Abstract
Human CD4−8− T cells are a minor subset quantitatively but potentially important in immunity because they are predominantly distributed at body surfaces, and their number and activities increase in autoimmune diseases and decrease with aging. Distinguishing characteristics of CD4−8− T cells are found to include a unique profile of cytokines, including Serpin E1, which is not generated by other T cells, MIF, and TGF-β. At 2–5% of the total in mixtures with CD4 + CD8 T cells, CD4−8− T cells enhance the generation of IFN-γ and IL-17 by up to 12- and 5-fold, respectively, without contributing either cytokine or affecting cytokine production by NK/NKT cells. CD4−8− T cell-derived MIF is their major enhancer and TGFβ their principal inhibitor of CD4 and CD8 T cell cytokine production. Decreases in CD4−8− T cell effects may diminish protective immunity in aging, whereas increases may augment the severity of autoimmune diseases.—Huang, M.-C., Patel, K., Taub, D. D., Longo, D. L., Goetzl, E. J. Human CD4−8− T cells are a distinctive immunoregulatory subset.
Keywords: cytokines, autoimmunity, immunosenescence
CD4−8− T cells are a quantitatively minor subset of the T cell constellation, and little is known of their cellular and immunological properties. Mammalian CD4−8− T cells represent only 2–5% of blood T cells and fewer than 5% of all T cells trafficking through lymphoid organs and lymphatics (1, 2). The majority of CD4−8− T cells express T-cell antigen receptors (TCRs) of the γδ-type and are distributed at body surfaces in the gastrointestinal and respiratory systems and in skin (3,4,5). In humans and mice, CD4−8− T cells expressing αβ-type TCRs have a restricted range of Vβ usage with a predominance of Vβ8; recognize antigens, including microbially derived proteins and lipids, presented principally by CD1 proteins; and exhibit high levels of autoreactivity (2, 6,7,8).
CD4−8− T cells are distinctively capable of defending mammalian hosts against intracellular bacteria. The presentation of Mycobacterium tuberculosis antigens to TCRαβ CD4−8− T cells by CD1b of antigen-presenting cells results in proliferation and preparation of the CD4−8− T cells for specific cytotoxic activity against intracellular M. tuberculosis (7). Specifically sensitized TCRαβ CD4−8− T cells block growth of M. tuberculosis and Francisella tularensis inside macrophages and transfer such antimicrobial immunity to naive recipients with resultantly increased resistance to infections by these microbes (9).
An increased number of CD4−8− T cells are observed in some human diseases of the immune system, and CD4−8− T cells also may contribute to the pathogenesis of several autoimmune diseases. HIV-1 DNA was found in CD4−8− T cells only in patients who had failed to respond to a course of antiviral therapy, and in this group the level of HIV-1 DNA in CD4−8− T cells correlated with plasma viral load (10). Although the total number of CD4−8− T cells may not be unusually high in most patients with systemic lupus erythematosus (SLE), there is a striking increase in the TCRαβ CD4−8− T cell subset that expresses higher than normal levels of surface protein markers of T cell activation and enhances the production of anti-DNA antibodies as efficiently as CD4 Th cells (11). A fascinating but still controversial recent observation of IL-17-containing CD4−8− T cells in kidney tissues of SLE patients with nephritis is complicated by the lack of differences in IL-17 positivity of CD4−8− T cells in blood from SLE patients compared to normal control subjects, whether T cells were freshly isolated, cultured, or stimulated in culture (12). The greatest increases in blood and tissue levels of TCRαβ CD4−8− T cells are found in patients with the autoimmune lymphoproliferative syndrome (ALPS) and MRL/lpr mice with a similar syndrome (13,14,15). ALPS is attributable to diverse defects in lymphocyte Fas-mediated apoptosis leading to lymphadenopathy, splenomegaly, B lymphocytosis, hypergamma globulinemia, and numerous immune cytopenias, in association with very high circulating levels of TCRαβ CD4−8− T cells that may rise to 1000/μl and thus represent 25–30% of total T cells (14). This preferential increase in the level of TCRαβ CD4−8− T cells in ALPS suggests that they are normally distinctively susceptible to Fas-mediated apoptosis.
Our present findings demonstrate that human CD4−8− T cells express a unique profile of surface immune proteins, secrete a distinctive array of immune cytokines, and are major regulators of the generation of IFN-γ and IL-17 by CD4 and CD8 T cells but do not alter their production of TGFβ1 or affect NK/NKT cell generation of cytokines. These findings provide a basis for new considerations of the roles of CD4−8− T cells in host defense and a different understanding of their contributions to immunopathogenesis in several human disease states.
MATERIALS AND METHODS
Immunomagnetic bead isolation and fluorescence-activated cell sorting (FACS) purification of sets of normal human blood lymphocytes
Venous blood was obtained from healthy human donors who had signed an informed consent document. Blood was diluted 1:1 (v:v) with Ca2+- and Mg2+-free Dulbecco’s balanced salt solution and centrifuged in 35-ml aliquots on 10-ml cushions of Ficoll-Paque (GE Healthcare Lifesciences, Pittsburgh, PA, USA) at 400 g for 30 min at room temperature to yield a mixture of mononuclear leukocytes (MLs). All cell sets were purified from MLs by immunomagnetic bead methods. The total T-cell population was isolated with a human T-cell negative selection kit, NK/NKT cells were recovered after sequential binding of biotinylated anti-human CD56 antibodies and anti-biotin antibody-coated beads, and CD8 and CD4 T cell sets were obtained after incubation with anti-human CD8 antibody-coated beads and anti-human CD4 antibody-coated beads, respectively (Miltenyi Biotec, Auburn, CA, USA). CD4−8− T cells were purified from the total T-cell population by 2 cycles of absorption with a mixture of anti-human CD8 + anti-human CD4 antibody-coated beads on LD columns (Miltenyi). For FACS isolation of human CD4−8− T cells, total T cells were purified with a T-cell enrichment kit (R&D Systems, Minneapolis, MN, USA) before staining with fluorescent antibodies to CD3, CD4, and CD8 and introduction into a MoFlow cell sorter (Beckman Coulter, Miami, FL, USA). The purity of all sets of T cells was >98% by flow cytometric analyses of their defining markers, and that of most preparations of CD4−8− T cells exceeded 99%.
