Abstract
Macrophage migration inhibitory factor (MIF) affects inflammation, glucose homeostasis, and cellular proliferation in mammals. Previously, we found that MIF was significantly elevated in multiple long-lived mouse models, including calorie restriction (CR), which led us to hypothesize that MIF might be important in the control of mammalian life span and be necessary for the life-extending effects of CR. To test this hypothesis, we examined the life span of mice with a targeted deletion of the Mif gene on a segregating B6 × 129/Sv background (MIF-KO) under ad libitum (AL) feeding and CR conditions. Control mice were generated by mating C57BL/6J females with 129/SvJ males to make an F1 hybrid, and crossing F1 males to F1 females to produce segregating F2 mice homozygous for the normal MIF allele. Not only did MIF-KO mice show a life span extension in response to CR, they were, unexpectedly, longer lived than controls under standard AL conditions. MIF-KO mice were significantly protected against lethal hemangiosarcoma, but more likely than controls to die of disseminated amyloid, an age-related inflammatory syndrome. Overall, these data refute the suggestion that MIF is required for the CR effect on life span, but raise the possibility that MIF may limit life span in normal mice.—Harper, J. M., Wilkinson, J. E., Miller, R. A. Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction.
Keywords: MIF, CR, life span, cancer, inflammation
Macrophage migration inhibitory factor (MIF) was first described as a T-cell-derived cytokine that could inhibit the migration of macrophages in vitro (1). Further work showed that MIF is expressed in many other cell types, most notably the pancreas and pituitary gland. MIF is unusual for a cytokine, in that it is constitutively produced and stored in intracellular pools, obviating the need for de novo synthesis prior to its release (2, 3). MIF is now considered to be an important component of the innate immune response, opposing the anti-inflammatory effects of glucocortioids on a host of cell/tissue types at both the local and systemic level (4). Pharmacological inhibition of the proinflammatory effects of MIF has shown promise as a clinical approach to protecting patients from potentially lethal septic shock and other inflammatory conditions (5).
In addition to its contribution to inflammation, there is a growing body of evidence to suggest that MIF is an important regulator of energy metabolism via its neuroendocrine effects on insulin signaling pathways in the pancreas, muscle, and adipocytes (6). More recently, MIF has also been implicated as a contributor to tumor growth and progression (7) through its effects on tumor vascularization and alteration of apoptotic signaling pathways (8, 9). Approaches that diminish MIF function are being tested to see whether they might have therapeutic value to treat a variety of cancers (10,11,12,13).
An early study showed that local production of MIF by stimulated immune cells was reduced by aging in guinea pigs (14, 15), but there is no significant change in serum MIF level during healthy aging in humans (16, 17). Studies on MIF effects in invertebrate models of delayed aging are now also in progress, in part because MIF is a mediator of hypoxia inducible factor-1α (HIF-1α) activity, a regulator of cellular senescence in vitro (18, 19), which can also modulate life span in invertebrates (20, 21).
In our own work, an unbiased study of gene expression profiles showed that the basal expression of MIF mRNA was significantly elevated in the liver of long-lived Snell dwarf and growth hormone receptor-knockout (GHR-KO) mice relative to their normal-lived counterparts (22). Moreover, we found that mice maintained on either of two antiaging diets, i.e., calorie restriction (CR) or a diet low in the essential amino acid methionine (Meth-R), also had significantly elevated levels of MIF mRNA in the liver (22, 23). In addition, MIF mRNA is significantly elevated in the liver of long-lived Idaho-derived wild mice compared to a genetically heterogeneous population of laboratory mice (unpublished data). The finding that liver MIF expression is significantly elevated by CR has recently been confirmed by an independent group, who found increased MIF expression in heart and skeletal muscle as well (24). Taken together, these data led us to hypothesize that MIF might be required for the life-span-extending effects of CR. To test this hypothesis, we examined the life span of mice with a targeted disruption of the Mif gene (MIF-KO) (25) under both ad libitum (AL) feeding and CR conditions.
MATERIALS AND METHODS
MIF-KO mice were produced as described previously (25, 26) and kindly provided to us by Dr. Abhay Satoskar (Ohio State University, Columbus, OH, USA) as homozygotes on a segregating (C57BL/6J×129/SvJae) background. Control mice were generated at Michigan by mating C57BL/6J females with 129/SvJ males to make an F1 hybrid, and crossing F1 males to F1 females to produce segregating F2 mice, homozygous for the normal MIF allele. We will refer to these control mice as (B6×129)F2. Only female mice were used for this study and were housed at a density of 4 mice/cage. Mice were maintained using standard specific pathogen-free (SPF) husbandry techniques; sentinel animals were exposed to spent bedding on a quarterly basis to check for possible pathogen infection, and all such tests came up negative over the course of the study. At the age of 6 wk, mice of each genotype in the CR groups were given an amount of Purina Lab Diet 5001 (PMI Nutrition International, St. Louis, MO, USA) equal to 90% of the amount consumed by mice in the respective AL group for 2 wk. They were then shifted to 75% food availability for 2 wk and then shifted to 60% food intake for the remainder of the experiment. The CR mice of either genotype in this study did not receive any vitamin or mineral supplements. Previous studies have demonstrated a robust CR effect on life span using this protocol (27). Mice were provided with tap water ad libitum. A total of 24, 24, 39, and 39 mice were used for the control AL, control CR, MIFKO AL, and MIFKO CR groups, respectively.
