGGCACCCGACAAACACGGCACATCTGGCACGG-5′ | |
| THF (AP-site analogue) | 5′-ATATACCGCGG(THF)CGGCCGATCAAGCTTATT-3′ |
| 3′-TATATGGCGCCGGCCGGCTAGTTCGAATAA-5′ |
Incisions of the substrates containing 5-OH-U and 8-oxoG on bubble oligonucleotides (Midland Certified Reagent, Midland, TX, USA; Table 1) were determined by incubating 10 μg WM with 100 fmol of the damage-containing DNA substrate in 10 μl reactions with 50 mM HEPES-KOH (pH 7.5), 5 mM EDTA, 1 mM DTT, 2.5% glycerol, 50 mM KCl, and 0.1 mg/ml BSA. Incision reactions with 5-OH-U on bubble were incubated for 1 h at 37°C, whereas reactions with 8-oxoG on bubble were incubated for 4 h.
All incision reactions were terminated by adding 10 μl formamide dye (80% formamide, 10 mM EDTA, 0.1 mg/ml xylene cyanol FF, and 0.1 mg/ml bromphenol blue), and heating at 90–95°C for 10 min. Samples were run on denaturating gels and reaction substrates and products were visualized by PhosphoImager (GE Healthcare Bio-Sciences, Uppsala, Sweden) and quantified using ImageQuant™(GE Healthcare). For sequence of oligonucleotides used see Table 1.
AP endonuclease activity
Mitochondria proteins were extracted by dilution of whole mitochondria in a buffer containing 20 mM HEPES/KOH (pH 7.4), 1 mM EDTA, 2 mM DTT, 5% glycerol, and 300 mM KCl for 2 min followed by dilution in the same buffer excluding KCl to give a final concentration of 100 mM KCl. APE activity was measured by incubation of 50–170 ng WM with 90 fmol 32P end-labeled DNA substrate containing THF (Table 1) for 30 min at 37°C, in 20 μl reaction volume, containing 100 mM HEPES/KOH (pH 7.4), 25 mM EDTA, 25 mM DTT, 5% glycerol, 75 mM KCl, 5 mM MgCl2, and 0.5 mg/mL BSA. Reactions were terminated by addition of 0.2 mg/ml Proteinase K, 0.4% SDS, and incubation at 55°C for 30 min, followed by dilution in formamide-containing buffer and separation on a denaturating gel. Reaction substrate and products were visualized by PhosphoImager and quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA). We used 50 ng WM for the incisions represented in Fig. 4A.
Western blotting
WM was diluted in SDS protein loading buffer with DTT and heated at 90°C for 10 min. Extracts were separated using SDS-PAGE. Proteins were transferred to PVDF membranes (0.2-μm pore size) in transfer buffer containing 10–20% methanol. Membranes were blocked in 5% milk in TBST, either 1–2 h at room temperature or overnight at 4°C. Incubations with primary antibodies were performed in 5% milk in TBST, either 1–2 h at room temperature or overnight at 4°C, with the following antibodies: anti-Lamin B (1:1000; goat from Novocastra Laboratories, Newcastle on Tyne, UK, or mouse, Abcam, Cambridge, MA, USA), anti-COX IV antibody (1:1000; mouse from Molecular Probes), anti VDAC (1:1000; goat from Santa Cruz Biotechnology), anti-CDC47 (Neomarkers; Lab Vision Corporations, Fremont, CA, USA), anti-SOD-2 (Santa Cruz Biotechnology), and anti-CSB antibody (1:1000; rabbit from Santa Cruz Biotechnology). After washing with TBST, appropriate secondary antibodies linked to horseradish peroxidase were applied at 1:1000 to 1:40,000 (donkey anti-goat and mouse anti-rabbit: Santa Cruz Biotechnology; goat anti-mouse: Pierce, Thermo Fisher Scientific LSR, Rockford, IL, USA; goat anti-rabbit: Sigma; rabbit anti-mouse: Sigma-Aldrich) in 5% milk in TBST. Membranes were then again washed repeatedly with TBST and visualized using Amersham ECLPlus (GE Healthcare).
Chloramphenicol resistance assay
Chloramphenicol (CAP) resistance was determined as a measurement of mtDNA mutagenesis, as described previously (31), with small modifications. In short, mock-transfected and CSB shRNA-transfected HeLa cells were seeded onto 6-well dishes in DMEM supplemented with 10% fetal bovine serum, 2 μg/ml puromycin, and 1 mM sodium pyruvate at 300 cells/well, and 24 h later the growth medium was replaced with medium containing 200 μg/ml CAP. For measurement of plate efficiency, wells subjected to the same experimental conditions were incubated in the absence of CAP. The cultures were maintained until colonies formed; the colonies were then fixed with methanol and stained with 0.5% methylene blue. The number of colonies per well was measured using a Typhoon (GE Healthcare Bio-Sciences) with the colony-counting mode. The relative survival rate was calculated by dividing the average number of colonies in the presence of CAP by the average number of colonies in absence of CAP for each experimental condition.
Statistics
All statistical comparisons were done using Student’s t test. The minimum level of statistical significance was P < 0.05.
