Abstract
A major source of “high-output” NO in inflammation is inducible nitric oxide synthase (iNOS). iNOS is primarily transcriptionally regulated and is thought to function as an uncontrolled generator of high NO. We found that iNOS in cytokine-stimulated human lung microvascular endothelial cells (HLMVECs) is highly regulated post-translationally via activation of the B1 kinin G protein-coupled receptor (B1R). We report here that B1R-mediated iNOS activation was significantly inhibited by knockdown of β-arrestin 2 with siRNA in cytokine-treated HLMVECs or HEK293 cells transfected with iNOS and B1R. In contrast, β-arrestin 1 siRNA had no effect. The prolonged phase of B1R-dependent ERK activation was also inhibited by β-arrestin 2 knockdown. Furthermore, robust ERK activation by the epidermal growth factor receptor (a β-arrestin 2 independent pathway) had no effect on iNOS-derived NO production. β-arrestin 2 and iNOS coimmunoprecipitated, and there was significant fluorescence resonance energy transfer between CFP-iNOS and β-arrestin 2-YFP (but not β-arrestin 1-YFP) that increased 3-fold after B1R stimulation. These data show that β-arrestin 2 mediates B1R-dependent high-output NO by scaffolding iNOS and ERK to allow post-translational activation of iNOS. This could play a critical role in mediating endothelial function in inflammation.—Kuhr, F. K., Zhang, Y., Brovkovych, V., Skidgel, R. A. β-Arrestin 2 is required for B1 receptor-dependent post-translational activation of inducible nitric oxide synthase.
Keywords: kinins, G-protein-coupled receptor, extracellular signal-regulated kinase, inflammation, endothelial cells
NO is a pleiotropic molecule generated by 3 isoforms of NO synthase: neuronal (nNOS or NOS I), inducible (iNOS or NOS II), and endothelial (eNOS or NOS III). The physiological effects of NO in the vascular system depend on its location and concentration. The constitutively expressed eNOS is highly regulated by receptor activation and increased intracellular Ca2+, generating NO that is primarily utilized as a signaling molecule to regulate vascular tone and endothelial barrier function (1,2,3). eNOS activity can also be positively or negatively regulated by direct interaction with proteins such as caveolin-1 or hsp90 (4). In addition, G-protein-coupled receptors (GPCRs), such as the kinin B2, endothelin-1 ETB, and angiotensin AT1 receptors, were reported to negatively regulate eNOS activity through a basal interaction of their fourth intracellular domain with eNOS (5,6,7).
Under inflammatory conditions, the major source of “high-output” NO in the vascular system is iNOS (4, 8,9,10). The prevailing view is that iNOS is transcriptionally regulated, functioning at the protein level as a constitutively active and uncontrolled generator of high NO (4, 11, 12). In support of this view, iNOS, unlike eNOS or nNOS, contains tightly bound Ca2+/calmodulin that does not dissociate in resting cells, thus rendering it constitutively active once synthesized (4, 13). However, we found that the generation of high-output, iNOS-derived NO in cytokine-stimulated human lung microvascular endothelial cells (HLMVECs) is also regulated post-translationally via activation of the G-protein-coupled B1 kinin receptor (B1R) (9, 10); that is, the control of iNOS activity is more subtle than previously appreciated. Stimulation of the B1R results in activation of ERK which, in turn, phosphorylates iNOS at Ser745, leading to a 3- to 5-fold further increase in NO production beyond its basal activity (10, 14).
β-Arrestins, originally discovered for their role in terminating GPCR signaling by facilitating desensitization and internalization, are now appreciated for their additional functions as GPCR effectors, e.g., by scaffolding members of the MAPK cascade, enhancing and prolonging MAPK signaling (15, 16). Recently, eNOS was found to indirectly down-regulate GPCR function via interactions with β-arrestin 2. β-Arrestin 2 and eNOS were basally associated in transfected HEK cells and, after β-adrenergic receptor stimulation, activated eNOS generated NO, resulting in S-nitrosylation of β-arrestin 2 (17). This modification resulted in dissociation of β-arrestin 2 from eNOS and promoted its association with clathrin heavy chain and β-adaptin, accelerating internalization of the β-adrenergic receptor (17). However, whether other NOS isoforms interact with β-arrestin 2 is unknown. We report here that B1R-mediated post-translational iNOS activation via ERK is critically dependent on β-arrestin 2. β-Arrestin 2 mediates both the prolonged phase of B1R-dependent ERK activation and importantly interacts with iNOS to facilitate its ERK-mediated stimulation, resulting in iNOS-derived high-output NO.