Mice were weighed at 1, 2, 3, 6, 12, 18, and 24 mo of age and were inspected daily, and the date of death was recorded for each animal. Mice were euthanized if they were found to be so severely ill that in the opinion of an experienced caretaker they were unlikely to survive more than another few days. For each group, slightly more than half of the mice were found dead (14/24, 15/24, 21/39, and 26/39 for control AL, control CR, MIFKO AL, and MIFKO CR mice, respectively) while the remainder were euthanized when moribund. At the time of death, the body cavity and the base of the skull were opened, and the mice were immersed in 10% formalin and stored for up to 3 yr prior to necropsy. A complete necropsy was done on each animal. The skin, subcutis, and mammary glands were examined carefully for inflammatory lesions or tumors. Sections of kidneys, adrenal glands, liver, spleen, heart, skeletal muscle, lung ovary, and uterus were embedded in paraffin, cut, and stained with hematoxylin and eosin (H&E) for histopathologic examination. In addition, any grossly detectable lesion was processed for histopathology.
RESULTS
The initial hypothesis was that MIF-KO mice would not respond to CR. We did not expect to see any difference in life span between the two stocks fed the AL diet. Both working hypotheses proved incorrect (Fig. 1). When fed the AL diet, MIF-KO mice were significantly longer-lived than their (B6×129)F2 controls (log-rank test, P<0.001), and MIF-KO mice exhibited a robust life-span extension in response to CR (P=0.003). As expected, CR resulted in a significant increase in life span in the (B6×129)F2 controls (P<0.001). There was no significant difference in life span of MIF-KO and (B6×129)F2 mice fed the CR diet (P>0.3; Fig. 1).
Figure 1.
MIF-KO mice are significantly long lived (log-rank, P<0.001) relative to (B6×129)F2 control mice, and exhibit a significant life-span extension in response to CR. Kaplan-Meier survival curves for each genotype and dietary condition; each point represents a single mouse.
Median survival in the 24 AL control mice was 774 d (95% confidence interval, 714–834 d); median survival in the 39 AL MIF-KO mice was 16% higher (895 d, CI 843–947). Among CR mice, median survival of the 24 controls was 1045 d (CI 960–1130), and was 1064 d (CI 1021–1107) in the 39 CR MIF-KO, an increase of 35 and 19%, respectively, relative to AL mice of the same genotype. The increase in longevity seen with CR in the (B6×129)F2 control stock is typical of that normally seen in mice undergoing this degree of CR (28). As a test of maximum life span, we used the method of Wang and Allison (29), which applies the Fisher exact test to the proportion of mice alive in each group at the age when 90% of the pooled population has died. Among AL mice, 0/24 of the controls were alive at the cutoff date (1043 d), compared to 7/39 of the MIF-KO mice (P=0.01). As expected, CR led to a significant increase in maximum life span among control mice (0/24 of the AL mice and 5/24 of CR mice were alive at 1275 d; P=0.05). There was a trend toward a CR effect on maximal longevity even in the long-lived MIF-KO mice, with 2/39 of the control vs. 6/39 of the MIF-KO mice still alive at 1178 d, but the effect did not reach statistical significance in this fairly small study.
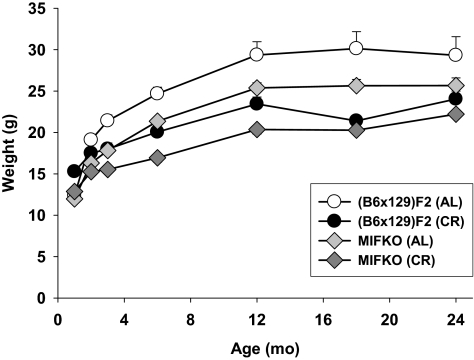
Phenotypically, MIF-KO mice appear normal on multiple genetic backgrounds. Offspring are generated in the expected 1:2:1 genotypic ratio, and the litter sizes of heterozygous and homozygous matings are normal. In addition, gross and histopathological analyses of multiple organs have revealed no abnormalities (25, 30) except that testes weight is reduced in conjunction with ultrastructural abnormalities in the acrosome, apical ectoplasmic specialization and spermatids; both Leydig and Sertoli cells are normal (31). Moreover, counts of blood cells have revealed no differences in the ratio of cell types between genotypes, while flow cytometric analyses of splenocytes and thymocytes indicated normal ratios of lymphocyte populations (25, 30). However, although a difference in body weight had not previously been reported, we found that MIF-KO mice were lighter in weight than the control stock for at least the first 24 mo of life under both AL and CR conditions (Fig. 2). A repeated-measures ANOVA indicated that there are significant main effects on body weight for both genotype (P<0.0001) and diet (P<0.0001; n=12–35/point dependent on genotype and diet), but the interaction term was not significant (P>0.4), indicating that each stock responded to the CR diet in a similar way. Body length was not measured. In addition, pilot studies conducted in a second, distinct population of 6- to 12-mo-old (B6×129)F2 and MIF-KO mice indicated there is no difference in serum insulin-like growth factor-1 (IGF-I), food-deprived glucose, or fecal corticosteroid level in control vs. MIF-KO mice under both the AL and CR feeding regimens (P>0.4 for all); although CR did result in a significant reduction in glucose and IGF-I level (P≤0.02) concomitant with an increase in fecal corticosteroid level (P=0.04) as expected (data not shown).