RESULTS
CSB localizes to mitochondria in HeLa cells
Our previous observation that mitochondrial 8-oxoG incision activity is decreased in CSB-deficient cells, and the overlap in phenotype between CS patients and patients with mitochondrial dysfunction, lead us to investigate whether CSB is present in mitochondria before and after oxidative stress. Immunocytochemical experiments were used for this purpose, and the mitochondrial transcription factor A (TFAM), which localizes exclusively to the mitochondrial nucleoid (32), was used as a mitochondrial marker. HeLa cells were seeded on slides, grown for 24 h, and treated for 1 h either under mock conditions or with 200 μM menadione. After treatment, cells were costained with TFAM and CSB antibody (see Materials and Methods). Without menadione, a weak cytosolic localization of CSB was seen in some cells. In cells treated with menadione, a noticeable relocalization of CSB was observed, resulting in CSB partly colocalizing with TFAM (Fig. 1A, arrows). Volocity’s colocalization software indicated a 3-fold greater colocalization of CSB and TFAM in cells treated with menadione compared to mock treatment, which was statistically significant (Fig. 1B). To make sure that this redistribution of CSB was not limited to transformed cells, primary human skin fibroblasts were mock treated or treated with 200 μM menadione. Some relocalization of CSB was observed, although not as significant as with HeLa cells (Fig. 1C). This supports that the relocalization of CSB in response to menadione treatment is not limited to HeLa cells.
To further confirm the presence of CSB in mitochondria, mitochondrial extracts were prepared from HeLa cells and analyzed by Western blotting. Antibodies against the nuclear protein Lamin B confirmed that the mitochondrial extract was essentially free of nuclear contamination (Fig. 2A, top panel, lanes 2–5), whereas a clear signal was seen in the nuclear-containing pellet (Fig. 2A, lanes 1 and 6). Mitochondrial enrichment was confirmed by probing for mitochondrial protein cytochrome C oxidase subunit IV (CoxIV) (Fig. 2A, lower part, lanes 2–5). Using the same mitochondrial extract, we detected a band at the expected size of CSB (Fig. 2B, top panel, lanes 2–5), which comigrated with CSB in the nuclear containing pellet (Fig. 2B, lanes 1 and 6). The same membrane was probed for the mitochondrial protein VDAC1 (Fig. 2B, bottom panel, lanes 2–5), and the ratio of band intensity of CSB to VDAC was measured (numbers are given below Western blot in Fig. 2B). A higher ratio was found in the mitochondrial extract from menadione-treated cells (Fig. 2B, lanes 4 and 5) compared to control cells (Fig. 2B, lanes 2 and 3), indicating an increase in the amount of CSB in the mitochondria after menadione exposure. These results demonstrate that CSB is present in mitochondria in human cells and that its level increases in response to menadione-induced oxidative stress.
To further determine the generality of CSB presence in mitochondria in different cells and tissues, we investigated whether CSB was localized in primary human skeletal muscle mitochondria from a healthy individual. This is also relevant because some CS patients exhibit muscle weakness. The mitochondria were isolated from a muscle biopsy as described in Materials and Methods. The muscle mitochondria preparation was checked for nuclear contamination using antibody against the nuclear protein CDC47 with nuclear HeLa extract as a positive control. The samples were tested for mitochondrial enrichment using an antibody against the mitochondrial protein SOD-2 with mitochondrial extract and nuclear extract from HeLa for comparison. The muscle mitochondria were essentially free of contamination (Fig. 2C). When the muscle mitochondria were tested for presence of CSB protein, a band was seen at the same size as purified CSB (Fig. 2D). This result shows that in addition to the above described presence of CSB in transformed cells, CSB is localized to the mitochondria in human muscle cells isolated directly from a muscle biopsy.
Using a 2-hybrid screen for interacting partners of full-length CSB (see Materials and Methods; additional results to be reported elsewhere), “normal” and “weak” associations with the mitochondrial proteins 3-hydroxyisobutyryl-coenzyme A hydrolase, mitochondrial ribosomal protein L3, and mitochondrial ribosomal protein L51 were detected (Table 2). Although these studies require further validation outside the scope of this report, the results support the notion of CSB localizing in a functionally significant way to the mitochondria.
TABLE 2.
Mitochondrial proteins that interact with CSB in a yeast 2-hybrid system
| Gene name | Description | Growth on SD-his | LacZ positive | Interaction strength |
|---|---|---|---|---|
| HIBCH (HIBYL-COA-H) | 3-hydroxyisobutyryl-coenzyme A hydrolase | + | ++ | Normal |
| MRPL3 (MRL3, RPML3) | Mitochondrial ribosomal protein L3 | + | ++ | Normal |
| MRPL51 (CDA09, MRP64, bMRP64, HSPC241) | Mitochondrial ribosomal protein L51 | + | + | Weak |
Alternate gene names are in parentheses. Initial screen was for interactors that enable histidine prototrophy. Positive clones were subsequently investigated for their ability to drive transcription of a second reporter, the LacZ gene, and are recorded here as + (weak) or ++ (normal) based on visual assessment of the PXG assay (DualSystems).