Flow cytometric analyses of T-cell surface immune proteins
T cells were washed in FACS buffer (1% fetal bovine serum in PBS) and incubated for 30 min at 4°C with diverse fluorochrome-labeled antibodies and fluorochrome-labeled isotype control immunoglobulins. Fluorescent immunospecifically labeled surface proteins characteristic of CD4−8− T cells and control CD4 and CD8 T cells were analyzed either with a FACSCantoI flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) or a FACScan flow cytometer (BD Biosciences) and analyzed using either a FlowJo software program (Tree Star, Ashland, OR, USA) or CellQuest Pro software (BD Biosciences) as described previously (16). The fluorochrome-labeled antibodies were purchased from BD Biosciences (San Jose, CA, USA), R&D Systems, and eBiosciences (San Diego, CA, USA).
Lymphocyte incubation protocols
To assess the cytokine products of CD4−8− T cells alone, replicate aliquots of 50,000–200,000 cells/0.5 ml of complete RPMI 1640 medium with 10% FBS, 100 U/ml of penicillin, 50 μg/ml of streptomycin, and 292 μg/ml of glutamine were added to 48-well plates and incubated without and with 1 or 3 μg each of adherent anti-human CD3 plus anti-human CD28 antibodies or anti-human CD3 plus anti-human CD161 antibodies, or IL-12 plus IL-18 at 5 and 50 ng/ml, respectively. Studies of the effects of CD4−8− T cells on the generation of cytokines by individual target cells employed the same conditions, except that suspensions of the target cells were at 106/ml, T cells were stimulated with 1 or 3 μg each of anti-human CD3 plus anti-human CD28 antibodies, and NK/NKT cells were stimulated with 5 ng/ml of IL-2 plus 2 ng/ml of IL-12. The cytokine inhibitory mouse monoclonal antibodies were anti-TGFβ1 IgG1 (clone 9016.2; Abcam, Cambridge, MA, USA), anti-RANTES IgG2b (clone VL-1; ProSci, Poway, CA, USA), anti-MIF receptor IgG1 (CD74, clone LN2; Biolegend, San Diego, CA, USA) together with anti-MIF IgG1 (clone 4E4; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-Serpin E1 IgG1 (clone 242816; R&D Systems). Neither anti-MIF or anti-MIF receptor antibody alone was inhibitory. The MIF receptor antagonist Iso-1 was obtained from EMD Biosciences (San Diego, CA, USA).
Recombinant human IL-2, IL-12, TGFβ1, CCL5 (RANTES), and CCL1 (I-309) (Peprotech, Rocky Hill, NJ, USA), IL-18 (MBL International, Woburn, MA, USA), and Serpin E1 and MIF (R&D Systems) were obtained from the cited suppliers. This MIF has an N106S substitution in an amino-terminal oligomerization site but has shown full cellular activity in other studies at the same concentrations as used here (17, 18). Activity of the MIF was proven by inhibition of migration of U937 cells through 8-μm-pore filters in Transwell chambers (Costar-Corning, Lowell, MA, USA); mean inhibition (n=2) was 34% at 10 ng/ml and 69% at 100 ng/ml.
Cytokine profiler assays
T-cell supernatant media were analyzed for concentrations of diverse chemokines and other cytokines using the Proteome Profiler Array Human Cytokine Array Panel A (product ARY005; R&D Systems). Membranes were developed according to the manufacturer’s protocol, and the relative level of immune cytokine was determined by measuring the diameter and density of each spot for comparison with those variables of negative and positive standards.
ELISA quantification of cytokines
T-cell supernatant media were analyzed by a panel of ELISAs to determine concentrations of cytokines suggested to be products of CD4−8− T cells by the results of cytokine profiler assays. The ELISA kits were IL-17A, TGFβ1, and IFN-γ (range 4–500 pg/ml) from eBiosciences; Serpin E1 (range 0.06–20 ng/ml) and RANTES (range 2–1000 pg/ml) from R&D Systems; and migration inhibitory factor (MIF; range 8–6000 pg/ml) from RayBiotech (Norcross, GA, USA). Color intensity of each well was determined in an ELISA plate reader (MRX Revelation; Dynex Technologies, Chantilly, VA, USA).
RESULTS
Surface protein antigen expression by CD4−8− T cells
Human blood CD4−8− T cells have a distinctive surface immune protein phenotype that differs substantially from that of CD4 and CD8 T cells. As for CD4 and CD8 T cells, CD4−8− T cells nearly universally express CD2, CD3, CD5, CD47, CD50, and CD59 (Table 1; Supplemental Fig. 1). CD4−8− T cells differ from both CD4 and CD8 T cells in their greater extent of expression of TCRγδ, IL-18Rα, and CCR5 chemokine receptor, but narrower expression of TCRαβ (Table 1). CD4−8− T cells are more like CD4 than CD8 T cells in the extent of their expression of CD28 and CD45RO. In contrast, CD4−8− T cells resemble CD8 more than CD4 T cells in the greater breadth of their expression of CD54, CD210, and CD58, as well as narrower expression of CD161, which defines a memory set of pre-Th17 cells, CD38, CD26, and the chemokine receptors CCR7 and CXCR4 (Table 1). CD154 (CD40L) is expressed on a slightly higher percentage of newly isolated CD4−8−T cells than CD4 or CD8 T cells (Table 1), but stimulation with anti-CD3 + anti-CD28 antibodies for 24 h increases the extent of expression to a mean of only 11% (n=3, range=10–12%) for CD4−8− T cells as contrasted with a mean of 26% for CD4 T cells and 24% for CD8 T cells (n=4, range=19–31%).
TABLE 1.