Figure 2.
MIF-KO mice are significantly smaller than (B6×129)F2 mice. Data represent mean ± se body weight as a function of age in each of the two genotypes under each dietary condition (n=12–35 mice/point dependent on genotype and diet).
Necropsies that included histopathological analysis were conducted on 29 control mice and 49 MIF-KO mice. Excluding those in which autolysis was too advanced to allow diagnosis, 26 controls (14 AL and 12 CR) and 45 MIF-KO cases (22 AL and 23 CR) produced useful findings. The pathologist attempted to assign a most likely cause of death, i.e., to determine whether any specific disease or combination of diseases was sufficiently advanced that it could plausibly have led to the death of the mouse (for those found dead) or to the symptoms that led to the decision to euthanize mice in found to be severely ill. Results are shown in Table 1. There were no statistically significant effects of the CR diet on occurrence of any tabulated lesion in both genotypes, and therefore AL and CR groups were combined for further analysis. The case was classified as open (i.e., no disease deemed likely to account for death) in 2/26 (8%) of control and 8/45 (18%) of MIF-KO mice; these proportions are not significantly different. Seven of 45 (16%) of the MIF-KO mice, but none of the controls, were judged to have died of amyloidosis (P=0.03 by Fischer exact test). All of these animals had severe amyloidosis of the renal glomeruli, characterized by the deposition of homogenous, acellular, pink, fibrillar material with expansion and complete destruction of the normal glomeruli. The renal tubules contained significant amounts of protein, with secondary degeneration of the tubular epithelium. Two mice also had amyloid deposition in the adrenal gland, two in the spleen, and one in the liver (Fig. 3). This pattern of amyloid deposition is consistent with amyloid derived from the acute-phase protein serum amyloid A (SAA) and is commonly associated with an aberrant immune response (32). In MIF-KO mice, it would be expected that the lack of MIF leads to an increase in glucocorticoid-mediated immunosuppression (33) and increased SAA expression (34).
TABLE 1.
Results of histopathology studies
| Likely cause of death | Control
|
MIF-KO
|
||
|---|---|---|---|---|
| AL | CR | AL | CR | |
| Open (no diagnosis reached) | 1 | 1 | 3 | 5 |
| Adrenal cancer | 1 | 1 | ||
| Amyloidosis | 2 | 5 | ||
| Fibrosarcoma | 1 | |||
| Glomerulonephritis | 1 | |||
| Cardiac failure | 1 | |||
| Hemangiosarcoma | 3 | 7 | 3 | 3 |
| Histiocytic sarcoma | 1 | |||
| Hydronephrosis | 1 | |||
| Lymphoma | 4 | 2 | 5 | 2 |
| Mammary carcinoma | 1 | 1 | ||
| Ovarian carcinoma | 1 | |||
| Pancreatic islet cell carcinoma | 1 | |||
| Pneumonia | 1 | 1 | ||
| Pulmonary carcinoma | 2 | 1 | 2 | 4 |
| Uterine carcinoma | 1 | 1 | 1 | |
| Total | 14 | 12 | 22 | 23 |
Figure 3.
AA-amyloidosis in kidney, spleen, liver, and adrenal gland of MIF-KO mice. A) Kidney: diffuse, severe glomerular AA-amyloidosis (×20). B) Glomerular AA-amyloidosis with expansion and destruction of the glomeruli (×40). C) Glomerular AA-amyloidosis (×80). D) Spleen: AA-amyloidosis (×40). E) Liver: perivascular AA-amyloidosis (×40). F) Adrenal-medullary amyloidosis (×40).
Hemangiosarcoma was judged to have been the cause of death in 10/26 (38%) of controls but only 6/45 (13%) of MIF-KO mice (P=0.01). MIF is capable of inducing angiogenesis (35) and the absence of MIF in the KO mice may result in fewer tumors of endothelial origin. Increased longevity due to dietary restriction has previously been shown to be associated with a decreased incidence of hemangiosarcoma in mice (34). The basis for this difference in both models of increased longevity is unclear.
The extent of glomerulonephritis was also evaluated on a scale of 0–4 points and found not to show significant effects of either genotype or diet, although there was a trend toward reduced severity in CR mice in both the control group (from 0.71±0.19 in AL to 0.29±0.08 in CR, given as mean±se), and in the MIF-KO mice (from 0.33±0.07 in AL to 0.25±0.05 in CR).