MATERIALS AND METHODS
Materials
Human iNOS cDNA cloned into pcDNA3 was a gift from Dr. Timothy Billiar (University of Pittsburgh, Pittsburgh, PA, USA). iNOS cDNA was further subcloned into pECFP-C1 (Clontech Laboratories, Palo Alto, CA, USA) in frame between restriction sites 5′-BglII and 3′-SalI to create N-terminally tagged CFP-iNOS. Human B1R cDNA was a gift from Dr. Fredrik Leeb-Lundberg (University of Lund, Lund, Sweden). β-Arrestin 2 was cloned from human heart RNA (BD Laboratories, San Jose, CA, USA), using primers that contain the NheI and BamH I restriction sites in a PCR amplification reaction. PCR primers were designed based on β-arrestin 2 mRNA from GenBank (accession no. 39812034). The β-arrestin 2 PCR fragment was subcloned into the pcDNA6-A vector containing C-terminal-tagged V5-His between the 5′-NheI and 3′-BamH I restriction sites. β-Arrestin 2-YFP was made by inserting β-arrestin 2 cDNA in frame into pEYFP-N1 vector (C-terminal YFP tag) without the stop codon between restriction sites 5′-EcoR I and 3′-BamH I. All constructs were verified by sequence analysis. Des-Arg10-kallidin (DAKD), DMEM, phenol red-free DMEM/F-12, and protein A-agarose were from Sigma (St. Louis, MO, USA). Epidermal growth factor receptor (EGFR) kinase inhibitor PD153035 was from Upstate (Lake Placid, NY, USA). Anti-human iNOS antibody (H174; polyclonal; SC-8310) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA), β-arrestin antibody (polyclonal PAN arrestin ab2914)was from Abcam (Cambridge, MA, USA), anti-V5 monoclonal antibody (R960-25) was from Invitrogen (Carlsbad, CA, USA), GAPDH and β-actin antibodies (AM4300 and AM4302, respectively) were from Ambion (Austin, TX, USA) and antibody to phospho-p44/42 MAP kinase (ERK; 9101S) was from Cell Signaling (Beverly, MA, USA).
Cell culture and transfection
Human embryonic kidney (HEK293) cells were maintained in DMEM with 10% FBS, 100 μg/ml penicillin and 100 U/ml streptomycin. Human lung microvascular endothelial cells (HLMVECs; Cambrex, East Rutherford, NJ, USA), used from passages 5 to 7, were cultured on dishes coated with 0.1% gelatin in basal medium with growth factors and antibiotics from EGM-2 Bullet kits (Cambrex) and 10% FBS. HLMVECs were treated with 5 ng/ml IL-1β and 100 U/ml IFN-γ for 16–24 h to induce B1R and iNOS expression (10, 18). HEK293 cells were transiently transfected using Effectene (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. HEK293 cells stably expressing B1Rs (HEK-B1R cells) were established as previously reported (10).
Coimmunoprecipitation assays
HEK-B1R cells were transfected with both iNOS and β-arrestin 2-V5 cDNA for 36–48 h. Cells were then treated with B1R agonist 100 nM DAKD for the indicated times and collected, and after washing 2 times in PBS with 1 mM Na3VO4, cells were lysed in lysis buffer: 50 mM Tris/HCl (pH 7.5), 137 mM NaCl, 10% glycerol, 1 mM Na3VO4, 50 mM NaF, 20 mM β-glycerol phosphate, 1 mM phenylmethylsulfonyl fluoride, 1% protease inhibitor cocktail (Sigma p8340), 1% Triton X-100, 0.1% SDS, and 0.5% sodium deoxycholate. After lysis, cells were centrifuged at 14,000 g for 15 min. iNOS was precipitated with rabbit anti-NOS2 (H174) and pulled down with protein A beads. Samples were resolved on 10% SDS-PAGE gels, and β-arrestin 2 was detected with anti-V5 monoclonal antibody.
Fluorescence microscopy and fluorescence resonance energy transfer (FRET) analysis
Fluorescence imaging and FRET were performed using an LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany), as described previously (19, 20). HEK-B1R cells were transfected with CFP-iNOS and β-arrestin 1-YFP or β-arrestin 2-YFP on polylysine-coated glass coverslips. Thirty-six to 48 h post-transfection, cells were stimulated with B1R agonist and fixed with 4% paraformaldehyde. For fluorescence imaging, CFP-iNOS and β-arrestin 2-YFP were expressed separately to generate a calibrated spectrum for each emission profile, using an excitation wavelength of 458 nm. For FRET, cells were scanned in λ mode and visualized at 458-nm excitation. Selective photobleaching of YFP was performed by repeatedly scanning the region of interest (ROI) using 100 iterations set at 514-nm wavelength with maximum intensity to photobleach ≥85% of the original acceptor fluorescence. FRET efficiency in the selected bleach area was determined using the average pixel intensity of the CFP signal from the unmixed pre- and postbleach images using Zeiss software. Relative FRET efficiency was calculated as (CFP postbleach − CFP prebleach)/CFP postbleach. As a control, CFP-iNOS was cotransfected with YFP alone. Any increase in donor emission of the control after acceptor photobleaching was subtracted from original FRET efficiency for each time point.
RNA interference
siRNA duplexes (Sigma) with sequences specifically targeting β-arrestin 1 and β-arrestin 2 RNA were 5′-AAAGCCUUCUGCGCGGAGAAU-3′ and 5′AAGGACCGCAAAGUGUUUGUG-3′, respectively, as reported previously (21, 22). These sequences have been extensively validated with regard to specificity for β-arrestin 1 and 2 knockdown, effects on signaling and ERK phosphorylation mediated by angiotensin AT1 and β2-adrenergic receptors, and by similarity of results with siRNA to those obtained in mouse embryo fibroblasts from β-arrestin 1- and 2-knockout mice (21, 22). A scrambled RNA duplex (sc-37007; Santa Cruz Biotechnology) was used as a control. A day before siRNA treatment, cells were seeded in 12-well plates at 150,000 cells/well. siRNA duplexes at various concentrations were then transfected into HEK cells using DharmaFECT DUO (Dharmacon, Lafayette, CO, USA) for 72–96 h before experiments. Transfection of HLMVECs was achieved using an Amaxa Nucleofector (Lonza, Allendale, NJ, USA) and the manufacturer’s buffer kit and protocol optimized for HLMVECs. Knockdown of expression of the target was determined by Western blotting.