Decreased BER activities in mitochondrial extracts from CSB-deficient cells
Given the previous results indicating an involvement of CSB in nuclear BER, and in light of the observation that CSB localizes to the mitochondria (see above), the participation of CSB in mitochondrial BER was examined using isogenic CSB-deficient and CSB-proficient cell lines. The CSB-deficient cell line CS1AN was stably transfected with empty pcDNA3.1 vector or with pcDNA3.1 containing wt CSB. Mitochondria from the 2 cell lines were isolated, and whole mitochondrial extracts were prepared. The purity of these extracts was assessed using nuclear protein Lamin B as a marker for nuclear contamination and CoxIV for mitochondrial enrichment. Overexposure of the Western blot against Lamin B showed essentially no nuclear contamination, and CoxIV detection confirmed mitochondrial enrichment (Fig. 3). Incision assays were performed using double-stranded oligodeoxynucleotide substrates carrying a single 8-oxoG, uracil (U), or 5-hydroxy-uracil (5-OH-U) lesion (Table 1). Partially single-stranded “bubble” DNA substrates were also used, in which the 8-oxoG or 5-OH-U residue was located in an 11-nt bubble. Following separation of the substrate from the reaction products on a denaturating polyacrylamide gel, the amount of product relative to the total amount of oligodeoxynucleotide was quantified to determine incision efficiency. As previously reported (3), incision of 8-oxoG was lower in mitochondrial extracts from CSB-deficient cells than from wt cells (Fig. 4A, first set of bars). In addition, incision of uracil and 5-OH-U was lower in extracts from CSB-deficient cells than from wt cells (Fig. 4A). However, incision of 8-oxoG and 5-OH-U in bubble substrates was not decreased when comparing CSB-deficient to wt cells (Fig. 4A). This suggests that the effect of a CSB defect on incision capacity is substrate-specific, and not restricted to an involvement in 8-oxoG incision activity, which is the only mitochondrial repair activity previously found to be affected by CSB deficiency.
Apurinic endonuclease (APE) activity, likely to be the product of APE1 function (33, 34), was also measured in whole mitochondrial extracts using a tetrahydrofuran (THF)-containing DNA substrate. THF is an analog of an abasic (AP) site that can be cleaved by APE, but not by bifunctional glycosylases. The results show that APE activity is lower in mitochondrial extracts from CSB-deficient cells than from wt cells (Fig. 4A, last set of bars; B). Thus, CSB appears to be required for normal levels of glycosylase activity toward certain base lesions and AP site incision activity in mitochondrial extracts.
CSB deficiency alters the association of BER with the mitochondrial membrane
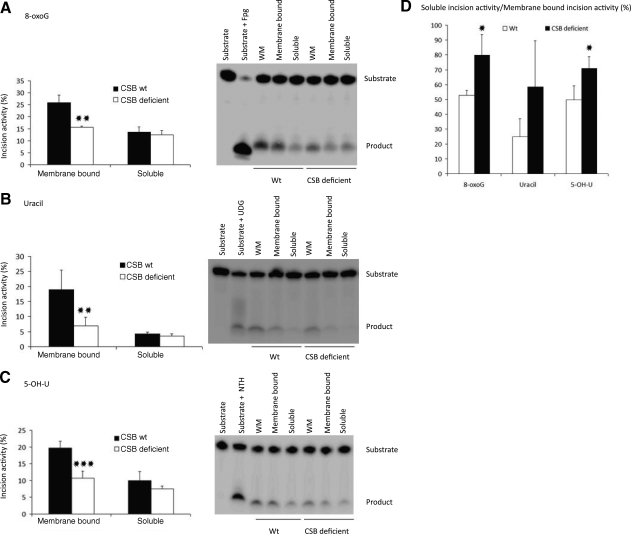
Mitochondrial BER activity is mainly associated with the inner membrane (26). We therefore investigated whether the presence of CSB affected the distribution of BER activities between the inner membrane-bound and soluble fractions, or if there was a general decrease in both. Whole mitochondria were fractionated into a membrane-bound and a soluble fraction by sonication and ultracentrifugation (ref. 26, described in Material and Methods and shown in Fig. 5A). Representative gels for the different incision assays using the fractionated mitochondria are shown in the right panels of Fig. 6A–C for 8-oxoG, uracil, and 5-OH-U, respectively. Incision activities were quantified and presented as percentage incision (Fig. 6A–C, left panels). In agreement with earlier findings (26), the majority of incision activity for 8-oxoG, uracil, and 5-OH-U was found in the membrane-bound fraction in mitochondria from wt cells (compare “CSB wt” bars for membrane-bound incision with bars for soluble incision activity in Fig. 6). Interestingly, the 8-oxoG incision activity in the membrane-bound fraction was ∼40% lower in CSB-deficient cells compared to wt cells, whereas no difference was seen for the incision activity in the soluble fraction (Fig. 6A, left panel). This pattern was also observed for incision activity toward uracil and 5-OH-U, with a ∼60% and ∼45% decrease in the membrane-bound incision activity, respectively, in CSB-deficient cells; no clear difference was observed for the soluble fraction (Fig. 6B, C). When comparing the ratio of soluble to membrane-bound incision activity for the 3 different lesions (Fig. 6D), we found that this ratio was 1.5-to-2.3-fold higher in CSB-deficient cells for 8-oxoG, uracil, and 5-OH-U incision relative to wt. This indicates that the decrease in repair capacity in whole mitochondrial extract from CSB-deficient cells (Fig. 4) is due mainly to a decrease in activity associated with the inner membrane. These results suggest that CSB may play a role in recruiting, stabilizing, and/or retaining BER enzymes at the mitochondrial inner membrane.
Figure 6.