Distinctive profile of expression of surface immune proteins by CD4−8− T cells
| Category/protein | Expression by T cells (%)
|
||||
|---|---|---|---|---|---|
| Functions | CD4−8− | CD4+ | CD8+ | ||
| Immune cell interactions | |||||
| CD58 (LFA-3) | T-cell–APC, CTL-target interactions | 87 | 54 | 81 | |
| CD161 | Th17 differentiation, survival | 50 | 75 | 54 | |
| CD40 | B-cell differentiation, Ig class switch | 8 | 14 | 25 | |
| CD154 (CD40L) | Ligand for CD40 | 3 | 1 | 1 | |
| Immune cellular adhesion | |||||
| CD50 (ICAM-3) | Binds to LFA-1, T–B-cell interactions | 100 | 100 | 100 | |
| CD47 (IAP) | ECM-induced [Ca2+]i, migration | 99 | 100 | 100 | |
| CD54 | Binds to ICAM-1, T-cell-endothelial interactions | 75 | 30 | 80 | |
| CD38 | Cyclic ADP-ribose ectoenzyme, many functions | 25 | 46 | 20 | |
| Immune cellular proliferation | |||||
| CD5 (Leu 1) | T-cell proliferation, Th functions | 97 | 100 | 92 | |
| CD45RA (PTP) | Roles in differentiation, coactivation | 69 | 60 | 87 | |
| CD4RO (PTP) | Roles in differentiation, activation | 49 | 39 | 16 | |
| Antigen recognition | |||||
| TCRαβ | T-cell activation, signaling | 35 | 100 | 100 | |
| TCRγδ | T-cell activation, signaling | 65 | 0 | 0 | |
| Costimulation of T-cell responses to antigens | |||||
| CD2 | Adhesion and costimulatory protein | 100 | 100 | 100 | |
| CD28 | Broadly activates T cells with TCRs | 84 | 96 | 51 | |
| Receptors for chemokines, other mediators | |||||
| CCR4 | Chemokine GPCR | 31 | 39 | 32 | |
| CCR5 | Chemokine GPCR | 41 | 6 | 16 | |
| CCR7 | Chemokine GPCR | 27 | 79 | 35 | |
| IL-18Rα | IL-18R-binding unit | 60 | 36 | 35 | |
| IL-18Rβ | IL-18R expression, IL-18 high-affinity binding | 8 | 2 | 6 | |
| CD210 | IL-10 receptor | 22 | 6 | 27 | |
| CXCR3 | Chemokine GPCR | 56 | 46 | 55 | |
| CXCR4 | Chemokine GPCR | 27 | 67 | 40 | |
| CXCR5 | Chemokine GPCR | 8 | 15 | 5 | |
| Other | |||||
| CD59 | Complement membrane attack complex inhibitor | 87 | 96 | 71 | |
| CD26 | Multifunctional serine dipeptidyl peptidase-4 | 43 | 85 | 39 | |
Values are means of results of 2 to 4 determinations. ECM, extracellular matrix; GPCR, G-protein-coupled receptor; PTP, protein tyrosine phosphatase.
CD4−8− T-cell enhancement of generation of cytokines by CD4 and CD8 T cells
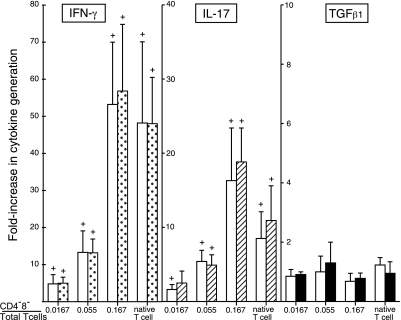
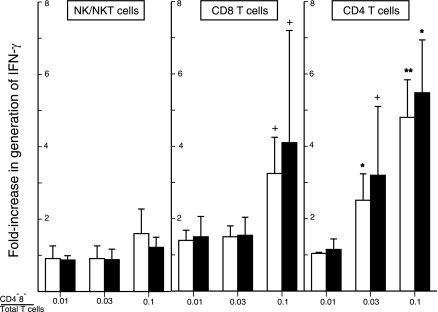
The addition of purified autologous CD4−8− T cells to a CD4−8− T-cell-free mixture of CD4 and CD8 T cells in different ratios prior to stimulation with adherent anti-CD3 plus anti-CD28 antibodies significantly enhanced production of IFN-γ and IL-17 (Fig. 1). The native mixture of CD4, CD8, and CD4−8− T cells generated nearly 50-fold more IFN-γ and 10-fold more IL-17 than the same number of CD4 and CD8 T cells without CD4−8− T cells (Fig. 1). Progressive significant increases in generation of both cytokines were achieved when the percentage of CD4−8− T cells to CD4 plus CD8 T cells was raised, and the maximal levels attained exceeded those of the native T cell mixture. Stimulation of the greatest number of CD4−8− T cells used in this study alone led to generation of less than 20 pg/ml of IFN-γ and 2 pg/ml of IL-17, which effectively excluded a direct cytokine contribution from CD4−8− T cells. CD4−8− T cells had no effects on the generation of TGFβ1 by CD4 plus CD8 T cells at the ratios examined here after correction for the quantity of TGFβ1 contributed by CD4−8− T cells alone (Fig. 1). To determine the target cell specificity of CD4−8− T-cell enhancement of cytokine generation, autologous CD4−8− T cells were added to separately purified NK/NKT, CD4, and CD8 cells before stimulation. There was no increment in generation of IFN-γ by NK/NKT cells stimulated by IL-2 plus IL-12 (Fig. 2). The enhancement of IFN-γ generation by isolated subsets of CD4 and CD8 T cells was significant but less than for CD4 plus CD8 T cell mixtures (Figs. 1 and 2). At 3 and 10% of the level of CD4 T cells and 10% of that of CD8 T cells, CD4−8− T cells augmented IFN-γ generation significantly.
Figure 1.
CD4−8− T-cell enhancement of generation of IFN-γ and IL-17, but not TGFβ, by mixed CD4 and CD8 T cells stimulated with anti-CD3 + anti-CD28 antibodies. Columns and bars depict mean ± sd results of studies of T cells from 4 healthy human donors, expressed as fold increase above the cytokine concentration attained by CD4−8− T-cell-depleted mixtures of CD4 and CD8 T cells. Native T-cell mixture contained relative numbers of CD4, CD8, and CD4−8− T cells normally present in the blood of each donor. Left-hand (open) and right-hand (shaded) bars of each pair show results for 2 and 3 d of incubation, respectively. CD4 + CD8 T cells depleted of CD4−8− T cells and stimulated with 1 μg each of anti-CD3 + anti-CD28 antibodies achieved a range of concentrations of IFN-γ of 65–281 pg/ml on d 2 and 175–1076 pg/ml on d 3, IL-17 of 32–75 pg/ml on d 2 and 58–94 pg/ml on d 3, and TGFβ of 610–930 pg/ml on d 2 and 862–1215 pg/ml on d 3. +P < 0.05; t test.