Real-time measurement of NO
NO was measured directly in real time with a porphyrinic microsensor, as described previously (18, 23). Cell culture medium was changed to phenol red-free DMEM/F12 + 0.5% FBS for 1–3 h before measurement. The sensor was positioned with a micromanipulator close to the cell culture surface (20±1 μm). To initiate B1R-dependent iNOS activation and NO production, 100 nM DAKD was added, and the current was recorded continuously. Current generated was proportional to the NO released, and a computer-based Gamry VP600 potentiostat (Gamry Instruments, Warminster, PA, USA) was used to monitor NO concentration over time. Each electrode was calibrated with an NO standard. The concentration of NO achieved 20 min after the addition of agonist was used to quantitate the results.
ERK activation assays
HEK-B1R cells transfected with scrambled or β-arrestin siRNA (see above) were stimulated with B1R agonist 100 nM DAKD for various times. To determine ERK activation mediated by EGF, cells were stimulated with 100 ng/ml EGF, with or without preincubation (30 min) with 100 nM EGFR kinase inhibitor PD153035. Cells were then washed with ice-cold PBS containing 1 mM Na3VO4 and then disrupted with lysis buffer (see above) and spun at 14,000 g for 15 min. Supernatant was removed, protein content was determined by Bradford protein assay, and samples containing equal amounts of protein were probed for active ERK by Western blotting (see below). Equal loading was assessed by probing for GAPDH.
Western blot analysis
Cell lysates were separated on 10% SDS-PAGE gels and electrophoretically transferred to Immobilon PVDF membranes (Millipore). Blots were blocked with 5% evaporated milk in PBS containing 0.1% Tween 20 and then incubated with the appropriate primary antibody. After washing and secondary antibody incubation, membranes were developed with Pierce SuperSignal Extended Duration Substrate (Pierce, Rockford, IL, USA) to detect the protein of interest.
RESULTS
Role of β-arrestins in the B1R-dependent activation of iNOS
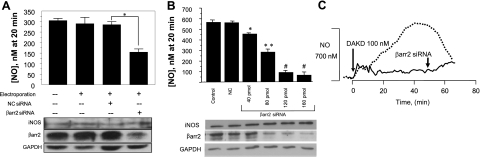
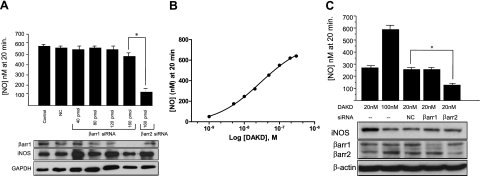
To understand the unique characteristics of the post-translational activation of iNOS by ERK phosphorylation at Ser745 (10), we investigated the role of β-arrestins. HLMVECs were transfected with siRNA specific for β-arrestin 2 and, after cytokine treatment to induce expression of the B1R and iNOS (10, 18, 24), cells were stimulated with B1R agonist (100 nM DAKD), and NO was measured in real time. Depletion of β-arrestin 2 by siRNA in HLMVECs resulted in a decrease in the iNOS-derived NO by ∼50%, compared with controls (Fig. 1A). To confirm the role of β-arrestin 2 in this pathway, we repeated these experiments in HEK293 cells stably expressing B1Rs (HEK-B1R cells) and coexpressing iNOS, a model system that we showed reproduces the B1R signaling pathways in HLMVECs (10, 14). In HEK-B1R cells coexpressing iNOS, increasing concentrations of siRNA for β-arrestin 2 resulted in a dose-dependent decrease in NO production, with an 87% reduction at the highest concentration (Fig. 1B). Figure 1C shows the effect of β-arrestin 2 knockdown on the real-time output of iNOS-derived NO over the whole 65-min time course of the response. Western blot analysis confirmed that the decrease in β-arrestin 2 expression correlated with the increasing concentration of siRNA and decreased NO production (Fig. 1B). In these experiments, β-arrestin 1 expression was not affected (not shown). To determine whether β-arrestin 1 is also involved in this signaling pathway, a similar experiment was conducted using siRNA against β-arrestin 1. As shown in Fig. 2, β-arrestin 1 siRNA had no effect on NO production in response to B1R stimulation, despite a significant knockdown of its expression, whereas β-arrestin 2 siRNA greatly inhibited the response as above.
Figure 1.
B1R-mediated NO production is dependent on β-arrestin 2. A) HLMVECs were transfected by electroporation (Amaxa Nucleofector) where indicated with vehicle, 160 pmol control siRNA, or 160 pmol β-arrestin 2 (βarr2) specific siRNA. At 50 h postelectroporation, cells were treated with 5 ng/ml IL-1β and 100 U/ml IFN-γ (22 h) to up-regulate B1R and iNOS expression. Cells were then stimulated with 100 nM DAKD, and NO was measured in real time with a porphyrinic microsensor. Cells were then lysed and subjected to Western blot analysis to detect iNOS and β-arrestin 2 expression. Data are expressed as means ± se of NO concentration achieved at 20 min. (n=3). *P < 0.05. B) HEK-B1R cells were transiently transfected with iNOS alone (control) or iNOS and control siRNA (NC) or increasing amounts of siRNA specific for β-arrestin 2. After transfection (48–72 h), cells were serum starved overnight. Cells were then treated with 100 nM DAKD, and NO was measured in real time with a porphyrinic microsensor. Data are expressed as means ± se of NO concentration achieved at 20 min (n=3). *P < 0.05, **P<0.01 vs. control; #P < 0.001 vs. control. C) HEK-B1R cells were transiently transfected with iNOS alone or with iNOS and β-arrestin 2 siRNA (75 pmol for 72 h). Cells were stimulated with 100 nM DAKD, and NO production was measured with porphyrinic microsensor for 65 min. Vertical bar on left axis represents electrode response to 700 nM NO standard.