Decreased association of incision capacity for 8-oxoG, uracil, and 5-OH-U with the inner mitochondrial membrane. Mitochondria from CS1AN stably transfected with empty vector (CSB deficient) or wild-type CSB (wt) were isolated and fractionated into a soluble and a membrane-bound fraction. A) Incision activity for 8-oxoG. Right panel: representative gel for incision activity in whole mitochondria (WM), membrane-bound fraction 1, and soluble fraction 1. Left panel: quantified incision activity in the membrane-bound fraction 1 and soluble fraction 1. B) Incision activity for uracil. Right panel: representative gel for incision. Left panel: quantified incision activity. C) Incision activity for 5-OH-U. Right panel: representative gel for incision activity. Left panel: quantified incision activity. D) Soluble fraction 1 incision activity relative to membrane-bound fraction 1 incision activity. Results are means ± sd for 3–4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. wt.
Decreased interaction of BER enzymes with the mitochondrial inner membrane in CSB-deficient cells
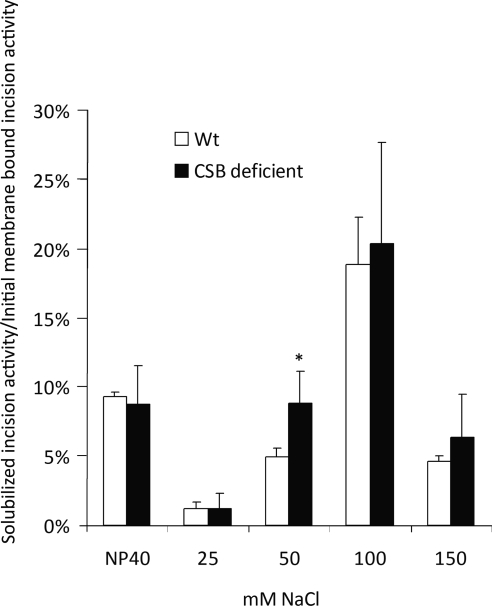
Stuart et al. (26) showed that the association of BER activities with the mitochondrial inner membrane was mostly of electrostatic nature and disrupted by increasing salt concentrations. To further investigate the role of CSB in mediating the link between BER glycosylase activities and the inner mitochondrial membrane, we examined the ionic strength dependence of the association of uracil incision activity to the inner membrane in CSB-deficient and wt cells. The membrane-bound fraction 1 used for the experiments shown in Fig. 6 was further subfractionated as outlined in Fig. 5B and as previously described (26). In short, the membrane-bound fraction 1 was incubated with Nonidet P-40 and refractionated by ultracentrifugation, resulting in a membrane-bound fraction 2 and a soluble fraction 2. The membrane-bound fraction 2 was next incubated with 25 mM NaCl and refractionated. This cycle was continued with the subsequent membrane-bound fractions 3, 4, and 5 being incubated with 50, 100, and 150 mM NaCl, respectively (Fig. 5B). The resulting soluble fractions 2–6 were used for uracil incision assays. Uracil incision activity was normalized to the incision activity of the membrane-bound fraction 1, the same fraction that was used for the experiments shown in Fig. 6. In agreement with what was reported by Stuart et al. (26) most of the uracil incision activity was released at salt concentrations below 150 mM NaCl for wt cells (Fig. 7). Notably, a significantly higher uracil incision activity was released by 50 mM salt from the inner membrane of CSB-deficient cells (Fig. 7). This finding indicates that the association of uracil incision activity to the inner mitochondrial membrane is weaker in CSB-deficient cells than the isogenic wt counterpart.
Figure 7.
Release of uracil incision activity by increasing salt concentration. Mitochondrial proteins were fractionated from membrane-bound fraction 1, as depicted in Fig. 5B. Uracil incision capacity in resulting soluble fractions was measured. Incision activity in soluble fraction relative to incision capacity of membrane-bound fraction 1 is presented. *P < 0.05 vs. wt.
CSB deficiency results in increased mitochondrial DNA mutation rate
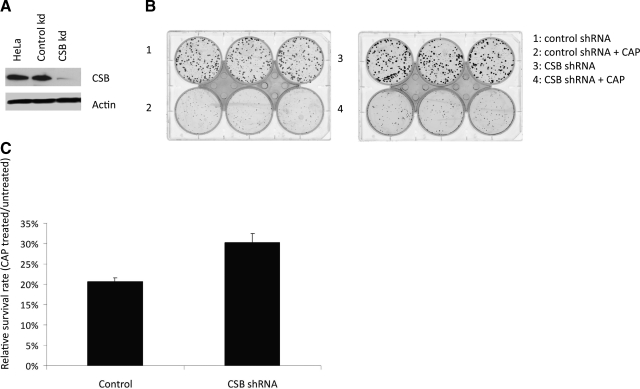
The results presented above indicated that mitochondria from CSB-deficient cells have decreased uracil, 5-OH-U, and 8-oxoG incision activity. Because these lesions are highly mutagenic (reviewed in ref. 35) we reasoned that CSB deficiency could result in a higher mtDNA mutation rate. We therefore measured the mtDNA mutation rate in HeLa cells with a stable CSB knockdown using shRNA compared to HeLa cells with control shRNA using a CAP resistance assay (31). Efficient CSB knockdown was verified by Western blot (Fig. 8A). CAP inhibits mitochondrial protein synthesis, and mutations in mitochondrial ribosomal RNA genes results in resistance to the antibiotic (reviewed in ref. 36). CAP resistance can therefore be used as a measurement for mtDNA mutation rate. CSB and control shRNA knockdown HeLa cells were seeded and grown in the presence or absence of CAP, and surviving colonies were quantified after methylene blue staining (Fig. 8B). The survival rate of CAP treated to CAP untreated was calculated for both CSB knockdown and control cells. We observed a ∼50% increase in survival rate for CSB knockdown cells compared to control cells (Fig. 8C). This observation indicates an increased mtDNA mutation rate in CSB-deficient cells compared to control cells, thereby showing biological relevance for CSB in mtDNA maintenance.
