Abstract
We have shown previously that prostatic stem/progenitor cells can be purified from isolated prostate ducts, based on their high expression of the Sca-1 surface antigen. We now report that high levels of aldehyde dehydrogenase (ALDH) activity are present in a subset of prostate epithelial cells that co-express a number of antigens found on stem/progenitor cells of other origins (CD9, Bcl-2, CD200, CD24, prominin, Oct 3/4, ABCG2 and nestin). Almost all of these ALDH hi cells also express Sca-1 and a third of them express high levels of this antigen. The ALDH hi cells have greater in vitro proliferative potential than cells with low ALDH activity. Importantly, in an in vivo prostate reconstitution assay, the ALDH hi expressing cell population was much more effective in generating prostatic tissue than a population of cells with low enzymatic activity. Thus, high levels of ALDH activity can be considered a functional marker of prostate stem/progenitor cells and allows for simple, efficient isolation of cells with primitive features. The elucidation of the role of ALDH in prostate stem/progenitor cells may lead to the development of rational therapies for treating prostate cancer and benign prostatic hyperplasia.
Keywords: Adult stem cells, FACS, progenitor cells, Sca-1
Introduction
Aldehyde dehydrogenases (ALDHs) are a family of enzymes that oxidize aldehydes. Different ALDH family members play diverse roles in detoxification pathways and retinoic acid biosynthesis, as well as folate, amino acid, ethanol and cyclophosphamide metabolism [1]. Stem cells from a variety of tissues express high levels of ALDH activity and this may be a characteristic of “stemness” [2, 3]. Hematopoietic and neural stem cells are enriched in cells expressing high levels of ALDH activity (ALDH hi cells) [4–7] and ALDH hi cells with low side scatter are self-renewing and multipotent [6, 8]. The adipose-derived stem cell population also contains a subset of ALDH hi cells [9] and the ALDH hi subpopulation of bone marrow cells is enriched in hematopoietic, mesenchymal and endothelial progenitor cells [10], indicating that high ALDH activity may be a general characteristic of stem/progenitor cells.
Normal stem cells and tumor cells have many common features [11], including expression of high levels of ALDH. Both normal and malignant human mammary [12, 13] and colon [14] stem/progenitor cells can be detected based on ALDH-1 activity. High ALDH activity has also been used as a marker for cancer stem/progenitor cells in prostate cancer cell lines [15]. Tumor initiating cells from colorectal cancer xenografts have also been isolated based on levels of ALDH activity [16] and a small fraction of cells from retinoblastoma tumors express the stem cell markers ABCG2, ALDH1 and Sca-1 [17]. Thus, the expression of high levels of ALDH activity may identify both tumor-initiating and normal stem cells.
We have shown that prostatic stem cells are concentrated in the proximal region of prostatic ducts [18] and can be purified from this region by virtue of their high expression of the Sca-1 surface antigen [19]. We now show that prostate stem/progenitor cells also express high levels of ALDH activity and that almost all of these cells co-express Sca-1. Furthermore, the ALDH hi cells have higher in vitro and in vivo proliferative potential than cells expressing low levels of this enzyme.
Materials and Methods
Animals
C57BL/6 mice, athymic nude mice and CDIGS rats were housed in the animal research facilities of the University of Cape Town or New York University and all experiments were performed in compliance with institutional review board requirements.
Cell preparation
Animals (6 to 8-week old C57BL/6 mice) were sacrificed and the urogenital tract was removed en block into Hank’s balanced salt solution (HBSS), pH 7.4. The dorsal, ventral, lateral and anterior prostates were dissected under a dissecting microscope using 25 gauge needles. In some instances, only the proximal region of prostatic ducts (those ducts nearest the urethra) was harvested [18, 19]. Cells were dissociated by incubation with 0.5% collagenase Type II (Sigma-Aldrich, St Louis, MO) in HBSS plus 7.5% fetal calf serum (FCS) for 45 min at 37°C, followed by digestion in 0.05% trypsin for 8 min at 37°C.
Separation of ALDH hi and ALDH lo cells
Cell digests were treated with lysing solution to lyse red blood cells (NH4Cl 0.15M, KHCO3 10mM, EDTA 0.1mM), washed with HBSS plus 5% FCS, resuspended in Aldefluor® buffer (Aldagen Inc., Durham, NC) and passed through a 40 μm nylon cell strainer (BD Biosciences, Bedford, MA). Cell viability was determined by Trypan Blue exclusion and cells were incubated with Aldefluor substrate for 30 min at 37°C, with and without the ALDH inhibitor, diethylaminobenzaldehyde (DEAB), according to the manufacturer’s instructions. Aldefluor substrate, buffer and DEAB is supplied in kit form by Aldagen. The substrate is converted by ALDH into a green fluorescent product that is retained in the cell and detected in the FITC channel. After incubation, the cells were kept on ice and either separated into ALDH hi and ALDH lo populations by a FACS Vantage SE cell sorter (Becton-Dickinson, San Jose, CA) for in vitro and in vivo growth analysis or incubated with antibodies or isotype-matched immunoglobulins for fluorescence activated cell sorter (FACS) analysis, to determine the co-expression of ALDH activity with various other antigens (see below).