DISCUSSION
Our principal finding is that ablation of MIF leads to increased life span in mice. Our confidence in this conclusion must be tempered by uncertainty about possible differences in proportions of background genes between the MIF-KO mice and the (B6×129)F2 mice used as controls. The MIF-KO mice were created by a cross between a chimera derived from a 129S4/SvJae mouse (36), and mice of the C57BL/6 strain (whose substrain is unstated in the original publication (25). Investigations of immune function in the MIF-KO mice (37, 38) have typically used F2 hybrids between C57BL/6 and 129/SvJ as controls, because these F2 mice, like the original MIF-KO mice, are expected to contain C57BL/6 and 129 genes segregating in equal proportions. We followed this established strategy in our own set of experiments. It is possible, however, that some alleles from either parental stock may have become fixed, and others lost, during the 10 yr over which the MIF-KO mice have been propagated at Harvard University, Ohio State University, and now the University of Michigan. Thus, we cannot exclude the possibility that the differences in life span, weight, and pathology we described above might reflect differences at loci other than MIF, and our conclusions should be considered tentative until similar work has been done using littermate controls in a population in which the MIF-KO allele is segregating.
To the best of our knowledge, this is the first time a reduction in level of a cytokine has been shown to extend life span in a mammal. Such a finding, if confirmed, opens new avenues for investigation in rodents, and, potentially, in human preventive medicine. Drugs or antibodies that can reduce serum MIF levels or interfere with MIF action on one or more of its target cell types might, in principle, duplicate some of the effects of the MIF-KO mutation on health and life span. A number of studies have already indicated the utility of neutralizing MIF activity through the use of monoclonal antibodies or small chemical inhibitors (e.g., ISO-1) in the control of autoimmune and inflammatory disease (5, 39,40,41) and as a cancer chemotherapeutic strategy (8, 10).
Our data give only a few, preliminary, hints as to the pathways by which ablation of MIF extends life span in these mice. Our evidence that MIF-KO mice show a further life span extension when treated with CR suggests that the lack of MIF does not completely duplicate the health benefits of CR. On the other hand, we see no additive effect, i.e., no evidence that MIF-KO mice fed CR diets live longer than controls fed CR diets. The observation that a CR diet extends life span in the Ames pituitary dwarf (27) has been interpreted as evidence that the Ames mutation and CR diets extend life span through mechanisms that are at least partially distinct. In contrast, the lack of any CR effect on the life span of GHR-KO mice has suggested that CR and GHR-KO mice share closely similar pathways for life-span extension, arguably based on extreme insulin sensitivity (42, 43). The MIF-KO survival curves (Fig. 1) are an intermediate case, in which CR does extend life span of a long-lived mutant, but not beyond the extent seen in CR control mice themselves. Elucidation of the pathways by which these diets and mutations postpone lethal illnesses and the other signs of aging will benefit from replication studies using a wider range of background genes and dietary formulations, to test the robustness of the phenomena, as well as evaluation of mice in which one of more of the purported response pathways has been inhibited in specific tissues or at specific developmental stages.
There is a growing body of evidence linking aberrant MIF expression to inflammatory disease processes and tumorigenesis (41, 44), and it is plausible that a loss of MIF function either delays, or slows, the progression of age-related inflammatory or neoplastic disease in MIF-KO mice. For example, MIF has been directly implicated as a mediator of disease progression and severity in a number of inflammatory conditions, such as colitis, atherosclerosis, or pancreatitis (45, 47), while models of experimental inflammation are suppressed in MIF-KO mice (45, 48, 49). Interestingly, analyses of genetic polymorphisms in the Mif gene in human populations have indicated that genetic variants associated with a reduction in MIF level and/or activity result in a corresponding reduction in disease severity (50,51,52).
With regard to tumorigenesis, elevated MIF has been linked to an increase in tumor incidence and aggression in a variety of cancers. The list includes prostate (53), gastric (54), skin (13, 55), breast (56), bladder (57), colorectal (58), and nervous system (10) cancers. Genetic variants that result in differential MIF activity can significantly alter cancer trajectories (11, 59), presumably due to an influence of MIF on cell growth and cell cycle regulatory proteins, such as p53 and Akt, as well as its proangiogenic effects (60). In this context, it is noteworthy that the incidence of hemangiosarcoma was significantly reduced in our MIF-KO mice.
In addition, there is emerging evidence that MIF is an important mediator of glucose metabolism and diabetes through its influence on insulin release by pancreatic islets, and through modulation of glucose uptake and insulin signaling cascades in target cells (e.g., adipocytes, myocytes). MIF may also influence immunoinflammatory responses involved in the pathogenesis of Type 1 diabetes (6). Although it is still somewhat controversial, MIF deficiency generally appears to be antidiabetic (61,62,63,64). For example, loss of MIF protects diabetes-prone LDL receptor deficient (Ldlr−/−) mice against the development of age-related insulin resistance and glucose intolerance, apparently through a reduction in inflammatory pathways in white adipose tissue (64). MIF-KO mice are also less prone to streptozotocin-induced diabetes (63). In humans, MIF is significantly elevated in diabetics (65), and chronic elevation of MIF precedes the onset of type 2 diabetes (66). Similarly, there is a clear association between MIF gene polymorphisms, MIF activity, and diabetes (62, 67) in humans.