Figure 2.
B1R and iNOS-dependent NO production is not affected by β-arrestin1 knockdown. A) HEK-B1R cells were transiently transfected with iNOS and siRNA specific to β-arrestin 1 (βarr1) or β-arrestin 2 (βarr2) as above (Fig. 1), and NO production stimulated with 100 nM DAKD was measured in real time with a porphyrinic microsensor. Data are expressed as means ± se of NO concentration achieved at 20 min. (n=3). *P < 0.001. B) HEK-B1R cells were transiently transfected with iNOS and then stimulated with increasing concentrations of B1R agonist DAKD, and the NO concentration achieved at 20 min was measured. C) HEK-B1R cells were transiently transfected with iNOS and 75 pmol of siRNA for β-arrestin 1 (βarr1), β-arrestin2 (βarr2), or nonspecific control (NC). Cells were then stimulated with 20 or 100 nM DAKD, and the NO concentration achieved at 20 min was measured. Samples were subjected to Western blot analysis and probed for iNOS, β-arrestin 1, β-arrestin 2, and β-actin. Data are expressed as means ± se (n=3). *P < 0.05.
Previous studies have shown that β-arrestins can, in some cases, have reciprocal effects on GPCR-mediated activation of the MAPK pathway, where one inhibits and the other activates (22, 25). Because 100 nM DAKD produces a near-maximal NO response, if β-arrestin 1 normally inhibits the response, its knockdown might not result in a further increase in NO production under these conditions. To address this issue, we measured the dose-response relationship between NO production and DAKD concentration and found that 20 nM DAKD elicited a submaximal NO response, ∼50% of that achieved with 100 nM DAKD (Fig. 2B). HEK-B1R cells were transiently transfected with iNOS and siRNA for β-arrestin 1 or β-arrestin 2 and then activated with 20 nM DAKD, and NO was measured in real time. When stimulated with 20 nM DAKD, cells transfected with control siRNA or β-arrestin 1 siRNA produced NO at the same level as in control cells, whereas β-arrestin 2 siRNA significantly reduced B1R-dependent NO output (Fig. 2C). These data show that β-arrestin 1 is not involved in augmenting or decreasing B1R-mediated NO production.
ERK activation is not sufficient for stimulation of iNOS-mediated NO production
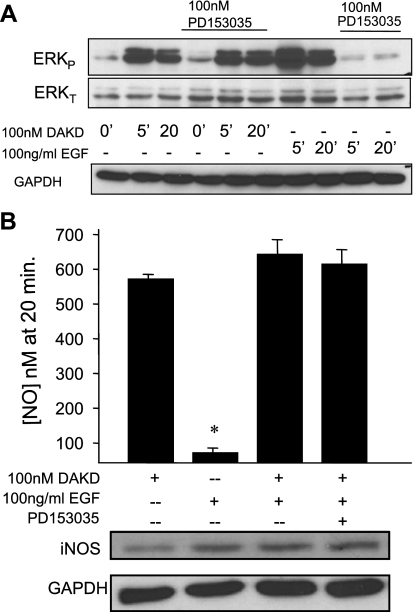
To determine whether activation of ERK by another pathway can also activate iNOS-dependent NO production, HEK cells stably expressing B1Rs were treated with 100 ng/ml EGF for 5 or 20 min and probed for phospho-ERK. As shown in Fig. 3A, EGF stimulated a robust activation of ERK at both time points, which was blocked by the specific EGFR tyrosine kinase inhibitor PD153035. As a control, the B1R agonist, 100 nM DAKD, also stimulated substantial ERK phosphorylation at the same time points (Fig. 3A), as we showed previously (10). Although the B2 kinin receptor can activate ERK via transactivation of the EGFR (26), the B1R has not been reported to have this capability. Pretreatment with the EGFR kinase inhibitor PD153035 did not diminish B1R-dependent ERK activation in response to DAKD (Fig. 3A), indicating that transactivation of the EGFR was not involved. To determine whether ERK activation mediated by the EGFR could stimulate iNOS-mediated NO production, HEK-B1R cells were transfected with iNOS and then stimulated with either B1R agonist DAKD or EGF. As shown in Fig. 3B, 100 ng/ml EGF did not stimulate significant NO production, whereas 100 nM DAKD resulted in high-output NO as previously reported (10, 18). Furthermore, pretreatment of cells for 10 min with 100 ng/ml EGF did not alter the NO production generated by 100 nM DAKD, nor did pretreatment with PD153035 followed by 100 ng/ml EGF (Fig. 3B). Taken together, these data indicate that ERK activation alone is not sufficient to stimulate iNOS-mediated NO production and that the B1R and EGFR activate two independent pools of ERK, consistent with the requirement for β-arrestin 2 in the B1R pathway.
Figure 3.