Figure 8.
Increased mitochondrial mutagenesis in CSB knockdown cells. A) Western blot of 50-μg cell extracts from HeLa cells and HeLa cells either stably transfected with control shRNA or CSB shRNA, as indicated, probed with CSB and actin antibody. CSB knockdown was calculated relative to loading control actin levels. B) Chloramphenicol (CAP) resistance assay. HeLa cells with a stable shRNA CSB knockdown and shRNA control cells were seeded and grown in presence or absence of CAP. C) Numbers of colonies formed with and without 200 μg/ml CAP treatment was quantified, and relative survival rate determined as colonies formed with CAP divided by the numbers of colonies formed without CAP. Results are presented as a representative experiment performed in triplicate.
DISCUSSION
CSB was found to colocalize with the mitochondrial nucleoid marker TFAM in HeLa cells and appears to translocate to mitochondria in response to menadione-induced oxidative stress, and the relocalization of CSB was seen, albeit to a lesser extent, in primary human fibroblasts. In addition, CSB was detected in mitochondrial extracts from HeLa cells by Western blotting, with increased amounts after menadione treatment. These data suggest that CSB plays a role in the cellular response to menadione, and that translocation of the protein to mitochondria may be an important feature of this response. Furthermore, human skeletal muscle mitochondria were also found to contain CSB, supporting the notion that CSB may be more generally present in mitochondria in various cell or tissue types.
Little is known about relocalization of DNA repair proteins to the mitochondria in response to mtDNA damage. One recent study showed that Saccharomyces cerevisiae DNA glycosylase Ntg 1 relocalizes to mitochondria in response to oxidative mtDNA damage (37); another study indicates that APE1 translocates to mitochondria in cells treated with hydrogen peroxide (38). Our current study demonstrates that localization of CSB to human cell mitochondria increases in response to menadione, which produces reactive oxygen species (ROS) within mitochondria, thereby oxidizing mtDNA. This is compatible with general dynamic relocalization of DNA repair proteins in response to genotoxic stress. A previous study from our group showed that CSB relocalizes within the nucleus in response to γ-irradiation (8) or H2O2, resulting in colocalization with sites of PARP activity (17). In addition, CSB may associate and colocalize with transcription elongation complexes in cells with DNA damage (39, 40). The observation that mouse embryonic fibroblasts from CSB-mutant mice are more sensitive to menadione compared to wild-type cells (27) also supports a role for CSB in response to menadione treatment.
Further support for a role of CSB in mitochondria was the identification, in yeast 2-hybrid screens of CSB interacting partners, of 3 mitochondrial proteins. The amino acid metabolism enzyme, 3-hydroxyisobutyryl-coenzyme A hydrolase (HIB-CoA), is very important for the valine catabolic pathway (41). There have been 2 reports of patients with HIB-CoA deficiency, which, among other clinical signs, resulted in ataxia and neurological degeneration (42) as seen with CS patients. Furthermore, another enzyme involved in the same valine catabolism pathway, the branched-chain alpha-keto acid dehydrogenase (BCKDH), is found to be a core part of the mitochondrial nucleoid in yeast, bacteria, Xenopus oocyte, and human cells (43, 44). Although it is not known whether HIB-CoA and BCKDH are associated in mitochondria, their involvement in the same pathway suggests a functional cooperation. It could be speculated that the interaction of CSB and HIB-CoA is somehow connected to the mitochondrial nucleoid structure. In addition, CSB was found to have a strong interaction with mitochondrial ribosomal protein L3 (MRPL3). About 50% of mitochondrial ribosomes are associated with the inner mitochondrial membrane and can be released by increasing salt concentration (45), much in the same way as the BER activity. In addition, the mitochondrial ribosome recycling factor mtRRF coimmunoprecipitated with ribosomal proteins, including MRPL3 and with nucleoid proteins (46), thus connecting ribosomal proteins to the mitochondrial nucleoids.
We have previously reported decreased mitochondrial 8-oxoG incision activity in CSB-deficient cells (3). Our results extend this observation by showing that 8-oxoG as well as uracil, 5-OH-U incision, and AP endonuclease activity are decreased in mitochondrial extracts from CSB-deficient cells compared to wt cells. Although a decrease in uracil incision was not observed by Stevnsner et al. (3), this difference could be the result of higher or saturated incision activity in the previous work. The observed lower mitochondrial AP endonuclease activity in CSB-deficient cells is the first demonstration of an effect of CSB activity on steps downstream of glycosylase activities in mitochondrial BER.
Our group reported earlier that a large part of mitochondrial BER activity is associated with the inner mitochondrial membrane (26), and here we show that this association is weakened in CSB-deficient cells. MtDNA is organized into large complexes called nucleoids (43), which associate with the inner mitochondrial membrane (47). Respiratory complex I and III reside in the inner mitochondrial membrane and leak 0.2–2% of the total electron flux directly to molecular oxygen resulting in the formation of ROS (reviewed in ref. 48). Thus, mtDNA is highly susceptible to ROS-induced DNA damage because of its association with the mitochondrial membrane. This susceptibility provides a rationale for association of BER with the inner mitochondrial membrane (26). This current study shows that CSB deficiency correlates with a specific decrease in BER activities associated with the mitochondrial inner membrane (Fig. 6D). We tried to complement mitochondrial extract from CSB-deficient cells with purified CSB, but did not detect restoration of activity (data not shown). This could indicate that CSB does not operate simply by interacting and stimulating specific glycosylases, but that the protein is part of an organizational complex connecting the BER process to the nucleoid. This notion may be supported by past studies of the role of CSB in the transcription complex, where its role has a structural component because a CSB lacking complex was much more easily disrupted by detergent than the wt complex (39). Because few of the oxidative lesions found in DNA appear to stall RNA polymerase II, and because these lesion are repaired without strand bias (25), we consider the role of CSB in BER is unrelated to its role in TCR of NER.