Finally, MIF has well-known effects on the hypothalamic-pituitary-adrenal (HPA) axis. It is released in conjunction with adrenocorticotrophin (ACTH) from the pituitary gland during times of physiological and psychological stress (1). On release, MIF acts primarily as a negative regulator of glucocorticoid (GC) activity, especially with respect to effects on immune cells (33), via a reduction in ACTH and GC production and/or secretion (68, 69). In this context, it is noteworthy that HPA axis responsiveness to restraint stress can predict life span in young adult inbred rats (70,71,72).
Acknowledgments
The authors thank Melissa Burns, Maggie Lauderdale, and Jessica Sewald for technical assistance. The work was supported by U.S. National Institutes of Health grants AG023122, AG024824, and AG013283. The authors report no conflict of interest.
References
- Bucala R. MIF rediscovered: cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response. FASEB J. 1996;10:1607–1613. doi: 10.1096/fasebj.10.14.9002552. [DOI] [PubMed] [Google Scholar]
- Bacher M, Meinhardt A, Lan H, Mu W, Metz C, Chesney J, Calandra T, Gemsa D, Donelly T, Atkins R, Bucala R. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol. 1997;150:12. [PMC free article] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Mitchell R, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:8. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerle-Rowson G R, Bucala R. Neuroendocrine properties of macrophage migration inhibitory factor (MIF) Immunol Cell Biol. 2001;79:368–375. doi: 10.1046/j.1440-1711.2001.01024.x. [DOI] [PubMed] [Google Scholar]
- Cvetkovic I, Stosic-Grujicic S. Neutralization of macrophage migration inhibitory factor—novel approach for the treatment of immunoinflammatory disorders. Int Immunopharmacol. 2006;6:1527–1534. doi: 10.1016/j.intimp.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Toso C, Emamaullee J, Merani S, Shapiro A. The role of macrophage migration inhibitory factor on glucose metabolism and diabetes. Diabetologia. 2008;51:1937–1946. doi: 10.1007/s00125-008-1063-3. [DOI] [PubMed] [Google Scholar]
- Fingerle-Rowson G, Petrenko O. MIF coordinates the cell cycle with DNA damage checkpoints. Lessons from knockout mouse models. Cell Div. 2007;2:22. doi: 10.1186/1747-1028-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon B E, Willer S S, Zundel W, Mitchell R A. Mechanisms of macrophage migration inhibitory factor (MIF)-dependent tumor microenvironmental adaptation. Exp Mol Pathol. 2009;86:180–185. doi: 10.1016/j.yexmp.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemajerova A, Mena P, Fingerle-Rowson G, Moll U M, Petrenko O. Impaired DNA damage checkpoint response in MIF-deficient mice. EMBO J. 2007;26:987–997. doi: 10.1038/sj.emboj.7601564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J-P, Deuster O, Balzer-Geldsetzer M, Meyer B, Dodel R, Bacher M. The role of macrophage inhibitory factor in tumorigenesis and central nervous system tumors. Cancer. 2009;115:2031–2040. doi: 10.1002/cncr.24245. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler K L, Iczkowski K A, Leng L, Bucala R, Vera P L. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–8739. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- Winner M, Meier J, Zierow S, Rendon B E, Crichlow G V, Riggs R, Bucala R, Leng L, Smith N, Lolis E, Trent J O, Mitchell R A. A novel, macrophage migration inhibitory factor suicide substrate inhibits motility and growth of lung cancer cells. Cancer Res. 2008;68:7253–7257. doi: 10.1158/0008-5472.CAN-07-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Duncan F J, Keiser T, Shin S, Kusewitt D F, Oberyszyn T, Satoskar A R, VanBuskirk A M. Macrophage migration inhibitory factor (MIF) plays a critical role in pathogenesis of ultraviolet-B (UVB) -induced nonmelanoma skin cancer (NMSC) FASEB J. 2009;23:720–730. doi: 10.1096/fj.08-119628. [DOI] [PubMed] [Google Scholar]
- Ganguly R, Waldman R, Craig C, Lockey R. Effects of age on respiratory tract immunity in guinea pigs. Allergol Immunopathol (Madr) 1986;14:1–7. [PubMed] [Google Scholar]
- Ganguly R, Lockey R. Age interference of lymphokine production by lung derived lymphocytes. Allerg Immunol (Leipz) 1985;31:203–206. [PubMed] [Google Scholar]
- Mizue Y, Nishihira J, Miyazaki T, Fujiwara S, Chida M, Nakamura K, Kikuchi K, Mukai M. Quantitation of macrophage migration inhibitory factor (MIF) using the one-step sandwich enzyme immunosorbent assay: elevated serum MIF concentrations in patients with autoimmune diseases and identification of MIF in erythrocytes. Int J Mol Med. 2000;5:397–403. doi: 10.3892/ijmm.5.4.397. [DOI] [PubMed] [Google Scholar]