Flow Cytometry
Cell digests were resuspended in Aldefluor buffer or in FACS buffer (phosphate buffered saline (PBS) containing bovine serum albumin (0.1%), sodium azide (0.01%) and aprotinin (20μg/ml)). Fc receptors were blocked with mouse CD16/32 antibodies and rat IgG and the cells were incubated with antibody or control IgG for 30 min on ice and washed with FACS buffer. In some experiments, the dye 7-aminoactinomycin D (7-AAD) (1 μg/ml) was added 5 min prior to analysis, so that dead cells could be excluded. Expression of intracellular antigens, such as Bcl-2, ALDH1/2, ALDH3A1, ABCG2, Oct3/4, nestin, CK5 and CK8 were determined in paraformaldehyde fixed cells, permeabilized with 0.2% Tween20 (Merck-Schuchardt, Hohenbrunn, Germany) in PBS. Cells were analyzed on a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA), using CellQuest software (Becton-Dickinson, San Jose, CA). Sca-1+ cells with fluorescent intensities in the upper 1/3 were defined as Sca-1 hi cells.
Antibodies and control immunoglobulins (IgGs)
Rat anti-mouse CD9 biotin (clone KMC8) was obtained from BD Biosciences. Phycoerythrin (PE) conjugated rat anti-mouse Sca-1 (clone D7), rat anti-mouse Sca-1 biotin (clone D7), rat anti-CD24 biotin (clone CT-HAS), rat IgG2a biotin, rat IgG2b biotin, rat IgM biotin, rat IgG2a PE, rat IgG, mouse anti-mouse CD16/32 and streptavidin conjugated allophycocyanin (SA-APC) were from Caltag Laboratories, Burlingame, CA. Rabbit anti-nestin (clone H85), rabbit anti-ABCG2 (clone M-70), rabbit anti-Oct 3/4 (clone H134), rabbit anti-Bcl-2 (clone N-19), rabbit anti-ALDH1/2 (clone H-85) and rabbit anti-ALDH3A1 (clone M-76) were purchased from Santa Cruz Biotechnology Inc., Santa Cruz, CA. Goat anti EpCam biotin and rat IgG2a biotin (clone 54447) were from R&D Systems Inc., Minneapolis, MN. Rat anti-prominin1 biotin (clone 13A4) and rat IgG1 biotin (clone eBRG1) were from eBioscience, Inc., San Diego, CA. Rat anti-mouse CD200 biotin (clone OX-90) was from AbD Serotec, Oxford, UK. Rabbit anti-mouse CK5 (clone AF138) was from Covance, Berkeley, CA., and mouse anti-mouse CK8 (clone Ks8.7) was from Fitzgerald Industries International Inc., Concord, MA. Mouse IgG1 and rabbit anti-mouse PE-Cy5 were obtained from Dako A/S, Glostrup, Denmark, goat anti-rabbit APC from Southern Biotech, Birmingham, AL and SA AlexaFluor 488 from Molecular Probes, Inc., Eugene, St Louis, MO.
In vitro growth of ALDH hi and ALDH lo cells
ALDH hi and ALDH lo populations were collected from the cell sorter into medium (HBSS, 25mM Hepes, 1mM EDTA, 2% BSA) and assessed for viability using Trypan Blue exclusion. For each experiment, cells (1,000–5,000 viable cells/well) were seeded in triplicate or quadruplicate in collagen coated 96 well plates, (PureCol: INAMED, Fremont, CA) in DMEM/F12 (1:1) (Gibco: Invitrogen Ltd., Paisley, UK) containing 10% FCS, basic fibroblast growth factor-2 (FGF-2) (10 ng/ml), glutamine (2mM), penicillin (100U/ml) and streptomycin (100 μg/ml). Half the medium was removed and replaced every 2–3 days and cell number was determined after approximately 13 days.
Implantation of grafts under the renal capsule
FACS sorted ALDH hi and ALDH lo cells (4 × 104) were combined with urogenital sinus mesenchyme (UGM) cells (2.5 × 105) and resuspended in 30μl of Type 1 collagen (BD Biosciences). The collagen grafts were inserted under the renal capsule [20]. Each experiment contained grafts of UGM alone to insure that tissue growth did not result from contaminating urogenital sinus epithelial cells. Grafts were harvested and weighed after 10 weeks. UGM was isolated from the urogenital sinus of 18 day old embryos from CDIGS rats [20–22].