EGF activates ERK without stimulating iNOS-dependent NO and does not affect B1R-mediated NO production. A) HEK-B1R cells were stimulated with either DAKD (100 nM) or EGF (100 ng/ml) for various times. Where indicated, cells were preincubated 30 min with 100 nM of EGFR kinase inhibitor PD153035. Cell lysates were analyzed by Western blot analysis for phospho-ERK, total ERK, and GAPDH. Results are representative of ≥3 independent experiments B) HEK-B1R cells were transiently transfected with iNOS. Cells were stimulated with either 100 nM DAKD, 100 ng/ml EGF, or 100 ng/ml EGF for 10 min, then 100 nM DAKD (without or with preincubation for 30 min with PD153035), and NO concentration was measured. Cells were then lysed, and iNOS expression was detected by Western blot analysis. Data are expressed as means ± se (n=3), *P < 0.01 vs. 100 nM DAKD.
iNOS and β-arrestin 2 interact
Because β-arrestin 2 acts as a scaffolding protein for members of the ERK-MAPK pathway (15), we wondered whether it might also function to scaffold iNOS, which would allow for efficient phosphorylation by ERK. HEK-B1R cells were cotransfected with iNOS and β-arrestin 2-V5, treated for various times with 100 nM DAKD, and lysed, and iNOS was then immunoprecipitated. Immunoblotting indicated that β-arrestin 2 coimmunoprecipitates with iNOS (Fig. 4A), suggesting an interaction between the two proteins. In contrast, β-arrestin 1 did not coimmunoprecipitate with iNOS either basally or after receptor stimulation (data not shown), consistent with the lack of effect of β-arrestin 1 knockdown on the response (Fig. 2).
Figure 4.
β-Arrestin 2 interacts with iNOS. A) HEK-B1R cells were transfected with iNOS and β-arrestin 2-V5 where indicated. After transfection for 36–48 h, cells were treated with B1R agonist at the indicated time points; cells were collected and lysed, and aliquots were removed for lysate control (bottom panel). Remaining supernatant was incubated with polyclonal antibody to iNOS and protein-G beads. After washing, immunoprecipitates were boiled in Laemmli sample buffer (2×) and analyzed by immunoblotting (IB) with antibody to iNOS (immunoprecipitation control) or the V5 epitope to detect coimmunoprecipitated β-arrestin 2. For controls, samples were immunoprecipitated with normal IgG (IgG) or with protein-G beads without primary antibody (bead). Results are representative of ≥3 independent experiments. B) HEK-B1R stable cells were transiently transfected with CFP-iNOS and β-arrestin 2 (βarr2)-YFP or CFP-iNOS and β-arrestin 1 (βarr1)-YFP (negative control). After treatment with 100 nM DAKD for various times, cells were fixed and analyzed for FRET using the acceptor photobleaching method. Top: CFP and YFP were visualized at excitation/emission wavelengths of 458/485 nm and 514/545 nm, respectively, after treatment with DAKD for 5 min. Images show enhanced CFP emission after YFP photobleaching in 2 ROIs (outlined in white) from cells transfected with CFP-iNOS and βarr2-YFP (left) but not β-arr1-YFP (right). Bottom: plot of time course of change in CFP emission corresponding to a decrease in YFP emission caused by photobleaching at 514 nm. Scale bars = 10 μm. C) FRET efficiency for CFP-iNOS and βarr2-YFP vs. CFP-iNOS and βarr1-YFP (negative control) in the absence of agonist (0 min) or after 100 nM DAKD stimulation for various times (15–20 cells/time point). FRET efficiency was calculated as described in Materials and Methods. Data are expressed as means ± se (n=20). *P < 0.05, **P < 0.001 vs. corresponding control.
To confirm the interaction, we next investigated whether it could be detected using FRET analysis. Donor CFP-iNOS and either β-arrestin 2-YFP or β-arrestin 1-YFP acceptor were transfected into HEK-B1R cells, and the acceptor photobleaching method was employed to detect the proximity of the two fluorophores. CFP-iNOS and β-arrestin 2-YFP cells treated with 100 nM DAKD for 5 min gave a significant FRET response (Fig. 4B). Plotting the time of agonist stimulation vs. the FRET efficiency revealed significant basal FRET (14%) that increased to a maximum FRET efficiency of 45% after 10–20 min of agonist treatment followed by a return to baseline in 2 h (Fig. 4C). In contrast, when CFP-iNOS was coexpressed with β-arrestin 1-YFP there was not significant basal FRET and little, if any, change after B1R activation (Fig. 4C). Since FRET requires that donor/acceptor pairs be within 10 nm of each other, these data are consistent with the conclusion that iNOS and β-arrestin 2 physically interact.
β-Arrestin 2 is required for B1R-mediated ERK activation
Because ERK activation is required for B1R-dependent enhancement of iNOS activity (10), we next determined whether β-arrestin 2 is required for B1R-mediated ERK activation. As shown in Fig. 5, knockdown of β-arrestin 2 with siRNA inhibited ERK activation after B1R stimulation, most prominently at the later time points (10–40 min). It has been reported that for some GPCRs, the early phase of ERK activation is G-protein dependent, whereas the later time points are β-arrestin dependent, consistent with our results with the B1R (Fig. 5).
Figure 5.
Depletion of β-arrestin 2 with siRNA reduces the prolonged phase of B1R-mediated ERK activation. HEK-B1R cells were transfected with 75 pmol of siRNA specific for βarr2 (open squares) or control siRNA (solid circles) for 72–96 h. Cells were then stimulated with 100 nM DAKD for the indicated time and lysed, and ERK activation was assayed by Western blot analysis for phospho-ERK. Membranes were also probed for β-arrestin 2 and GAPDH. Phospho-ERK was quantitated by densitometry using ImageJ software after correcting for loading based on intensity of the GAPDH bands. β-Arrestin 2-mediated ERK activation was determined by subtracting values for phospho-ERK in β-arrestin 2 siRNA treated cells from those obtained in cells treated with control siRNA (open triangles). Data are expressed as means ± se (n=5). *P < 0.05, **P < 0.01 vs. corresponding control.