- Hardman M J, Waite A, Zeef L, Burow M, Nakayama T, Ashcroft G S. Macrophage migration inhibitory factor: a central regulator of wound healing. Am J Pathol. 2005;167:1561–1574. doi: 10.1016/S0002-9440(10)61241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford S, Bedogni B, Gradin K, Poellinger Powell M, Giaccia A. HIF1α delays premature senescence through the activation of MIF. Genes Dev. 2006;20:3366–3371. doi: 10.1101/gad.1471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E L, Klimova T A, Eisenbart J, Schumacker P T, Chandel N S. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007;27:5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman J A. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans PLoS ONE. 2009;4:e6348. doi: 10.1371/journal.pone.0006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Steinkraus K A, Sutphin G L, Ramos F J, Shamieh L S, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal Regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–1198. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R A, Chang Y, Galecki A, Al-Regaiey K, Kopchick J J, Bartke A. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol Endocrinol. 2002;16:2657–2666. doi: 10.1210/me.2002-0142. [DOI] [PubMed] [Google Scholar]
- Miller R A, Buehner G, Chang Y, Harper J M, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach S, Teichert S, Niemann B, Franke C, Katschinski D. Caloric restriction counteracts age-dependent changes in prolyl-4-hydroxylase domain (PHD) 3 expression. Biogerontology. 2008;9:169–176. doi: 10.1007/s10522-008-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza M, Satoskar A R, Lin G, Lu B, Humbles A A, Gerard C, David J R. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoskar A R, Bozza M, Rodriguez Sosa M, Lin G, David J R. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect Immun. 2001;69:906–911. doi: 10.1128/IAI.69.2.906-911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Wright J C, Mattison J A, Ingram D K, Miller R A, Roth G S. Longevity: extending the lifespan of long-lived mice. Nature. 2001;414:412–412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford R L. Springfield, IL, USA: Charles C. Thomas; The Retardation of Aging and Disease by Dietary Restriction. 1988 [Google Scholar]
- Wang C, Li Q, Redden D T, Weindruch R, Allison D B. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Honma N, Koseki H, Akasaka T, Nakayama T, Taniguchi M, Serizawa I, Akahori H, Osawa M, Mikayama T. Deficiency of the macrophage migration inhibitory factor gene has no significant effect on endotoxaemia. Immunology. 2000;100:84–90. doi: 10.1046/j.1365-2567.2000.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anahara R, Toyama Y, Koda M, Honma S, Nishihira J, Toshimori K, Mori C. Deletion of macrophage migration inhibitory factor gene induces down regulation of sex hormones and ultrastructural abnormalities in mouse testes. Reprod Toxicol. 2006;21:167–170. doi: 10.1016/j.reprotox.2005.08.001. [DOI] [PubMed] [Google Scholar]
- HogenEsch H, Niewold T, Higuchi K, Tooten P, Gruys E, Radl J. Gastrointestinal AAPOAII and systemic AA-amyloidosis in aged C57BL/Ka mice. Virchows Arch B Cell Pathol. 1993;64:37–43. doi: 10.1007/BF02915094. [DOI] [PubMed] [Google Scholar]
- Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol Endocrinol. 2007;21:1267–1280. doi: 10.1210/me.2007-0065. [DOI] [PubMed] [Google Scholar]
- Turturro A, Duffy P, Hass B, Kodell R, Hart R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci. 2002;57:B379–B389. doi: 10.1093/gerona/57.11.b379. [DOI] [PubMed] [Google Scholar]
- Bach J P, Rinn B, Meyer B, Dodel R, Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. 2008;75:127–133. doi: 10.1159/000155223. [DOI] [PubMed] [Google Scholar]
- Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Paiva C N, Arras R H, Magalhaes E S, Alves L S, Lessa L P, Silva M H, Ejzemberg R, Canetti C, Bozza M T. Migration inhibitory factor (MIF) released by macrophages upon recognition of immune complexes is critical to inflammation in Arthus reaction. J Leukoc Biol. 2009;85:855–861. doi: 10.1189/jlb.0108009. [DOI] [PubMed] [Google Scholar]
- Magalhaes E S, Paiva C N, Souza H S P, Pyrrho A S, Mourao-Sa D, Figueiredo R T, Vieira-de-Abreu A, Dutra H S, Silveira M S, Gaspar-Elsas M I C, Xavier-Elsas P, Bozza P T, Bozza M T. Macrophage migration inhibitory factor is critical to interleukin-5-driven eosinophilopoiesis and tissue eosinophilia triggered by Schistosoma mansoni infection. FASEB J. 2009;23:1262–1271. doi: 10.1096/fj.08-124248. [DOI] [PubMed] [Google Scholar]