Immunohistochemistry of xenografts
Grafts were fixed in 3% paraformaldehyde and embedded in paraffin. Immunohistochemistry was performed as described previously [23]. Antibodies used for immunocytochemistry were: (a) for staining of basal cells:- rabbit anti-mouse CK5 (clone AF138) (1:200) from Covance, Berkeley, CA., followed by goat anti-rabbit AlexaFluor 594 (1:2000) from Molecular Probes, Inc., Eugene, St Louis, MO.; (b) for luminal cells:- mouse anti-mouse CK8 (clone Ks8.7) (undiluted) from Fitzgerald Industries International Inc., Concord, MA., followed by goat anti-mouse AlexaFluor 488 (1:2000) from Molecular Probes; (c) for neuroendocrine cells:- mouse anti-synaptophysin (clone SY38) (1:500) from Chemicon, Temecula, CA., followed by goat anti-mouse AlexaFluor 488 (1:2000) from Molecular Probes; (d) for prostate secretions:- rabbit polyclonal antibodies against dorsal prostate secretions (1:2500), a kind gift of Dr. C. Abate-Shen (Columbia University, New York, NY.), followed by goat anti-rabbit HRP (1:1000) from G E Healthcare UK Ltd., Little Chalfont, UK and detected using a SK4100 DAB detection kit (Vector Laboratories, Burlingame, CA); (e) for prostate specific proteins:- Nxk3.1 (1:600) was a gift from Dr. C. Abate-Shen, followed by ABC kit from Vector Laboratories. The specificity of staining was ascertained on sections using non-immune serum or IgG in place of primary antibodies.
Statistical analysis
The results are depicted as the means and standard deviations of each set of data. Comparisons between groups were made using the 2-tailed, paired Student’s t test or the Mann Whitney U test. A p value of < 0.05 is considered statistically significant.
Results
A minor population of murine prostate cells express high levels of ALDH activity
As stem cells from other origins express high levels of ALDH activity [4–10, 12], we examined the activity of this enzyme in prostate cells. Cells from prostate digests were incubated with Aldefluor substrate (this substrate is converted into a green fluorescent product by ALDH) in the presence and absence of dimethlyaminobenzaldehyde (DEAB: 10 μM), a potent inhibitor of ALDH, in order to determine the fraction of cells that express this enzyme. We found that 8.1 ± 2.1% of cells display high levels of ALDH activity, with the ALDH hi population having a mean fluorescence intensity (MFI) 5.2 fold higher (MFI = 348 ± 96) (Fig. 1B, R1,) than the ALDH lo population (MFI = 67 ± 34) (Fig. 1B, R2,) (p < 0.001; n = 7) (Fig. 1 depicts one of seven experiments), indicating that a subset of prostate cells express high levels of ALDH enzymatic activity. The majority of ALDH hi cells have medium to low side scatter properties (Fig. 1B, R1), indicating that the cells have medium to low granularity or cellular complexity. Phase contrast microscopy of DEAB treated (Fig. 1C) or FACS sorted ALDH hi (Fig. 1E) cells show that they are both composed of small round cells with an approximate diameter of 10 μm. Similarly, there is no difference in the sizes of the ALDH hi and ALDH lo cells (Supplementary Fig. S1). Under fluorescence microscopy, cells treated with DEAB exhibit no fluorescence (Fig. 1D), whereas in the absence of inhibitor ALDH hi sorted cells show bright green fluorescence (Fig. 1F), indicating that they contain high levels of ALDH activity.
Fig. 1. The prostate contains cells with high ALDH activity.

Prostate cell digests were incubated with Aldefluor in the presence (A) or absence (B) of DEAB. (A) Aldefluor substrate plus the inhibitor, DEAB, was used to establish baseline fluorescence (few cells in R1). (B) High levels of ALDH were expressed by ~8% of cells (R1). R2 denotes ALDH lo cells. Phase contrast photographs of cells in the presence (C) and absence (E) of DEAB show small round cells that under fluorescence microscopy exhibit no fluorescence in the presence of inhibitor (D) but bright green fluorescence in the absence of the inhibitor (F). Scale bars = 10 μm.
As we have shown that cells with stem cell features (quiescence, high proliferative potential) are concentrated in the proximal region of prostatic ducts [18], we determined the activity of ALDH in proximal cells compared with cells isolated from the whole prostate. We found that the proximal region contained 2.2 fold more cells (p < 0.01) expressing high levels of ALDH activity than cells isolated from the whole prostate (proximal region: 17.6 ± 3.6%, n = 4; whole prostate: 8.1 ± 2.1%, n = 7), indicating that ALDH hi cells are concentrated proximally (see Supplementary Fig. S2 for a representative plot). However, as we obtained more ALDH hi cells from digests of the whole prostate than from only the proximal region of ducts, we used cells from the whole prostate for phenotypic and growth assays, in order to maximize the total yield of ALDH hi cells.