Figure 2.
Target-cell specificity of CD4−8− T-cell enhancement of generation of IFN-γ. Columns and bars depict mean ± sd results of studies of cells from 3 healthy human donors, expressed as fold increase above the IFN-γ concentration attained by NK/NKT cell mixtures stimulated with 5 ng/ml of IL-2 and 2 ng/ml of IL-12, and CD4 and CD8 T cells stimulated with 2 μg each of anti-CD3 and anti-CD28 antibodies in the absence of CD4−8− T cells. Left-hand (open) and right-hand (solid) bars of each pair show results on d 1 and 2 for NK/NKT cells, and d 2 and 3 for CD4 and CD8 T cells, respectively. Range of concentrations of IFN-γ achieved in the absence of CD4−8− T cells on d 1 and 2 for NK/NKT cells were 21,250–29,300 and 75,200–80,350 pg/ml, respectively, and on d 2 and 3, 1650–2664 and 4030–4684 pg/ml for stimulated CD8 T cells, and 362–2315 and 1474–4861 pg/ml for stimulated CD4 T cells, respectively. +P < 0.05, *P < 0.01, **P < 0.001; t test.
Mechanism of CD4−8− T-cell enhancement of generation of cytokines
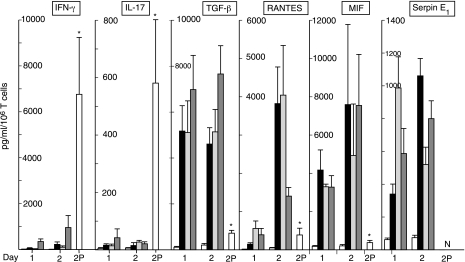
Purified CD4−8− T cells were irradiated with 5000 rads before adding them to a mixture of CD4 and CD8 cells. Without irradiation, 3% CD4−8− T cells enhanced anti-CD3 + anti-CD28 antibody-evoked generation of IFN-γ significantly by 228 ± 55% on d 1 (mean ± sd; n=3; P<0.01). Irradiation completely eliminated CD4−8− T cell-elicited enhancement of IFN-γ production. This result suggested that secreted products of CD4−8− T cells, rather than surface protein ligands, are responsible for enhancement of cytokine generation. The predominant cytokines consistently identified by semiquantitative membrane array profiler assays (n=3) were Serpin E1, MIF, CCL5 (RANTES), and CCL1 (I-309), and other screening studies had detected TGFβ1. The quantitatively principal products of CD4−8− T cells stimulated either by antibodies to TCR proteins or by IL-12 + IL-18 were TGFβ1 = MIF > RANTES > Serpin E1 (Fig. 3). MIF appeared at a more rapid rate from stimulated CD4−8− T cells than CD4 + CD8 T cells, whereas the time course of generation of TGFβ1 was similar for the two T-cell sources (Table 2). CD4−8−T cells obviously produce much more of both cytokines than the CD4 + CD8 T-cell mixture. In a native mixture of 5% CD4−8− T cells and 95% CD4 + CD8 T cells, Serpin E1 would originate solely from CD4−8− T cells, and they also would be the major source of MIF at early time points after stimulation. However, RANTES and TGFβ1 would be derived in significant quantities from both T-cell sources in activated mixtures.
Figure 3.
Generation of immune cytokines by purified CD4−8− T cells. Columns and bars depict mean ± sd results of studies of CD4−8− T cells on d 1 and 2 or of CD4 + CD8 T cells on d 2 (bar 2P) from 5 healthy human donors, expressed as pg/ml/106 T cells. Repetitive sets of 4 columns for CD4−8− T cells on d 1 and 2 represent, in order from left to right: no stimulus, anti-CD3 + anti-CD28 antibodies, anti-CD3 + anti-CD161 antibodies, and IL-12 (5 ng/ml)+ IL-18 (50 ng/ml). Single 2P bar in each frame depicts values on d 2 for CD4 + CD8 T cells from 5 donors (IFN-γ and IL-17) or 3 donors (other cytokines) stimulated with anti-CD3 + anti-CD28 antibodies. *P < 0.01 vs. d 2 level for anti-CD3 + anti-CD28 antibody-stimulated CD4−8− T cells. N for the Serpin E1 2P bar indicates not detectable.
TABLE 2.
Time courses of generation of the principal regulatory cytokines by CD4−8−T cells and a mixture of CD4 + CD8 T cells
| T-cell type | Cytokine | Time of incubation (h)
|
||
|---|---|---|---|---|
| 6 | 12 | 24 | ||
| CD4−8− | MIF | 1324 ± 180 | 2759 ± 174 | 4214 ± 434 |
| TGFβ1 | 515 ± 80 | 1170 ± 138 | 5286 ± 384 | |
| CD4 + CD8 | MIF | 43 ± 8 | 103 ± 16 | 277 ± 102 |
| TGFβ1 | 58 ± 8 | 109 ± 17 | 539 ± 59 | |
Values are mean ± sd results (pg/106 T cells) for 3 studies.
To further characterize the CD4−8− T-cell source of the principal cytokines, the TCRγδ and TCRαβ subsets were separated by an immunomagnetic method (Miltenyi) and stimulated for 1 d with adherent anti-CD3 + anti-CD28 antibodies or IL-12 + IL-18. All levels of TGFβ1 and MIF were lower than for the mixture of CD4−8− T cells, but demonstrated contributions from both subsets. For the two stimuli, respectively, mean concentrations (n=2) of TGFβ1 were 348 and 690 pg/ml/106 TCRαβ T cells and 1181 and 890 pg/ml/106 TCRγδ T cells, and mean concentrations of MIF were 256 and 246 pg/ml/106 TCRαβ T cells and 377 and 367 pg/ml/106 TCRγδ T cells.