DISCUSSION
The activities of constitutively expressed eNOS and nNOS are highly regulated, for example, by changes in intracellular calcium, protein-protein interactions, or phosphorylation. The relatively low NO output resulting from their activation is primarily used for signaling functions (27,28,29,30). In contrast, iNOS is typically viewed as being expressed only under inflammatory conditions and, once synthesized, functioning as a constitutively active generator of high NO until it is degraded (4, 11, 12). This high-output NO can be toxic to invading microorganisms but may also be beneficial or deleterious to the host (4, 11, 12, 31). For example, iNOS-derived NO can be either cytoprotective or proapoptotic, depending on the state of the cell and its interaction with other molecules (32,33,34). However, iNOS is expressed constitutively in some cells, such as certain types of epithelium, lymphocytes, or in normal human or mouse platelets (35,36,37,38). We found low constitutive levels of iNOS in control HLMVECs (14) and high basal iNOS expression in lungs and neutrophils of Mpo−/− mice, where it reduced sepsis-induced lung injury and mortality (39). In addition, we have observed that iNOS activity is also highly regulated post-translationally via B1R-dependent signaling in endothelial cells and transfected HEK cells (9, 10, 14). Receptor-mediated activation of iNOS has also been found in platelets (38), although the signaling pathway has not been delineated.
We previously identified ERK-dependent phosphorylation of iNOS on Ser745 as a key post-translational modification mediating B1R-dependent activation of iNOS. However, ERK activation alone is not sufficient, as stimulation of the EGFR, which robustly activates ERK, did not drive iNOS-mediated NO production. The missing element appears to be β-arrestin 2, which is required for B1R-mediated activation of iNOS but does not participate in EGFR-mediated ERK activation. It is now clear that β-arrestins not only mediate GPCR desensitization but can also function as GPCR effectors (40,41,42,43). GPCR activation of ERK can result from both a rapid G-protein-dependent and a slower, more sustained β-arrestin-dependent mechanism (40, 41, 44). G-protein-dependent ERK activation generally results in translocation of ERK to the nucleus to control transcription. In contrast, β-arrestin retains active ERK in the cytosol via an endosomal signaling complex, and is proposed to facilitate ERK phosphorylation of cytosolic substrates, which are yet to be characterized (15, 16). Consistent with this model is our present finding that β-arrestin 2 is required for prolonged B1R-dependent ERK activation and our previous identification of cytosolic iNOS as an ERK substrate (10).
One mechanism by which β-arrestin 2 enhances ERK activation is by its ability to scaffold members of the ERK-MAPK pathway (15, 16). We recently found that upstream mediators of ERK-dependent iNOS activation include Gαi- and Gβγ-mediated activation of Src, Raf, Ras, and MEK (14), consistent with a scaffolding role for β-arrestin 2 in B1R signaling as found here. However, the present studies reveal an additional critical function for β-arrestin 2; it also binds iNOS, which would enhance the efficiency and specificity of its phosphorylation by ERK. The scaffolding of ERK and iNOS by β-arrestin 2 helps explain our previous finding of colocalization and coimmunoprecipitation of iNOS and ERK in cytokine-treated HLMVEC or transfected HEK293 cells (10). Coimmunoprecipitation data revealed a stable association between β-arrestin 2 and iNOS before and after B1R activation, whereas FRET efficiency increased significantly after agonist stimulation. However, FRET efficiency depends not only on the distance between the two fluorophores, but on their relative orientation as well (45). Thus, the increased FRET efficiency might reflect a dynamic conformational rearrangement between iNOS and β-arrestin 2, altering the relative orientation of the fluorophores without changing association of the two proteins. For example, β-arrestin 2 undergoes a global conformational change on binding to phosphorylated receptors (46).
S-nitrosylation (also called S-nitrosation) is an important mediator of NOS-dependent signaling and can regulate the activity of numerous signaling molecules (47). There is accumulating evidence that protein S-nitrosylation plays important roles in regulating GPCR signaling and trafficking (47, 48). For example, several GPCRs can be directly nitrosylated, resulting in varying effects on receptor affinity and function (48, 49). eNOS binds directly to dynamin-2 and nitrosylates Cys607, increasing dynamin self-assembly and GTPase activity, thereby enhancing receptor endocytosis (50, 51). In contrast, nitrosylation of G protein-coupled receptor kinase 2 at Cys340 inhibits its kinase activity, reducing GPCR phosphorylation, β-arrestin 2 recruitment and desensitization after agonist stimulation (52). Recently, S-nitrosylation of β-arrestin 2 was shown to regulate GPCR trafficking. In these studies, eNOS was constitutively bound to β-arrestin 2 and, after β-adrenergic receptor stimulation, eNOS-derived NO nitrosylated β-arrestin 2 at Cys409, causing dissociation from eNOS, facilitating its binding to clathrin/β-adaptin and promotion of β-adrenergic receptor internalization (17). Our studies provide the first example of functional iNOS-β-arrestin 2 interaction involved in GPCR signaling. In this case, β-arrestin 2 plays a critical role in iNOS activation. Whether β-arrestin 2 is nitrosylated via this interaction and thereby alters B1R trafficking will require further study. It has been proposed that the effect of β-arrestin 2 nitrosylation on receptor trafficking might depend on which receptor class the GPCR belongs to (17), and in this regard, the B1R differs from the β-adrenergic receptor and other class A GPCRs because it is resistant to endocytosis and desensitization (53).
β-Arrestin scaffolding may provide an important mechanism to regulate the specificity of NOS-mediated S-nitrosylation in response to activation of different signaling pathways (17). However, compared with the constitutive NOSs, iNOS generally produces larger amounts of NO, especially when further activated by B1R signaling (10, 14). Because NOS-mediated S-nitrosylation can regulate GPCR function and signaling via several different proteins, it is possible that iNOS activation in inflammatory conditions could lead to general effects on other GPCR signaling pathways with either beneficial or deleterious outcomes.