ALDH hi cells are of epithelial origin and express antigens present on stem/progenitor cells of other origins
In order to determine if ALDH hi cells were of epithelial origin and contained markers expressed by stem cells of other origins, we examined these cells for the expression of EpCam, a marker of epithelial cells [24], as well as for antigens present in stem cells. We found that almost all (98.1 ± 1.5%, n = 3) ALDH hi cells co-express EpCam, confirming the epithelial nature of these cells (Fig. 2B, C). In addition, 70.2 ± 10.0% (n = 5) of ALDH hi cells express the basal cell cytokeratin, CK5, indicating that most of the ALDH hi cells are of basal origin [25], while 31.0 ± 20.8% (n = 3) express the luminal cell marker, CK8 (Fig. 2C). These data show that 2.3 fold more cells with high ALDH activity express CK5 than CK8 (p = 0.03).
Fig. 2. ALDH hi cells express epithelial antigens as well as antigens expressed by stem/progenitor cells.

ALDH hi cells were stained with (A) goat IgG biotin or (B) goat anti EpCam antibody, followed by SA-APC. Plots are representative of three experiments. (C) ALDH hi cells express EpCam, CK5, CK8 and antigens present in stem cells of other origins. Data represent the mean and standard deviations from two or more experiments. Using rat IgG2a APC as the isotype control antibody (D), FACS analysis of ALDH hi cells shows that almost all (92%) ALDH hi cells express Sca-1 (E) and that 36% of ALDH hi cells express high levels of Sca-1 (G). Sca-1 data represent one of eight experiments.
We found that 93.7 ± 2.4% (n = 8) of ALDH hi cells expressed Sca-1 (Fig. 2C, E). Sca-1 is present on prostate stem cells [19, 26] as well as on stem cells from a variety of tissues, such as hematopoietic, cardiac, mammary gland, skin, muscle and testis [27]. Importantly, approximately 40% (36.3 ± 5.4%, n = 8) of ALDH hi cells express high levels of Sca-1 (Sca-1 hi) (Fig. 2C, G). We also found that 34.6 ± 3.6% (n = 3) of Sca-1 hi cells express high levels of ALDH activity. As prostatic cells with high levels of Sca-1 have high in vivo prostate regenerating potential [19], this indicates that the ALDH hi population is considerably enriched in stem cells.
Analysis of the expression of ALDH1/2 (74.8 ± 6.8%, n = 4) and ALDH3A1 (31.3 ± 17.0%, n = 3) (Fig. 2C) confirms that cells isolated based on high levels of ALDH enzymatic activity express various aldehyde dehydrogenase isozyme proteins (the ALDH1/2 antibody detects ALDH1A1, ALDH1A2 and ALDH1A3 as well as ALDH2). ALDH3A1 and ALDH2 are involved in detoxification processes, ALDH1A1 is active in both detoxification and the retinoic acid pathway, while ALDH1A2 and ALDH1A3 participate in the retinoic acid signalling pathway [1].
The majority of ALDH hi cells also express high levels of CD9 (97.5 ± 2.1%, n = 3) (Fig. 2C), a protein present on embryonic [28] and spermatogonial stem cells [29], as well as neural progenitor cells [30]. Bcl-2, an anti-apoptotic protein [31], that is expressed by prostate, hematopoietic, keratinocyte and colon stem cells [19, 32–35], is present in 82.7 ± 5.8% (n = 4) of ALDH hi cells (Fig. 2C). CD200, an antigen expressed by hair follicle [36, 37] and mesenchymal stem cells [38] is found on 70.5 ± 5.7% of ALDH hi cells (n = 4) (Fig. 2C). Almost half of the ALDH hi cells express Oct 3/4 (47.7 ± 2.5%, n = 2), while 42.9 ± 13.5% (n = 5) express ABCG2, both of which are present in primitive cells [39–42]. Approximately a third (37.3 ± 10.4%, n = 3) of ALDH hi cells co-expressed CD24, an antigen present on murine mammary stem cells [43], while prominin, a protein present on hematopoietic [44] and neural stem cells [45], as well as on primitive prostate cells [46, 47], is expressed by 32.3 ± 5.6% of ALDH hi cells (n = 3) (Fig. 2C). A small percentage of ALDH hi cells (6.8 ± 4.9%, n = 4) express nestin, a protein present on stem cells of prostate and neural origin [48, 49] (Fig. 2C).
We next compared the expression of the various antigens found on ALDH hi cells with those on cells with low ALDH activity (Table 1). The expression of ALDH1/2 was increased 1.8 fold (p <0.05) in the ALDH hi population compared with the ALDH lo population, demonstrating that ALDH 1/2 protein levels correlate with ALDH enzymatic activity (Table 1). CK5 expression was also increased 1.8 fold (p <0.05) in the ALDH hi population, showing that this population is enriched in basal cells (Table 1). The expression of Sca-1 was increased 1.6 fold (p <0.01), while the fraction of cells with high levels of Sca-1 was increased 1.8 fold (p <0.01) in the ALDH hi samples (Table 1), confirming that primitive Sca-1 hi cells are concentrated in the population with high levels of ALDH activity. The ALDH hi population was also enriched in cells expressing Bcl-2 (2.0 fold; p <0.05), Oct 3/4 (2.1 fold; p <0.01), ABCG2 (2.5 fold; p <0.05), CD24 (2.7 fold; p <0.05) and CD200 (2.9 fold; p <0.001) compared with the ALDH lo population (Table 1). Representative dot plots of the expression of surface antigens together with the levels of Aldefluor present in ALDH hi versus ALDH lo cells, as well as isotype controls, are presented in Supplementary Fig. S3. For FACS analysis of the intracellular antigens, the ALDH hi and ALDH lo populations were first separated by FACS sorting, with subsequent permeabilization of the cells prior to antigen visualization (Supplementary Fig. S4). This was done as permeabilization releases the Aldefluor substrate from the cells. Thus, it is not possible to simultaneously visualize ALDH activity in conjunction with intracellular antigens by FACS.