The possibility that coculturing of CD4−8− T cells with CD4 + CD8 T cells might reciprocally stimulate generation of cytokines, such as IFN-γ and IL-17, by the former regulatory set was examined by ELISPOT quantification of intracellular IFN-γ (eBioscience kit) in each subset of T cells after 1 d of incubation on adherent anti-CD3 + anti-CD28 antibodies. After incubation of CD4 + CD8 T cells alone, CD4−8− T cells alone, or a mixture of the 2 populations of T cells, the CD4 + CD8 T cells and CD4−8− T cells in the mixture were resolved again by CD4 bead + CD8 bead immunomagnetic chromatography (Miltenyi). With an optimum T cell number of 1.3 × 104/well, the relative densitometric levels of intracellular IFN-γ were 1.0 and 7.3 (mean, n=3), respectively, for CD4−8− T cells and CD4 + CD8 T cells incubated separately, and 1.1 and 9.7 for CD4−8− T cells and CD4 + CD8 T cells incubated as a mixture and then resolved prior to performing the ELISPOT assay. Thus, the quantity of intracellular IFN-γ was 7-fold higher for CD4 + CD8 T cells than CD4−8− T cells without an interaction, was not increased in CD4−8− T cells by incubation with CD4 + CD8 T cells, and was increased from 7- to 10-fold higher in CD4 + CD8 T cells after incubation with CD4−8− T cells.
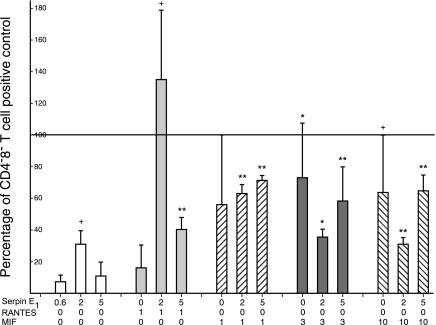
To delineate mechanisms of augmentation of cytokine generation by CD4 and CD8 T cells, purified recombinant human cytokines were introduced alone or in pairs into replicate suspensions of CD4−8− T-cell-depleted mixtures of CD4 + CD8 T cells, at concentrations that would be attained by CD4−8− T cells alone, followed by stimulation with anti-CD3 + anti-CD28 antibodies (Fig. 4). A frame of reference for interpreting the effects of the cytokines alone was established by adding 3% CD4−8− T cells to identical suspensions of CD4 + CD8 T cells before stimulation (100% mean control level of IFN-γ production). Neither Serpin E1 nor RANTES alone substantially altered IFN-γ production by stimulated CD4 + CD8 T cells in the absence of CD4−8− T cells. The combination of 1 ng/ml of RANTES with 2 and 5 ng/ml of Serpin E1 elevated IFN-γ production significantly to above the control level and to 40% of the control level, respectively (Fig. 4). MIF alone at 3 and 10 ng/ml, but not at 1 ng/ml, significantly augmented IFN-γ production to respective means of 73 and 64% of the CD4−8− T cell-added control level. The combination of 2 or 5 ng/ml of Serpin E1 with 1 ng/ml of MIF raised IFN-γ production to significant mean levels of 63 and 71% of control, whereas the same concentrations of Serpin E1 had either no effect or decreased the level of enhancement of IFN-γ production attained by 3 and 10 ng/ml of MIF alone (Fig. 4). TGFβ1 at 1–10 ng/ml had no significant effect on IFN-γ production by mixtures of CD4 + CD8 T cells depleted of CD4−8− T cells before stimulation for 2 d with anti-CD3 plus anti-CD28 antibodies. However, when MIF was added at 3 ng/ml to enhance their generation of IFN-γ, then 3 ng/ml of TGFβ1 suppressed CD4 + CD8 T-cell production of IFN-γ by a mean of 84% (n=2).
Figure 4.
CD4−8− T-cell-type cytokine enhancement of generation of IFN-γ by mixed CD4 and CD8 T cells in the absence of CD4−8− T cells. Columns and bars depict mean ± sd enhancement of IFN-γ generation attained by adding 1 or 2 cytokines to CD4−8− T-cell-free CD4 + CD8 T cells from 3 healthy donors before stimulation by anti-CD3 + anti-CD28 antibodies for 3 d. The 100% control value represents level of IFN-γ production by same number of CD4 + CD8 T cells mixed with 3% CD4−8− T cells before stimulation. +P < 0.05, *P < 0.025, and **P < 0.01; t test.
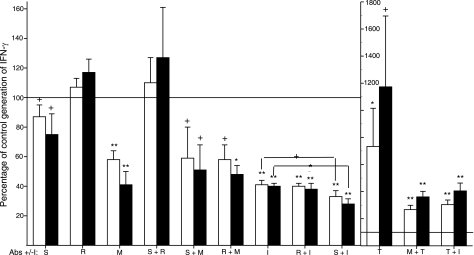
In a second approach, replicate suspensions of CD4 + CD8 T cells with 3% CD4−8− T cells received either monoclonal antibodies that neutralize Serpin E1, RANTES, TGF-β1, and/or MIF and its receptor or the MIF receptor antagonist Iso-1 (I) before stimulation with anti-CD3 + anti-CD28 antibodies (Fig. 5). The mean level of IFN-γ production achieved in the absence of anticytokine antibodies or Iso-1 was set at 100%. Only Iso-1 and the combination of antibodies to MIF and its receptor substantially suppressed generation of IFN-γ by up to 60%, confirming the enhancing activity of MIF observed in the absence of CD4−8− T cells (Fig. 4). The combination of antibodies to Serpin E1 or RANTES with antibodies to the MIF system did not further suppress IFN-γ production below the level attained by antibodies to MIF alone (Fig. 5). However, the addition of antibodies to Serpin E1 significantly augmented the suppressive effect of Iso-1 on IFN-γ generation. The apparent stimulatory activity of Serpin E1 plus RANTES (Fig. 4) was not supported by combined application of their neutralizing antibodies, which had no effect on IFN-γ production (Fig. 5). The introduction of neutralizing antibodies to TGF-β1 alone very significantly elevated IFN-γ generation (Fig. 5, right side). The enhancement of IFN-γ generation attained by reduction of the suppressive effect of TGFβ1 was much less when the level of IFN-γ produced was diminished by concurrent inhibition of the stimulatory activity of MIF using either Iso-1 or antibodies to the MIF system. Isotype control immunoglobulins had no effects on IFN-γ generation. Thus CD4−8− T cells regulate generation of IFN-γ by CD4 and CD8 T cells principally through the suppressive effect of TGF-β1 and the enhancing effect of MIF, with possible augmenting activity of Serpin E1 at lower concentrations of MIF.
Figure 5.