The interaction of CSB with mitochondrial proteins associated with the nucleoid could point to a mechanism for the association of complexes with the inner membrane, as we observed for the mitochondrial BER enzymes. A recent study supports such a more general role for CSB in complex organization in the inner mitochondrial membrane (27). They found the association of complex I, III, and IV (supercomplex) to be decreased in mouse embryonic cells from CSB mutated mice compared to wt mice. They also found the recovery after transient ATP depletion to be slower in the CSB mutated cells compared to wt cells, supporting that lack of CSB in mitochondria could play an important role in the CS phenotype.
In summary, this study reports evidence for the presence of CSB protein in the mitochondria of human cells, by immunocytochemistry, by Western blotting of mitochondrial extracts from mock or menadione-treated HeLa cells, and by Western blotting of mitochondria from human skeletal muscle biopsies. These data suggest that CSB could modulate mitochondrial BER through a direct presence in the mitochondria. Moreover, the results presented herein are consistent with a role of CSB in organizing mitochondrial BER activity to the inner mitochondrial membrane, and possibly other mitochondrial complexes. The increased mtDNA mutation rate in CSB knockdown cells compared to control cells provides an important biological link between the observed decrease in mitochondrial BER activity in CSB-deficient cells and the segmental premature aging phenotype of CS patients, because a link between mtDNA mutation, mitochondrial dysfunction, and premature aging has been proposed (49). A function for CSB in mitochondria could explain the reduced mitochondrial BER activity in CSB-deficient cells as well as the similarity in clinical symptoms of patients with CS or a mitochondrial defect.
Acknowledgments
Alfred May and Ulla Henriksen provided excellent technical assistance. The authors thank John Vissing (Department of Neurology, Rigshospitalet, Copenhagen, Denmark) and co-workers for providing human muscle mitochondria. This research was supported by the Intramural Research Program of the National Institute of Health, National Institute on Aging, the Danish Research Council (grant 271-08-0697), the Velux Foundation (grant 24715), the European Commission (LSHM-CT-2004-512020), the Danish Cancer Society (DP05118), and the Lundbeck Foundation (4-55951-95094019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Balajee A S, Proietti De Santis L, Brosh R M, Jr, Selzer R, Bohr V A. Role of the ATPase domain of the Cockayne syndrome group B protein in UV induced apoptosis. Oncogene. 2000;19:477–489. doi: 10.1038/sj.onc.1203372. [DOI] [PubMed] [Google Scholar]
- Lehmann A R. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie (Paris) 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Stevnsner T, Nyaga S, de Souza-Pinto N C, van der Horst G T, Gorgels T G, Hogue B A, Thorslund T, Bohr V A. Mitochondrial repair of 8-oxoguanine is deficient in Cockayne syndrome group B. Oncogene. 2002;21:8675–8682. doi: 10.1038/sj.onc.1205994. [DOI] [PubMed] [Google Scholar]
- Wong H K, Muftuoglu M, Beck G, Imam S Z, Bohr V A, Wilson D M., 3rd Cockayne syndrome B protein stimulates apurinic endonuclease 1 activity and protects against agents that introduce base excision repair intermediates. Nucleic Acids Res. 2007;35:4103–4113. doi: 10.1093/nar/gkm404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianov G, Bischoff C, Sunesen M, Bohr V A. Repair of 8-oxoguanine in DNA is deficient in Cockayne syndrome group B cells. Nucleic Acids Res. 1999;27:1365–1368. doi: 10.1093/nar/27.5.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunesen M, Stevnsner T, Brosh R M, Jr, Dianov G L, Bohr V A. Global genome repair of 8-oxoG in hamster cells requires a functional CSB gene product. Oncogene. 2002;21:3571–3578. doi: 10.1038/sj.onc.1205443. [DOI] [PubMed] [Google Scholar]
- Tuo J, Chen C, Zeng X, Christiansen M, Bohr V A. Functional crosstalk between hOgg1 and the helicase domain of Cockayne syndrome group B protein. DNA Repair. 2002;1:913–927. doi: 10.1016/s1568-7864(02)00116-7. [DOI] [PubMed] [Google Scholar]
- Tuo J, Jaruga P, Rodriguez H, Dizdaroglu M, Bohr V A. The Cockayne syndrome group B gene product is involved in cellular repair of 8-hydroxyadenine in DNA. J Biol Chem. 2002;277:30832–30837. doi: 10.1074/jbc.M204814200. [DOI] [PubMed] [Google Scholar]
- Muftuoglu M, Souza-Pinto N C, Dogan A, Aamann M, Stevnsner T, Rybanska I, Kirkali G, Dizdaroglu M, Bohr V A. Cockayne syndrome group B protein stimulates repair of formamidopyrimidines by NEIL1 DNA glycosylase. J Biol Chem. 2009;284:9270–9279. doi: 10.1074/jbc.M807006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp C, Reite K, Klungland A, Epe B. Deficiency of the Cockayne syndrome B (CSB) gene aggravates the genomic instability caused by endogenous oxidative DNA base damage in mice. Oncogene. 2007;26:4044–4048. doi: 10.1038/sj.onc.1210167. [DOI] [PubMed] [Google Scholar]