- Stosic-Grujicic S, Stojanovic I, Nicoletti F. MIF in autoimmunity and novel therapeutic approaches. Autoimmun Rev. 2009;8:244–249. doi: 10.1016/j.autrev.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Morand E F. New therapeutic target in inflammatory disease: macrophage migration inhibitory factor. Int Med J. 2005;35:419–426. doi: 10.1111/j.1445-5994.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- Morand E F, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov. 2006;5:399–411. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- Masternak M M, Al-Regaiey K A, Del Rosario Lim M M, Jimenez-Ortega V, Panici J A, Bonkowski M S, Kopchick J J, Wang Z, Bartke A. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006;41:417–429. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Regaiey K A, Masternak M M, Bonkowski M S, Panici J A, Kopchick J J, Bartke A. Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J Gerontol A Biol Sci Med Sci. 2007;62:18–26. doi: 10.1093/gerona/62.1.18. [DOI] [PubMed] [Google Scholar]
- Mitchell R A. Mechanisms and effectors of MIF-dependent promotion of tumourigenesis. Cell Signal. 2004;16:13–19. doi: 10.1016/j.cellsig.2003.07.002. [DOI] [PubMed] [Google Scholar]
- De Jong Y P, Abadia-Molina A C, Satoskar A R, Clarke K, Rietdijk S T, Faubion W A, Mizoguchi E, Metz C N, Sahli M A, Ten Hove T, Keates A C, Lubetsky J B, Farrell R J, Michetti P, Van Deventer S J, Lolis E, David J R, Bhan A K, Terhorst C. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–1066. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- Burger-Kentischer A, Goebel H, Seiler R, Fraedrich G, Schaefer H E, Dimmeler S, Kleemann R, Bernhagen J, Ihling C. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation. 2002;105:1561–1566. doi: 10.1161/01.cir.0000012942.49244.82. [DOI] [PubMed] [Google Scholar]
- Yoshitaka S, Atsushi M, Akihiko S, Jun N, Tetsuya Y, Tooru S. Macrophage migration inhibitory factor is a critical mediator of severe acute pancreatitis. Gastroenterology. 2003;124:725–736. doi: 10.1053/gast.2003.50099. [DOI] [PubMed] [Google Scholar]
- Ohkawara T, Mitsuyama K, Takeda H, Asaka M, Fujiyama Y, Nishihira J. Lack of macrophage migration inhibitory factor suppresses innate immune response in murine dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2008;43:1497–1504. doi: 10.1080/00365520802273017. [DOI] [PubMed] [Google Scholar]
- Pan J H, Sukhova G K, Yang J T, Wang B, Xie T, Fu H, Zhang Y, Satoskar A R, David J R, Metz C N, Bucala R, Fang K, Simon D I, Chapman H A, Libby P, Shi G P. Macrophage migration inhibitory factor deficiency impairs atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:3149–3153. doi: 10.1161/01.CIR.0000134704.84454.D2. [DOI] [PubMed] [Google Scholar]
- Rachelle D, Zaynab A, Fabrizio De B, Cristina M, Eleftheria Z, Mark L, Adam S, Emma S, Rebecca L, William E R O, Wendy T, David R. Mutation screening of the macrophage migration inhibitory factor gene: Positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402–2409. doi: 10.1002/art.10492. [DOI] [PubMed] [Google Scholar]
- Radstake T R, Sweep F C, Welsing P, Franke B, Vermeulen S H, Geurts-Moespot A, Calandra T, Donn R, van Riel P L. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum. 2005;52:3020–3029. doi: 10.1002/art.21285. [DOI] [PubMed] [Google Scholar]
- Awandare G A, Martinson J J, Were T, Ouma C, Davenport G C, Ong'echa J M, Wang W, Leng L, Ferrell R E, Bucala R, Perkins D J. MIF (macrophage migration inhibitory factor) promoter polymorphisms and susceptibility to severe malarial anemia. J Infect Dis. 2009;200:629–637. doi: 10.1086/600894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denz A, Pilarsky C, Muth D, Rückert F, Saeger H-D, Grützmann R. Inhibition of MIF leads to cell cycle arrest and apoptosis in pancreatic cancer cells. J Surg Res. 2010;151:298–298. doi: 10.1016/j.jss.2009.03.048. [DOI] [PubMed] [Google Scholar]
- Harry Hua-Xiang X, Yi Y, Kent-Man C, Qing G, Yuan-Yuan Z, Hua H, Wai Man W, Suet-Yi L, Siu-Tsan Y, Man-Fung Y, Annie O O C, Benjamin C Y W. Serum macrophage migration-inhibitory factor as a diagnostic and prognostic biomarker for gastric cancer. Cancer. 2009;115:5441–5449. doi: 10.1002/cncr.24609. [DOI] [PubMed] [Google Scholar]