Prevention of CD4−8− T-cell regulation of mixed CD4 + CD8 T-cell generation of IFN-γ by antagonism of cytokines. Columns and bars depict mean ± sd change induced by adding anti-cytokine/cytokine receptor antibodies and/or the MIF receptor antagonist Iso-1 (I) to CD4 + CD8 T cells mixed with 3% CD4−8− T cells from 3 healthy donors before stimulation by anti-CD3 + anti-CD28 antibodies (no additions control=100%). Antibodies were to Serpin E1 (S; 2 μg/ml), RANTES (R; 10 μg/ml), and MIF + MIF receptor (M; 30 and 6 μg/ml, respectively); Iso-1 (I) level was 40 μM. Bars in each pair depict data from d 2 and 3 in that order. Statistical analyses and symbols as in Fig. 2, except that brackets over Iso-1 data in left frame show significantly greater inhibition at both days when S is added to Iso-1.
Diminished blood level and T-cell-enhancing activity of CD4−8− T cells in aging
As the blood concentration of CD4−8− T cells and their immune effects are elevated in many autoimmune diseases, where some T-cell responses are heightened, the status of CD4−8− T cells was examined in older humans who manifest diminished adaptive immune responses. First, the blood level of CD4−8− T cells was determined by flow cytometry for 12 healthy subjects aged 68 yr or older. Each older subject was studied concurrently with a matched healthy individual aged 35 yr or younger, who was of the same gender, race, and national origin. The blood level of CD4−8− T cells for the older set was a mean ± sd percentage of 2.20 ± 0.46% of the total T cells, which contrasted significantly with that for the younger control set of 4.64 ± 1.82% (P=0.0035 by a 2-sample t test). Second, CD4−8− T cells from 6 subjects of each set were incubated with autologous CD4 + CD8 T cells at the same level of 5% of the total T cells on adherent anti-CD3 plus anti-CD28 antibodies for 2 d. Compared to IFN-γ generation by CD4 + CD8 T cells alone, the mixture with 5% CD4−8− T cells generated a maximal mean level 14.3-fold higher for the younger subjects and 6.2-fold higher for the older subjects (P=0.0018 for the difference between younger and older subjects by a 2-sample t test). Thus both the blood level of CD4−8− T cells and the effectiveness of their T-cell cytokine enhancement are significantly lower for older than younger healthy subjects.
DISCUSSION
Previous investigations of CD4−8− T cells have characterized their frequency, tissue distribution, TCRs, distinctive requirements for antigen recognition, special microbicidal capabilities, and greater prevalence in autoimmune diseases. The current results describe, first, their novel array of immunologically relevant surface proteins, second, a unique profile of cytokine products, and, third, their selective capacity to regulate generation of immune cytokines by CD4 and CD8 T cells, but not NK/NKT cells, by cytokine-dependent mechanisms. Our consequent designation of CD4−8− T cells as a regulatory subset is based on their capacity to control cytokine generation by other T cells, while lacking the ability to produce relevant amounts of the major effector cytokines characteristic of their target cells.
Although some immune markers expressed by CD4−8− T cells are the same as those observed on CD4 or CD8 T cells, in aggregate their surface marker phenotype differs substantially from those of CD4 or CD8 T cells (Table 1; Supplemental Fig. 1). The predominance of TCRγδ over TCRαβ on CD4−8− T cells is consistent with their known microbicidal role at body surfaces. The breadth of expression of the CD40 ligand CD154 is slightly greater on unstimulated CD4−8− T cells than CD4 T cells and CD8 T cells. However, activation of the different sets of T cells by anti-TCR antibodies augmented expression of CD154 on CD4−8− T cells by only 3-fold, as contrasted with over 20-fold for CD4 T cells and CD8 T cells. The failure to detect production of functionally significant quantities of IFN-γ or IL-17 by blood CD4−8− T cells distinguishes their effector cytokine repertoire from that of CD4 T cells and CD8 T cells. Instead, CD4−8− T cells generate the novel combination of TGFβ1, MIF, CCL5 (RANTES), and the inhibitor of serine esterases/proteases termed Serpin E1 (Fig. 3), which had been detected predominantly in mononuclear phagocytes but not other immune cells (19, 20). The principal source of each cytokine in mixtures of activated CD4 + CD8 + CD4−8− T cells will depend on the relative number of each type of T cell and the time after stimulation.
The principal immune function of CD4−8− T cells discovered now is their ability to augment generation of IFN-γ by CD4 and CD8 T cells, but not NK/NKT cells (Figs. 1 and 2), and of IL-17 by CD4 T cells or mixtures of CD4 + CD8 T cells (Fig. 1). Elimination of this cytokine-enhancing effect by irradiation of CD4−8− T cells suggested initially a dependence on their synthesis of endogenous cytokines rather than through interacting cell-surface proteins. The capacity of CD4−8− T cells to regulate cytokine generation by CD4 and CD8 T cells is dependent primarily on their secretion of functional levels of the T cell function-enhancing factor MIF and the T cell function-inhibiting factor TGFβ1. Substitution of CD4−8− T-cell-derived cytokines for activated intact CD4−8− T cells demonstrated that MIF or Serpin E1 + RANTES, but not Serpin E1 or RANTES alone, could replace up to 70% of the total cytokine-enhancing ability of their CD4−8− T cell source (Fig. 4). The cytokine-enhancing mechanism of CD4−8− T cells was confirmed by showing that either antibody neutralization of the MIF system, which required antibodies to both MIF and its receptor, or the MIF receptor antagonist Iso-1 significantly suppressed CD4−8− T-cell enhancement of IFN-γ generation by activated CD4 + CD8 T cells (Fig. 5). Immunoneutralization of CD4−8− T cell-derived Serpin E1 further decreased MIF-dependent cytokine-enhancing effectiveness of CD4−8− T cells, suggesting possible concerted enhancing effects of the 2 mediators (Fig. 5). Serpin E1 is derived solely from CD4−8− T cells and MIF predominantly from CD4−8− T cells (Table 2), but all T cells in an activated mixture may contribute significantly to the effective concentrations of RANTES and TGFβ1. The relative involvement of each type of T cell in establishing the concentrations of these two cytokines will depend on the ratio of CD4−8− T cells to CD4 + CD8 T cells and the time after activation. There is a possibility of different ratios of CD4−8−T cells to CD4 + CD8 T cells in distinct immunological domains, as, for example, the likelihood that this ratio would be much higher just beneath body surfaces than in lymph and lymph nodes.