In summary, our finding of a GPCR-mediated, β-arrestin 2-dependent activation of iNOS supports the novel concept that iNOS activity can be more finely regulated than previously appreciated. It also adds to the growing evidence that β-arrestins provide critical platforms for the formation of “signalsomes” that result in both sustained and targeted signaling in response to GPCR activation (15, 16). The ability of endothelial cells to generate receptor-dependent prolonged and high output iNOS-derived NO in inflammatory conditions could have important pathological consequences in vascular inflammation.
Acknowledgments
The authors thank Dr. Fulong Tan (Department of Pharmacology, University of Illinois at Chicago) for cloning β-arrestin 2 and making initial constructs and Dr. Tiffany Sharma and Debra Salvi (Molecular Resources Core, Department of Pharmacology, University of Illinois at Chicago) for generating tagged β-arrestin 2 and iNOS constructs. The authors are grateful to Svitlana Brovkovych for excellent technical assistance. This work was supported by U.S. National Institutes of Health grants HL60678 and DK 41431. F.K.K. is the recipient of an American Heart Association predoctoral fellowship.
References
- Kubes P. Nitric oxide affects microvascular permeability in the intact and inflamed vasculature. Microcirculation. 1995;2:235–244. doi: 10.3109/10739689509146769. [DOI] [PubMed] [Google Scholar]
- Schubert W, Frank P G, Woodman S E, Hyogo H, Cohen D E, Chow C W, Lisanti M P. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik A B. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Rudic R D, Sessa W C. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc Res. 1999;43:509–520. doi: 10.1016/s0008-6363(99)00161-3. [DOI] [PubMed] [Google Scholar]
- Marrero M B, Venema V J, Ju H, He H, Liang H, Caldwell R B, Venema R C. Endothelial nitric oxide synthase interactions with G protein-coupled receptors. Biochem J. 1999;343:335–340. [PMC free article] [PubMed] [Google Scholar]
- Ju H, Venema V J, Marrero M B, Venema R C. Inhibitory interactions of the bradykinin B2 receptor with endothelial nitric-oxide synthase. J Biol Chem. 1998;273:24025–24029. doi: 10.1074/jbc.273.37.24025. [DOI] [PubMed] [Google Scholar]
- Golser R, Gorren A C, Leber A, Andrew P, Habisch H J, Werner E R, Schmidt K, Venema R C, Mayer B. Interaction of endothelial and neuronal nitric-oxide synthases with the bradykinin B2 receptor. Binding of an inhibitory peptide to the oxygenase domain blocks uncoupled NADPH oxidation. J Biol Chem. 2000;275:5291–5296. doi: 10.1074/jbc.275.8.5291. [DOI] [PubMed] [Google Scholar]
- Geiger M, Stone A, Mason S N, Oldham K T, Guice K S. Differential nitric oxide production by microvascular and macrovascular endothelial cells. Am J Physiol Lung Physiol. 1997;273:L275–L281. doi: 10.1152/ajplung.1997.273.1.L275. [DOI] [PubMed] [Google Scholar]
- Ignjatovic T, Stanisavljevic S, Brovkovych V, Skidgel R A, Erdös E G. Kinin B1 receptors stimulate nitric oxide production in endothelial cells: Signaling pathways activated by angiotensin I-converting enzyme inhibitors and peptide ligands. Mol Pharmacol. 2004;66:1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brovkovych V, Brovkovych S, Tan F, Lee B S, Sharma T, Skidgel R A. Dynamic receptor-dependent activation of inducible nitric-oxide synthase by ERK-mediated phosphorylation of Ser745. J Biol Chem. 2007;282:32453–32461. doi: 10.1074/jbc.M706242200. [DOI] [PubMed] [Google Scholar]
- Kleinert H, Pautz A, Linker K, Schwarz P M. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Alderton W K, Cooper C E, Knowles R G. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R G, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovkovych V, Zhang Y, Brovkovych S, Minshall R D, Skidgel R A. A novel pathway for receptor-mediated post-translational activation of inducible nitric oxide synthase [E-pub ahead of print]. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00992.x. doi: 10.1111/j.1582–4934.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire S M, Ahn S, Lefkowitz R J, Shenoy S K. β-Arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R J, Shenoy S K. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Whalen E J, Nelson C D, Mu Y, Hess D T, Lefkowitz R J, Stamler J S. S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangsree S, Brovkovych V, Minshall R D, Skidgel R A. Kininase I-type carboxypeptidases enhance nitric oxide production in endothelial cells by generating bradykinin B1 receptor agonists. Am J Physiol Heart Circ Physiol. 2003;284:H1959–H1968. doi: 10.1152/ajpheart.00036.2003. [DOI] [PubMed] [Google Scholar]
- Liu J, Ernst S A, Gladycheva S E, Lee Y Y, Lentz S I, Ho C S, Li Q, Stuenkel E L. Fluorescence resonance energy transfer reports properties of syntaxin1a interaction with Munc1–8-1 in vivo. J Biol Chem. 2004;279:55924–55936. doi: 10.1074/jbc.M410024200. [DOI] [PubMed] [Google Scholar]
- Siegel R M, Chan F K, Zacharias D A, Swofford R, Holmes K L, Tsien R Y, Lenardo M J. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Sci STKE. 2000;2000:PL1. doi: 10.1126/stke.2000.38.pl1. [DOI] [PubMed] [Google Scholar]
- Ahn S, Nelson C D, Garrison T R, Miller W E, Lefkowitz R J. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci U S A. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Wei H, Garrison T R, Lefkowitz R J. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J Biol Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- Brovkovych V, Stolarczyk E, Oman J, Tomboulian P, Malinski T. Direct electrochemical measurement of nitric oxide in vascular endothelium. J Pharm Biomed Anal. 1999;19:135–143. doi: 10.1016/s0731-7085(98)00090-9. [DOI] [PubMed] [Google Scholar]