- Honda A, Abe R, Yoshihisa Y, Makino T, Matsunaga K, Nishihira J, Shimizu H, Shimizu T. Deficient deletion of apoptotic cells by macrophage migration inhibitory factor (MIF) overexpression accelerates photocarcinogenesis. Carcinogenesis. 2009;30:1597–1605. doi: 10.1093/carcin/bgp160. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008;261:147–157. doi: 10.1016/j.canlet.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Taylor J, Kuchel G, Hegde P, Voznesensky O, Claffey K, Tsimikas J, Leng L, Bucala R, Pilbeam C. Null mutation for macrophage migration inhibitory factor (MIF) is associated with less aggressive bladder cancer in mice. BMC Cancer. 2007;7:135. doi: 10.1186/1471-2407-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J M, Coletta P L, Cuthbert R J, Scott N, MacLennan K, Hawcroft G, Leng L, Lubetsky J B, Jin K K, Lolis E, Medina F, Brieva J A, Poulsom R, Markham A F, Bucala R, Hull M A. Macrophage migration inhibitory factor promotes intestinal tumorigenesis. Gastroenterology. 2005;129:1485–1503. doi: 10.1053/j.gastro.2005.07.061. [DOI] [PubMed] [Google Scholar]
- Ding G X, Zhou S Q, Xu Z, Feng N H, Song N H, Wang X J, Yang J, Zhang W, Wu H F, Hua L X. The association between MIF-173 G>C polymorphism and prostate cancer in southern Chinese. J Surg Oncol. 2009;100:106–110. doi: 10.1002/jso.21304. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Robinson S C, Thompson R G, Charles K, Kulbe H, Balkwill F R. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Therapeutics. 2007;6:1993–2002. doi: 10.1158/1535-7163.MCT-07-0118. [DOI] [PubMed] [Google Scholar]
- Koska J, Stefan N, Dubois S, Trinidad C, Considine R V, Funahashi T, Bunt J C, Ravussin E, Permana P A. mRNA concentrations of MIF in subcutaneous abdominal adipose cells are associated with adipocyte size and insulin action. Int J Obes. 2009;33:842–850. doi: 10.1038/ijo.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herder C, Klopp N, Baumert J, Müller M, Khuseyinova N, Meisinger C, Martin S, Illig T, Koenig W, Thorand B. Effect of macrophage migration inhibitory factor (MIF) gene variants and MIF serum concentrations on the risk of type 2 diabetes: results from the MONICA/KORA Augsburg Case–Cohort Study, 1984–2002. Diabetologia. 2008;51:276–284. doi: 10.1007/s00125-007-0800-3. [DOI] [PubMed] [Google Scholar]
- Stosic-Grujicic S, Stojanovic I, Maksimovic-Ivanic D, Momcilovic M, Popadic D, Harhaji L, Miljkovic D, Metz C, Mangano K, Papaccio G, Al-Abed Y, Nicoletti F. Macrophage migration inhibitory factor (MIF) is necessary for progression of autoimmune diabetes mellitus. J Cell Physiol. 2008;215:665–675. doi: 10.1002/jcp.21346. [DOI] [PubMed] [Google Scholar]
- Verschuren L, Kooistra T, Bernhagen J, Voshol P J, Ouwens D M, van Erk M, de Vries-van der Weij J, Leng L, van Bockel J H, van Dijk K W, Fingerle-Rowson G, Bucala R, Kleemann R. MIF deficiency reduces chronic inflammation in white adipose tissue and impairs the development of insulin resistance, glucose intolerance, and associated atherosclerotic disease. Circ Res. 2009;105:99–107. doi: 10.1161/CIRCRESAHA.109.199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabunaka N, Nishihira J, Mizue Y, Tsuji M, Kumagai M, Ohtsuka Y, Imamura M, Asaka M. Elevated serum content of macrophage migration inhibitory factor in patients with type 2 diabetes. Diabetes Care. 2000;23:256–258. doi: 10.2337/diacare.23.2.256. [DOI] [PubMed] [Google Scholar]
- Herder C, Kolb H, Koenig W, Haastert B, Müller-Scholze S, Rathmann W, Holle R, Thorand B, Wichmann H E. Association of systemic concentrations of macrophage migration inhibitory factor with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:368–371. doi: 10.2337/diacare.29.02.06.dc05-1474. [DOI] [PubMed] [Google Scholar]
- Thorand B, Baumert J, Kolb H, Meisinger C, Chambless L, Koenig W, Herder C. Sex differences in the prediction of type 2 diabetes by inflammatory markers. Diabetes Care. 2007;30:854–860. doi: 10.2337/dc06-1693. [DOI] [PubMed] [Google Scholar]
- Bocheva A, Dzambazova E, Hadjiolova R, Traikov L, Mincheva R, Bivolarski I. Effect of Tyr-MIF-1 peptides on blood ACTH and corticosterone concentration induced by three experimental models of stress. Auton Autacoid Pharmacol. 2008;28:117–123. doi: 10.1111/j.1474-8673.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- Fingerle-Rowson G, Koch P, Bikoff R, Lin X, Metz C N, Dhabhar F S, Meinhardt A, Bucala R. Regulation of macrophage migration inhibitory factor expression by glucocorticoids in vivo. Am J Pathol. 2003;162:47–56. doi: 10.1016/S0002-9440(10)63797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli S A, McClintock M K. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli S A, Yee J R, McClintock M K. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav. 2006;50:454–462. doi: 10.1016/j.yhbeh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Yee J R, Cavigelli S A, Delgado B, McClintock M K. Reciprocal affiliation among adolescent rats during a mild group stressor predicts mammary tumors and lifespan. Psychosom Med. 2008;70:1050–1059. doi: 10.1097/PSY.0b013e31818425fb. [DOI] [PMC free article] [PubMed] [Google Scholar]