Table 1.
The expression of the indicated antigens by ALDH hi cells compared with the expression of these antigens by ALDH lo cells
| Antigen | ALDH hi cells | ALDH lo cells | Fold difference | p value | ||
|---|---|---|---|---|---|---|
| Expression (%) | n | Expression (%) | n | |||
| EpCam | 98.1 ± 1.5 | 3 | 83.9 ± 4.1 | 2 | 1.2 | ns |
| CK5 | 70.2 ± 10.0 | 5 | 38.0 ± 13.1 | 3 | 1.8 | <0.05 |
| CK8 | 31.0 ± 20.8 | 3 | 25.5 ± 21.1 | 2 | 1.2 | ns |
| Sca-1 | 93.7 ± 2.4 | 8 | 59.2 ± 2.9 | 4 | 1.6 | <0.01 |
| Sca-1 hi | 36.3 ± 5.4 | 8 | 20.1 ± 3.6 | 4 | 1.8 | <0.01 |
| ALDH 1/2 | 74.8 ± 6.8 | 4 | 42.6 ± 21.4 | 4 | 1.8 | <0.05 |
| ALDH 3A1 | 31.3 ± 17.0 | 3 | 23.5 ± 15.1 | 3 | 1.3 | ns |
| CD9 | 97.5 ± 2.1 | 3 | 90.4 ± 3.6 | 2 | 1.1 | ns |
| Bcl-2 | 82.7 ± 5.8 | 4 | 40.7 ± 21.0 | 4 | 2.0 | <0.05 |
| CD200 | 70.5 ± 5.7 | 4 | 24.4 ± 2.9 | 4 | 2.9 | <0.001 |
| Oct 3/4 | 47.7 ± 2.5 | 2 | 22.5± 3.0 | 2 | 2.1 | <0.01 |
| ABCG2 | 42.9 ± 13.5 | 5 | 17.3 ± 6.5 | 3 | 2.5 | <0.05 |
| CD24 | 37.3 ± 10.4 | 3 | 13.7 ± 3.8 | 3 | 2.7 | <0.05 |
| Prominin | 32.3 ± 5.6 | 3 | 23.2 ± 2.5 | 3 | 1.4 | ns |
| Nestin | 6.8 ± 4.9 | 4 | 7.4 ± 1.0 | 2 | 1.1 | ns |
n = number of experiments
ns = not significant
These data indicate that ALDH hi cells are epithelial in nature, predominantly basal and express a number of surface and intracellular antigens that are present on stem/progenitor cells of various origins. In addition, approximately a third of ALDH hi cells express high levels of Sca-1, a marker of prostate stem cells [19] and about a third of Sca-1 hi cells express high levels of ALDH. Compared with ALDH lo cells, the ALDH hi population is enriched in basal cells, in cells expressing high levels of Sca-1, ALDH 1/2, as well as a number of antigens expressed by stem/progenitor cells, such as Bcl-2, ABCG2, CD200, Oct 3/4 and CD24. Importantly, the results show for the first time, that ALDH activity is a functional marker of prostate epithelial cells that also express antigens associated with a primitive phenotype.
ALDH hi cells have greater proliferative potential in vitro than ALDH lo cells
As an important feature of stem cells is their high proliferative potential, we compared the growth potential of ALDH hi and ALDH lo cells. Prostate cells were isolated by FACS sorting based on the expression of high or low levels of ALDH activity (Fig. 1) and their growth on collagen films was compared. ALDH hi cells showed greater growth potential (3.3 fold; p = 0.012; n = 8) than ALDH lo cells (Fig. 3 depicts one such individual experiment). However, as the difference between the in vitro growth of the two ALDH populations could be due to a difference between the rate of proliferation rather than to a difference in growth potential, we compared the ability of these two populations to regenerate prostate tissue in vivo.
Fig. 3. ALDH hi cells have higher in vitro proliferative potential than ALDH lo cells.