High levels of Serpin E1 are found in macrophages, which increase after macrophage exposure to diverse stimuli (19, 20). In contrast, only one study detected Serpin E1 in extracts of human blood lymphocytes but did not examine distribution in different sets of lymphocytes or release with immune stimulation (21). Now we show that Serpin E1 is a major cytokine product of human CD4−8− T cells, whereas no Serpin E1 was generated by CD4 and CD8 T cells (Fig. 3). The few previous investigations of effects of Serpin E1 on T-cell immunity have been carried out in mouse models of allergic diseases, such as airway challenge with ovalbumin (OA) of mice sensitized systemically with OA. OA challenge evoked respiratory symptoms, anti-OA IgE antibody responses, airway eosinophilia, and splenocyte generation of IL-4 and IL-5 in wild-type mice, but not mice genetically deficient in Serpin E1, suggesting that Serpin E1 normally enhances Th2 cell activities (22). Inversely, Serpin E1-deficient mice produced more anti-OA IgG antibody and their splenocytes higher levels of IFN-γ than in wild-type mice, indicating that Serpin E1 usually suppresses Th1 activity in these systems. In support of Th1 cell inhibition by Serpin E1 are findings of higher levels of IFN-γ in plasma of Serpin E1-deficient mice and in their splenocyte cultures than in wild-type mice following challenge with staphylococcal enterotoxin B (23). These results conflict with our findings of a direct facilitatory role of human CD4−8− T-cell-derived Serpin E1 in enhancement of generation of IFN-γ by human CD4 T cells and CD8 T cells stimulated through their TCRs (Figs. 4 and 5).
The ability of MIF and RANTES from CD4−8− T cells to enhance generation of IFN-γ by CD4 and CD8 T cells is not unexpected, based on their known individual capacities to augment production of other T-cell cytokines at concentrations attained in cultures of human CD4−8− T cells. It had been shown more than a decade ago that the Th2 subset of CD4 T cells is a greater source of MIF than the Th1 subset (24). Our current findings now prove that CD4−8− T cells generate an order of magnitude more MIF per cell than CD4 + CD8 T cells, and more MIF than Th2 cells (Fig. 3). The roles of MIF in innate immunity, delayed-type hypersensitivity, and inflammation have been comprehensively defined, but less is known about how MIF participates in T-cell and B-cell components of adaptive immunity (25,26,27,28). MIF is required for optimal mouse T-cell proliferation and IL-2 production in response to stimulation with antigen or mitogen in vitro, maximal T-cell-dependent antibody generation in vivo, B-cell proliferation and survival necessary for maintenance of a mature B cell population, and antibody and Th2 cytokine responses in immune tissue inflammation (24, 29, 30). Now it also is clear that CD4−8− T cell-derived MIF alone and at low concentrations in combination with Serpin E1 also is required for maximal enhancement of production of IFN-γ and IL-17 by CD4 and CD8 T cells stimulated through their TCRs (Figs. 4 and 5).
It also had been shown that RANTES is one of the CCL chemokines that costimulates proliferation and IL-2 production by human T cells activated with anti-TCR antibodies (31). In mice genetically deficient in RANTES, there are striking decreases in proliferative responses and generation of IL-2 and IFN-γ by T cells stimulated with antigen or anti-TCR antibodies, which supports a requisite costimulatory role for RANTES (32). Now it has been demonstrated that a combination of RANTES and Serpin E1 from CD4−8− T cells is involved in enhancing production of IFN-γ and IL-17 by CD4 + CD8 T cells stimulated through their TCRs (Fig. 4). Mechanisms necessitating combinations of Serpin E1 with MIF or RANTES for optimal augmentation of T cell cytokine production have not been elucidated. The suppression of cytokine generation by TGFβ1 has no distinctive features, beyond what was known, when it is derived from CD4−8− T cells and/or CD4 or CD8 T cells in an activated mixture (Fig. 5).
The broader immunological and physiological significance of increases in CD4−8− T cells and their immune activities observed in some autoimmune diseases or of the decreases in CD4−8− T cells and their T-cell cytokine-enhancing effects that we now have found in human aging have not been elucidated. It is likely that altered proportions and/or activities of CD4−8− T cells to CD4 + CD8 T cells in autoimmunity and aging would at a minimum modify the generation of inflammatory cytokines by CD4 and CD8 T cells. Delineation of the mechanisms of consequent pathogenetic effects or deficiencies in host defense awaits further investigation.
Acknowledgments
This research was supported by a grant from the Kenneth Rainin Foundation and by U.S. National Institutes of Health RO-1 grant HL31809. The authors are grateful to Judith H. Goetzl for preparation of the figures and tables. The authors declare no conflicts of interest. Authors’ contributions: D.D.T., D.L.L., and E.J.G. designed research; M.-C. H., K.P., and E.J.G. performed research and analyzed data; E.J.G., M.-C. H., and D.L.L. wrote the paper.