- Stanisavljevic S, Ignjatovic T, Deddish P A, Brovkovych V, Zhang K, Erdös E G, Skidgel R A. Angiotensin I-converting enzyme inhibitors block protein kinase Cε by activating bradykinin B1 receptors in human endothelial cells. J Pharmacol Exp Ther. 2006;316:1153–1158. doi: 10.1124/jpet.105.093849. [DOI] [PubMed] [Google Scholar]
- Ren X R, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz R J. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adomeit A, Graness A, Gross S, Seedorf K, Wetzker R, Liebmann C. Bradykinin B(2) receptor-mediated mitogen-activated protein kinase activation in COS-7 cells requires dual signaling via both protein kinase C pathway and epidermal growth factor receptor transactivation. Mol Cell Biol. 1999;19:5289–5297. doi: 10.1128/mcb.19.8.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton J P, Sessa W C. Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- Ignarro L J, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Stuehr D J. Structure-function aspects in the nitric oxide synthases. Annu Rev Pharmacol Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y, Kim P K, Bagci E Z, Ermentrout G B, Chow C C, Bahar I, Billiar T R. Inflammatory modulation of hepatocyte apoptosis by nitric oxide: in vivo, in vitro, and in silico studies. Curr Mol Med. 2004;4:753–762. doi: 10.2174/1566524043359944. [DOI] [PubMed] [Google Scholar]
- Razavi H M, Hamilton J A, Feng Q. Modulation of apoptosis by nitric oxide: implications in myocardial ischemia and heart failure. Pharmacol Ther. 2005;106:147–162. doi: 10.1016/j.pharmthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Chung H-T, Pae H-O, Choi B-M, Billiar T R, Kim Y-M. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun. 2001;282:1075–1079. doi: 10.1006/bbrc.2001.4670. [DOI] [PubMed] [Google Scholar]
- Choy J C, Wang Y, Tellides G, Pober J S. Induction of inducible NO synthase in bystander human T cells increases allogeneic responses in the vasculature. Proc Natl Acad Sci U S A. 2007;104:1313–1318. doi: 10.1073/pnas.0607731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R A, Zhang G, Nussler N C, Gleixner S L, Ford H R, Simmons R L, Watkins S C. Constitutive expression of inducible nitric oxide synthase in the mouse ileal mucosa. Am J Physiol Gastrointest Physiol. 1997;272:G383–G392. doi: 10.1152/ajpgi.1997.272.2.G383. [DOI] [PubMed] [Google Scholar]
- Guo F H, De Raeve H R, Rice T W, Stuehr D J, Thunnissen F B, Erzurum S C. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A. 1995;92:7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanovic J A, Stojanovic A, Brovkovych V M, Skidgel R A, Du X. Signaling-mediated functional activation of inducible nitric-oxide synthase and its role in stimulating platelet activation. J Biol Chem. 2008;283:28827–28834. doi: 10.1074/jbc.M801646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovkovych V, Gao X-P, Ong E, Brovkovych S, Brennan M-L, Su X, Hazen S L, Malik A B, Skidgel R A. Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L96–L103. doi: 10.1152/ajplung.00450.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea K A, Vaughn Z D, O'Bryan E M, Nishijima D, Dery O, Bunnett N W. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L M, Roudabush F L, Choy E W, Miller W E, Field M E, Pierce K L, Lefkowitz R J. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S K, Drake M T, Nelson C D, Houtz D A, Xiao K, Madabushi S, Reiter E, Premont R T, Lichtarge O, Lefkowitz R J. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Tohgo A, Pierce K L, Choy E W, Lefkowitz R J, Luttrell L M. Beta-arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- DeFea K A, Zalevsky J, Thoma M S, Dery O, Mullins R D, Bunnett N W. β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M J, Bunemann M, Hoffmann C, Vilardaga J P, Nikolaev V O. Monitoring receptor signaling by intramolecular FRET. Curr Opin Pharmacol. 2007;7:547–553. doi: 10.1016/j.coph.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Gurevich V V, Gurevich E V. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D T, Matsumoto A, Kim S O, Marshall H E, Stamler J S. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. Vascular signaling through G protein-coupled receptors: new concepts. Curr Opin Nephrol Hypertens. 2009;18:153–159. doi: 10.1097/MNH.0b013e3283252efe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkola T, Savinainen J R, Monkkonen K S, Retamal M D, Laitinen J T. S-nitrosothiols modulate G protein-coupled receptor signaling in a reversible and highly receptor-specific manner. BMC Cell Biol. 2005;6:21. doi: 10.1186/1471-2121-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Moniri N H, Ozawa K, Stamler J S, Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci U S A. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Yao J, McCabe T J, Yao Q, Katusic Z S, Sessa W C, Shah V. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem. 2001;276:14249–14256. doi: 10.1074/jbc.M006258200. [DOI] [PubMed] [Google Scholar]
- Whalen E J, Foster M W, Matsumoto A, Ozawa K, Violin J D, Que L G, Nelson C D, Benhar M, Keys J R, Rockman H A, Koch W J, Daaka Y, Lefkowitz R J, Stamler J S. Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L M, Marceau F, Muller-Esterl W, Pettibone D J, Zuraw B L. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]