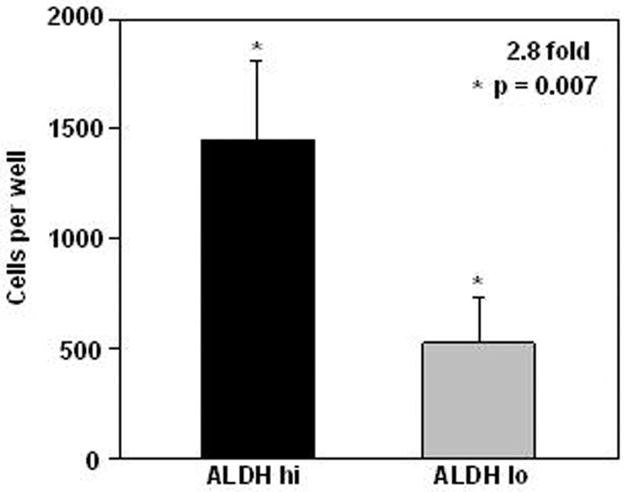
ALDH hi and ALDH lo cells were seeded in quadruplicate at 1,000 viable cells per well in collagen coated wells and cells were enumerated after 20 days. Data represent one of eight experiments.
ALDH hi cells have greater prostate regeneration capacity than ALDH lo cells
As the ability to regenerate tissue in vivo is a characteristic of stem cells, we determined the in vivo growth potential of ALDH hi cells, compared with that of cells with low levels of this enzyme. ALDH hi and ALDH lo cells were FACS sorted and these populations were combined with UGM cells (inductive mesenchyme for prostatic tissue [20–22]) and inserted under the renal capsule of male animals. Each experiment also contained grafts of UGM alone to control for the possibility that the UGM was not completely depleted of urogenital sinus epithelial cells. The amount of prostatic tissue generated was measured after 10 weeks.
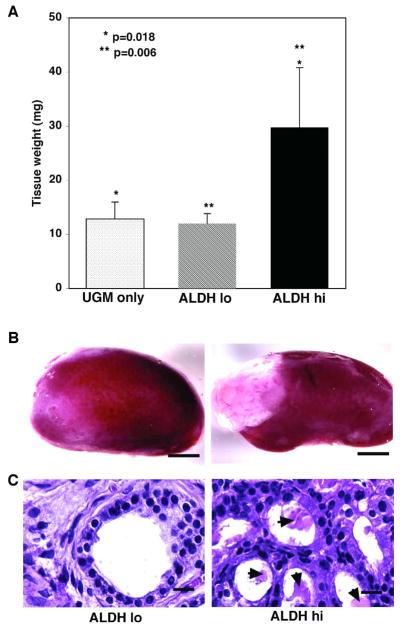
ALDH hi cells formed significantly more prostatic tissue (29.8 ± 11.0 mg: 2.5 fold) than ALDH lo cells (12.0 ± 1.8 mg: p = 0.006) and 2.3 fold more than UGM cells alone (12.9 ± 3.1 mg; p = 0.018) (Fig. 4A, B) (n = 2: 6 kidneys for each population). The prostatic tissue obtained from ALDH hi cells had normal prostatic morphology comprising basal and luminal cells lining ducts containing secretory products, while the sparse tissue arising from ALDH lo cells had minimal ductal structures lacking secretory material (Fig. 4C). UGM cells formed tissue that contained only stromal elements. Interestingly, whereas the prostatic tissue derived from ALDH hi cells contained both basal (CK5+) and luminal (CK8+) cells (Fig. 5A), the ducts formed by ALDH lo cells contained only luminal cells (Fig. 5B). Moreover, synaptophysin expressing cells were present only in grafts arising from ALDH hi cells (Fig. 5C) and not those formed from ALDH lo cells (Fig. 5D), indicating that only ALDH hi cells gave rise to cells of neuroendocrine origin. In addition, although the ductal tissue formed from ALDH hi cells contained abundant secretory products (Fig. 5E), little or no secretory material was present in ducts from ALDH lo grafts (Fig. 5F). However, tissue generated from ALDH hi and ALDH lo cells both show expression of Nkx3.1, indicating their prostatic origin (Fig. 5G and H). The data reveal that the ALDH hi population has the ability to regenerate functional prostatic tissue containing basal, luminal and neuroendocrine cells whereas ALDH lo cells have little growth and limited differentiation potential, indicating that cells with the greatest prostate regenerating ability reside within the compartment that contains high levels of ALDH.
Fig. 4. ALDH hi cells have greater in vivo proliferative potential than ALDH lo cells.
(A) ALDH hi cells formed 2.5 fold more prostatic tissue in an in vivo transplantation assay than ALDH lo cells (** p = 0.006) and 2.3 fold more tissue than UGM alone (* p = 0.018). (B) Prostate tissue formed by ALDH hi versus ALDH lo cells (Scale bars = 2 mm). (C) Paraffin sections stained with hematoxylin and eosin showing the morphology of prostatic tissue arising from ALDH hi or ALDH lo cells. Prostatic tissue obtained from ALDH hi cells had normal prostatic morphology comprising basal and luminal cells lining prostatic ducts, that contained abundant secretory material (arrowheads), while tissue obtained from ALDH lo cells was mainly stromal in nature, showing few ducts and no secretory material (Scale bars = 20 μm).