- Osterod M, Larsen E, Le Page F, Hengstler J G, Van Der Horst G T, Boiteux S, Klungland A, Epe B. A global DNA repair mechanism involving the Cockayne syndrome B (CSB) gene product can prevent the in vivo accumulation of endogenous oxidative DNA base damage. Oncogene. 2002;21:8232–8239. doi: 10.1038/sj.onc.1206027. [DOI] [PubMed] [Google Scholar]
- Venema J, Mullenders L H, Natarajan A T, van Zeeland A A, Mayne L V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A. 1990;87:4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muftuoglu M, Selzer R, Tuo J, Brosh R M, Jr, Bohr V A. Phenotypic consequences of mutations in the conserved motifs of the putative helicase domain of the human Cockayne syndrome group B gene. Gene. 2002;283:27–40. doi: 10.1016/s0378-1119(01)00870-8. [DOI] [PubMed] [Google Scholar]
- Tuo J, Jaruga P, Rodriguez H, Bohr V A, Dizdaroglu M. Primary fibroblasts of Cockayne syndrome patients are defective in cellular repair of 8-hydroxyguanine and 8-hydroxyadenine resulting from oxidative stress. FASEB J. 2003;17:668–674. doi: 10.1096/fj.02-0851com. [DOI] [PubMed] [Google Scholar]
- Hu J, de Souza-Pinto N C, Haraguchi K, Hogue B A, Jaruga P, Greenberg M M, Dizdaroglu M, Bohr V A. Repair of formamidopyrimidines in DNA involves different glycosylases: role of the OGG1, NTH1, and NEIL1 enzymes. J Biol Chem. 2005;280:40544–40551. doi: 10.1074/jbc.M508772200. [DOI] [PubMed] [Google Scholar]
- Flohr C, Burkle A, Radicella J P, Epe B. Poly(ADP-ribosyl)ation accelerates DNA repair in a pathway dependent on Cockayne syndrome B protein. Nucleic Acids Res. 2003;31:5332–5337. doi: 10.1093/nar/gkg715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorslund T, von Kobbe C, Harrigan J A, Indig F E, Christiansen M, Stevnsner T, Bohr V A. Cooperation of the Cockayne syndrome group B protein and poly(ADP-ribose) polymerase 1 in the response to oxidative stress. Mol Cell Biol. 2005;25:7625–7636. doi: 10.1128/MCB.25.17.7625-7636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H K, Kim D, Hogue B A, McNeill D R, Wilson D M., 3rd DNA damage levels and biochemical repair capacities associated with XRCC1 deficiency. Biochemistry. 2005;44:14335–14343. doi: 10.1021/bi051161o. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Schon E A. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Licht C L, Stevnsner T, Bohr V A. Cockayne syndrome group B cellular and biochemical functions. Am J Hum Genet. 2003;73:1217–1239. doi: 10.1086/380399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance M A, Berry S A. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992;42:68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- DiMauro S. Mitochondrial myopathies. Curr Opin Rheumatol. 2006;18:636–641. doi: 10.1097/01.bor.0000245729.17759.f2. [DOI] [PubMed] [Google Scholar]
- De Souza-Pinto N C, Wilson D M, 3rd, Stevnsner T V, Bohr V A. Mitochondrial DNA, base excision repair and neurodegeneration. DNA Repair (Amst) 2008;7:1098–1109. doi: 10.1016/j.dnarep.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard S, Schurman S H, Harboe C, de Souza-Pinto N C, Bohr V A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorslund T, Sunesen M, Bohr V A, Stevnsner T. Repair of 8-oxoG is slower in endogenous nuclear genes than in mitochondrial DNA and is without strand bias. DNA Repair. 2002;1:261–273. doi: 10.1016/s1568-7864(02)00003-4. [DOI] [PubMed] [Google Scholar]
- Stuart J A, Mayard S, Hashiguchi K, Souza-Pinto N C, Bohr V A. Localization of mitochondrial DNA base excision repair to an inner membrane-associated particulate fraction. Nucleic Acids Res. 2005;33:3722–3732. doi: 10.1093/nar/gki683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osenbroch P O, Auk-Emblem P, Halsne R, Strand J, Forstrom R J, van der Pluijm I, Eide L. Accumulation of mitochondrial DNA damage and bioenergetic dysfunction in CSB defective cells. FEBS J. 2009;276:2811–2821. doi: 10.1111/j.1742-4658.2009.07004.x. [DOI] [PubMed] [Google Scholar]
- Mayne L V, Priestley A, James M R, Burke J F. Efficient immortalization and morphological transformation of human fibroblasts by transfection with SV40 DNA linked to a dominant marker. Exp Cell Res. 1986;162:530–538. doi: 10.1016/0014-4827(86)90356-3. [DOI] [PubMed] [Google Scholar]
- Selzer R R, Nyaga S, Tuo J, May A, Muftuoglu M, Christiansen M, Citterio E, Brosh R M, Jr, Bohr V A. Differential requirement for the ATPase domain of the Cockayne syndrome group B gene in the processing of UV-induced DNA damage and 8-oxoguanine lesions in human cells. Nucleic Acids Res. 2002;30:782–793. doi: 10.1093/nar/30.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Quistorff B. Regulation of mitochondrial respiration by inorganic phosphate; comparing permeabilized muscle fibers and isolated mitochondria prepared from type-1 and type-2 rat skeletal muscle. Eur J Appl Physiol. 2009;105:279–287. doi: 10.1007/s00421-008-0901-9. [DOI] [PubMed] [Google Scholar]