References
- Lanier L L, Ruitenberg J J, Phillips J H. Human CD3+ T lymphocytes that express neither CD4 nor CD8 antigens. J Exp Med. 1986;164:339–344. doi: 10.1084/jem.164.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londei M, Verhoef A, De Berardinis P, Kissonerghis M, Grubeck-Loebenstein B, Feldmann M. Definition of a population of CD4−8− T cells that express the alpha beta T-cell receptor and respond to interleukins 2, 3, and 4. Proc Natl Acad Sci U S A. 1989;86:8502–8506. doi: 10.1073/pnas.86.21.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyamada N, Ohteki T, Abo T, Fukumori T, Ohkouchi N, Satomi S, Taguchi Y, Kusumi A, Mori S, Kumagai K. Induction of specific tolerance by hepatic double-negative CD4−8− alpha beta T cells of mice immunized with allogeneic cells via the portal vein in vivo [corrected] Cell Immunol. 1993;149:107–116. doi: 10.1006/cimm.1993.1140. [DOI] [PubMed] [Google Scholar]
- Pawankar R, Okuda M. A comparative study of the characteristics of intraepithelial and lamina propria lymphocytes of the human nasal mucosa. Allergy. 1993;48:99–105. doi: 10.1111/j.1398-9995.1993.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh C, Sprent J. Aging leads to disturbed homeostasis of memory phenotype CD8(+) cells. J Exp Med. 2002;195:283–293. doi: 10.1084/jem.20011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S, Morita C T, Brenner M B. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Thomssen H, Ivanyi J, Espitia C, Arya A, Londei M. Human CD4−CD8− alpha beta + T-cell receptor T cells recognize different mycobacteria strains in the context of CD1b. Immunology. 1995;85:33–40. [PMC free article] [PubMed] [Google Scholar]
- Haque M A, Kimoto M, Inada S, Tokunaga O, Kohashi O. Autoreactive CD4− CD8− alpha beta T cells to vaccinate adjuvant arthritis. Immunology. 1998;94:536–542. doi: 10.1046/j.1365-2567.1998.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S C, Hamilton E, Frelinger J A, Su J, Forman J, Elkins K L. CD4−CD8− T cells control intracellular bacterial infections both in vitro and in vivo. J Exp Med. 2005;202:309–319. doi: 10.1084/jem.20050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney K M, Kumar R, Purins A, Mundy L, Ferguson W, Shaw D, Burrell C J, Li P. HIV type 1 persistence in CD4− /CD8− double negative T cells from patients on antiretroviral therapy. AIDS Res Hum Retroviruses. 2006;22:66–75. doi: 10.1089/aid.2006.22.66. [DOI] [PubMed] [Google Scholar]
- Anand A, Dean G S, Quereshi K, Isenberg D A, Lydyard P M. Characterization of CD3+ CD4− CD8− (double negative) T cells in patients with systemic lupus erythematosus: activation markers. Lupus. 2002;11:493–500. doi: 10.1191/0961203302lu235oa. [DOI] [PubMed] [Google Scholar]
- Crispin J C, Oukka M, Bayliss G, Cohen R A, Van Beek C A, Stillman I E, Kyttaris V C, Juang Y T, Tsokos G C. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei M, Bach S, Di Cesare S, Fraziano M, Placido R, Poccia F, Sammarco I, Moras A M, Bardone M R, Colizzi V. CD4−8− T-cells increase in MRI/lpr mice treated with thymic factors. Int J Immunopharmacol. 1994;16:651–658. doi: 10.1016/0192-0561(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Sneller M C, Wang J, Dale J K, Strober W, Middelton L A, Choi Y, Fleisher T A, Lim M S, Jaffe E S, Puck J M, Lenardo M J, Straus S E. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. [PubMed] [Google Scholar]
- Straus S E, Sneller M, Lenardo M J, Puck J M, Strober W. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann Intern Med. 1999;130:591–601. doi: 10.7326/0003-4819-130-7-199904060-00020. [DOI] [PubMed] [Google Scholar]
- Dixit V D, Yang H, Sun Y, Weeraratna A T, Youm Y H, Smith R G, Taub D D. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Pigeon H, Jean C, Charruyer A, Haure M J, Baudouin C, Charveron M, Quillet-Mary A, Laurent G. UVA induces granzyme B in human keratinocytes through MIF: implication in extracellular matrix remodeling. J Biol Chem. 2007;282:8157–8164. doi: 10.1074/jbc.M607436200. [DOI] [PubMed] [Google Scholar]
- Wadgaonkar R, Somnay K, Garcia J G. Thrombin induced secretion of macrophage migration inhibitory factor (MIF) and its effect on nuclear signaling in endothelium. J Cell Biochem. 2008;105:1279–1288. doi: 10.1002/jcb.21928. [DOI] [PubMed] [Google Scholar]
- Liao H, Hyman M C, Lawrence D A, Pinsky D J. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1alpha, and C/EBPalpha. FASEB J. 2007;21:935–949. doi: 10.1096/fj.06-6285com. [DOI] [PubMed] [Google Scholar]
- Ngiam N, Post M, Kavanagh B P. Early growth response factor-1 in acute lung injury. Am J Physiol. 2007;293:L1089–L1091. doi: 10.1152/ajplung.00265.2007. [DOI] [PubMed] [Google Scholar]
- Kowalewska A, Rosc D, Szadziewska-Kowalska D, Goralczyk K. [T-PA and PAI-1 in lymphocytes of patients with chronic lymphoid leukemia] Pol Arch Med Wewn. 2006;115:197–202. [PubMed] [Google Scholar]
- Sejima T, Madoiwa S, Mimuro J, Sugo T, Okada K, Ueshima S, Matsuo O, Ishida T, Ichimura K, Sakata Y. Protection of plasminogen activator inhibitor-1-deficient mice from nasal allergy. J Immunol. 2005;174:8135–8143. doi: 10.4049/jimmunol.174.12.8135. [DOI] [PubMed] [Google Scholar]
- Renckens R, Pater J M, van der Poll T. Plasminogen activator inhibitor type-1-deficient mice have an enhanced IFN-gamma response to lipopolysaccharide and staphylococcal enterotoxin B. J Immunol. 2006;177:8171–8176. doi: 10.4049/jimmunol.177.11.8171. [DOI] [PubMed] [Google Scholar]
- Bacher M, Metz C N, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhagen J, Bacher M, Calandra T, Metz C N, Doty S B, Donnelly T, Bucala R. An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996;183:277–282. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz C N, Bucala R. Role of macrophage migration inhibitory factor in the regulation of the immune response. Adv Immunol. 1997;66:197–223. doi: 10.1016/s0065-2776(08)60598-2. [DOI] [PubMed] [Google Scholar]
- Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D F, Horak K. Macrophage migration inhibitory factor: controller of systemic inflammation. Crit Care. 2006;10:138–140. doi: 10.1186/cc4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizue Y, Ghani S, Leng L, McDonald C, Kong P, Baugh J, Lane S J, Craft J, Nishihira J, Donnelly S C, Zhu Z, Bucala R. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A. 2005;102:14410–14415. doi: 10.1073/pnas.0507189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore Y, Starlets D, Maharshak N, Becker-Herman S, Kaneyuki U, Leng L, Bucala R, Shachar I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- Taub D D, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- Makino Y, Cook D N, Smithies O, Hwang O Y, Neilson E G, Turka L A, Sato H, Wells A D, Danoff T M. Impaired T cell function in RANTES-deficient mice. Clin Immunol. 2002;102:302–309. doi: 10.1006/clim.2001.5178. [DOI] [PubMed] [Google Scholar]