Fig. 5. ALDH hi cells are able to regenerate secretory prostatic tissue containing basal, luminal and neuroendocrine cells.
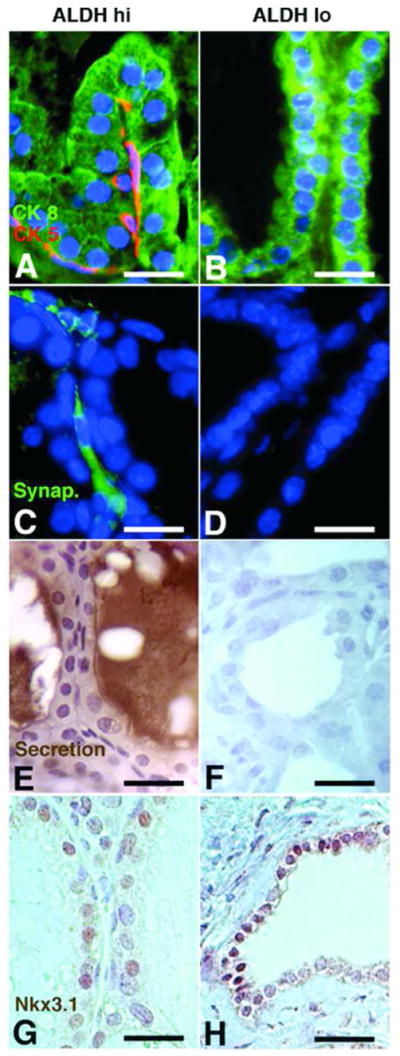
Grafts generated from ALDH hi cells contain cells expressing the cytokeratins, CK5 and CK8 (A), as well as synaptophysin (C). Secretory material is abundant within the ductal lumens of the ALDH hi grafts (E) and the expression of the prostate-specific protein, Nxk3.1 (G) confirms prostate generation. In contrast, the grafts generated from ALDH lo cells contain only CK8+ luminal cells but no CK5+ basal cells (B), no synaptophysin+ cells (D) or secretory products (F), while expressing Nxk3.1 (H). (Scale bars = 50 μm).
Discussion
We show, for the first time, that a subset of normal murine prostatic cells displays high levels of ALDH activity. Hematopoietic, neural, mesenchymal, endothelial, and mammary stem/progenitor cells are enriched in ALDH hi cells [4, 5, 10, 12] and it has been proposed that ALDH activity may be a functional marker of “stemness” [2, 3]. The ALDH hi cells from the prostate express Epcam, indicating that they are epithelial cells [24]. They also express the cytokeratins, CK5 and CK8, present in basal (CK5) and luminal (CK8) epithelial cells, in a ratio of 2.3 to1, indicating that the majority of ALDH hi cells are of basal origin.
Significant numbers of ALDH hi cells co-express antigens that are present on stem/progenitor cells of other origins, such as CD9 [28–30], Bcl-2 [19, 32–35] and CD200 [36–38]. A third to a half of ALDH hi cells express CD24, prominin, Oct 3/4 and ABCG2, antigens found on mammary, hematopoietic, neural and prostate stem cells, as well as by primitive cells exhibiting a side population [39–47], while a small subset of ALDH hi cells express nestin, an antigen also expressed by primitive cells [48, 49]. Compared with the ALDH lo population, ALDH hi cells are significantly enriched in antigens that are expressed by prostate basal epithelium, such as CK5 and Bcl-2, and also in antigens involved with protective and detoxifying functions, such as Bcl-2, ABCG2 and ALDH1/2. This may serve to protect stem/progenitor cells from toxic substances and ensure their long-term survival. The ALDH hi cells are also enriched, compared to ALDH lo cells, in antigens present in stem cells of other origins, such as CD200, Oct3/4 and CD24. In addition, the ALDH hi population contains more cells that express Sca-1, as well as cells with high levels of Sca-1, previously shown to be a feature of prostate stem cells [19], than the population with low ALDH activity. Furthermore, ALDH hi cells are enriched in the proximal region of prostatic ducts, a region in which stem cells are concentrated [18, 19, 50].
Interestingly, we do not find an increase in the expression of prominin/CD133 in the ALDH hi population versus the ALDH lo population. We also note no increase in prominin expression in the stem cell containing Sca-1 hi population versus the Sca-1+ population and prominin expression is not concentrated in the stem cell enriched proximal region of ducts compared with the remaining regions of ducts (unpublished observations). Although prominin has been described as a marker for hematopoietic, neural and prostate stem cells [44–47] as well as colon cancer initiating cells [51, 52], it has been suggested that its expression is not restricted to stem cells [53, 54]. Normal and malignant human colonic stem cells have recently been shown to express both high ALDH protein expression and high ALDH activity [14] and both these properties are more specific for colon stem cells than CD133 expression. Another stem cell marker that has recently been identified on adult murine prostate stem cells is c-kit (CD117) and a whole prostate can be reconstituted from a single cell that, among other surface antigens, also expresses c-kit [46]. It will be interesting to determine the incidence of c-kit expression by ALDH hi cells compared to cells with low ALDH activity.