- De Souza-Pinto N C, Mason P A, Hashiguchi K, Weissman L, Tian J, Guay D, Lebel M, Stevnsner T V, Rasmussen L J, Bohr V A. Novel DNA mismatch-repair activity involving YB-1 in human mitochondria. DNA Repair. 2009;8:704–719. doi: 10.1016/j.dnarep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido N, Griparic L, Jokitalo E, Wartiovaara J, van der Bliek A M, Spelbrink J N. Composition and dynamics of human mitochondrial nucleoids. Mol Biol Cell. 2003;14:1583–1596. doi: 10.1091/mbc.E02-07-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari M, Otterlei M, Pena-Diaz J, Krokan H E. Different organization of base excision repair of uracil in DNA in nuclei and mitochondria and selective upregulation of mitochondrial uracil-DNA glycosylase after oxidative stress. Neuroscience. 2007;145:1201–1212. doi: 10.1016/j.neuroscience.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Mitra S, Izumi T, Boldogh I, Bhakat K K, Chattopadhyay R, Szczesny B. Intracellular trafficking and regulation of mammalian AP-endonuclease 1 (APE1), an essential DNA repair protein. DNA Repair. 2007;6:461–469. doi: 10.1016/j.dnarep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Wilson D M, 3rd, Bohr V A. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair. 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Wallace D C, Bunn C L, Eisenstadt J M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975;67:174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths L M, Swartzlander D, Meadows K L, Wilkinson K D, Corbett A H, Doetsch P W. Dynamic compartmentalization of base excision repair proteins in response to nuclear and mitochondrial oxidative stress. Mol Cell Biol. 2008;29:794–807. doi: 10.1128/MCB.01357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossi B, Tell G, Spessotto P, Colombatti A, Vitale G, Pucillo C. H(2)O(2) induces translocation of APE/Ref-1 to mitochondria in the Raji B-cell line. J Cell Physiol. 2002;193:180–186. doi: 10.1002/jcp.10159. [DOI] [PubMed] [Google Scholar]
- Balajee A S, May A, Dianov G L, Friedberg E C, Bohr V A. Reduced RNA polymerase II transcription in intact and permeabilized Cockayne syndrome group B cells. Proc Natl Acad Sci U S A. 1997;94:4306–4311. doi: 10.1073/pnas.94.9.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Boom V, Citterio E, Hoogstraten D, Zotter A, Egly J M, van Cappellen W A, Hoeijmakers J H, Houtsmuller A B, Vermeulen W. DNA damage stabilizes interaction of CSB with the transcription elongation machinery. J Cell Biol. 2004;166:27–36. doi: 10.1083/jcb.200401056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Honda T, Goto H, Nonami T, Kurokawa T, Nagasaki M, Murakami T. Effects of liver failure on the enzymes in the branched-chain amino acid catabolic pathway. Biochem Biophys Res Commun. 2004;313:381–385. doi: 10.1016/j.bbrc.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Loupatty F J, Clayton P T, Ruiter J P, Ofman R, Ijlst L, Brown G K, Thorburn D R, Harris R A, Duran M, Desousa C, Krywawych S, Heales S J R, Wanders R J A. Mutations in the gene encoding 3-hydroxyisobutyryl-CoA hydrolase results in progressive infantile neurodegeneration. Am J Hum Genet. 2007;80:195–199. doi: 10.1086/510725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenhagen D F, Rousseau D, Burke S. The layered structure of human mitochondrial DNA nucleoids. J Biol Chem. 2008;283:3665–3675. doi: 10.1074/jbc.M708444200. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D F, Wang Y, Shen E L, Kobayashi R. Protein components of mitochondrial DNA nucleoids in higher eukaryotes. Mol Cell Proteomics. 2003;2:1205–1216. doi: 10.1074/mcp.M300035-MCP200. [DOI] [PubMed] [Google Scholar]
- Liu M, Spremulli L. Interaction of mammalian mitochondrial ribosomes with the inner membrane. J Biol Chem. 2000;275:29400–29406. doi: 10.1074/jbc.M002173200. [DOI] [PubMed] [Google Scholar]
- Rorbach J, Richter R, Wessels H J, Wydro M, Pekalski M, Farhoud M, Kuhl I, Gaisne M, Bonnefoy N, Smeitink J A, Lightowlers R N, Chrzanowska-Lightowlers Z M A. The human mitochondrial ribosome recycling factor is essential for cell viability. Nucleic Acids Res. 2008;36:5787–5799. doi: 10.1093/nar/gkn576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albring M, Griffith J, Attardi G. Association of a protein structure of probable membrane derivation with HeLa cell mitochondrial DNA near its origin of replication. Proc Natl Acad Sci U S A. 1977;74:1348–1352. doi: 10.1073/pnas.74.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaltowski A J, de Souza-Pinto N C, Castilho R F, Vercesi A E. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink J N, Rovio A T, Bruder C E, Bohlooly Y M, Gidlof S, Oldfors A, Wibom R, Törnell J, Jacobs H T, Larsson N G. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]