We show that cells with high ALDH activity have greater in vitro proliferative potential than cells expressing low levels of this enzyme. Importantly, ALDH hi cells have a greater capacity than ALDH lo cells to regenerate prostate tissue in an in vivo transplantation assay. Examination of the tissue regenerated by ALDH hi cells shows the presence of basal, luminal and neuroendocrine cells, as well as prostatic secretory material and the prostate-specific protein, Nxk3.1. These data confirm that ALDH hi cells are able to differentiate into multiple cell types. In contrast, the small amount of tissue derived from ALDH lo cells shows positivity for Nxk3.1 and for the presence of luminal cells, indicating a lack of pluripotency. These observations confirm that the cell population expressing high levels of ALDH enzyme activity contains stem/progenitor cells. ALDH activity may therefore be used as a new functional marker for the isolation of primitive prostate cells.
Although the interactions between Aldefluor substrate and various ALDH isoforms have not been systematically characterized, the substrate is recognized by ALDH1A1 and ALDH3 isoforms but not by mitochondrial forms of ALDH (Aldefluor kit information). It is unknown at present which isozyme(s) (ALDH1A1, ALDH1A2, ALDH1A3 and/or ALDH3A1) is responsible for the ALDH activity exhibited by stem cells and whether different isozymes function within various types of stem/progenitor cells (see [55] for review). ALDH family members play roles in diverse metabolic processes and a number of ALDH isoforms are involved in retinoic acid biosythesis [1]. Retinoids play an important role in the normal prostate: lack of vitamin A during rat embryogenesis inhibits mature prostate development [56], retinoic acid initiates prostatic bud formation [57], retinoids are necessary during early postnatal development for normal prostate branching [58] and for mature prostate homeostasis [59]. Conversely, it is thought that aberrant retinoic acid metabolism is involved in the development of prostate cancer: levels of retinoic acid are 5–8 fold lower in prostate carcinoma compared with normal prostate or benign prostatic hyperplasia [60], retinoic acid receptor gamma (RARδ) knockout mice have abnormal prostates [59], cellular retinoic acid binding protein 2 (CRABP2) mRNA and protein are downregulated in prostate carcinoma cells compared with normal tissue [61] and inactivation of RXR ∝ leads to preneoplastic lesions in the prostate [62]. In addition, ALDH1A2 is expressed in normal prostate epithelium but not in prostate cancer and is a candidate tumor suppressor gene in prostate cancer [63]. Furthermore, differences in the cellular distribution of RAR isoforms exist between normal, benign prostatic hyperplasia and prostate cancer epithelial cells [64]. These studies confirm the importance of the role of retinoid metabolism in the prostate. Various strategies currently being pursued to identify useful agents for the treatment of prostate cancer, include ALDH inhibitors [65] and retinoic acid metabolism blocking agents [66].
The presence of high levels of ALDH activity in a variety of stem cells acts as a functional rather than a phenotypic marker for stem cells. Hess et al [7] proposed that, as the cell phenotype can change during the cell cycle, the isolation of hematopoietic stem cells based on a conserved stem cell function such as ALDH activity, may be preferable to that based on a changing surface phenotype. Some ALDH isoforms act as detoxifying enzymes, which may serve to protect stem cells from toxic compounds. Other ALDH isozymes, involved in retinoic acid biosynthesis, may have a role in stem cell self-renewal or differentiation. Inhibition of ALDH promotes the self-renewal of human hematopoietic stem cellsthrough inhibition of retinoic acid signaling [67] and studies with RAR ∝ −/− and RAR δ −/− mice show that RAR δ is crucial for the regulation of HSC self-renewal and differentiation [68]. In the hair bulge (stem cell niche), ALDH1A2 is expressed throughout anagen and it is proposed that retinoic acid is involved in stem cell maintenance in the hair follicle [69]. Our data indicate that the murine prostate contains a subset of cells that express high levels of ALDH activity and that these cells have prostate regenerating activity and express many molecules previously identified in stem cells of other origins. ALDH activity can therefore be used as a new functional marker for the isolation of prostate stem/progenitor cells. Further research is necessary to elucidate the role of this functional marker in these cells.
Summary
We show that a subset of murine epithelial prostate cells has high ALDH activity, a functional marker of stem cells of other origins. The majority of ALDH hi cells express antigens associated with stem/progenitor cells, including several that serve a protective role, such as Bcl-2 and ABCG2. The ALDH hi population has greater in vitro and in vivo proliferative capacity than cells with low ALDH activity, indicating that this population contains prostate stem/progenitor cells. The expression of high ALDH activity by prostate cells is a novel finding that will assist with the isolation and further characterization of prostate stem cells and the development of therapeutic strategies for the treatment of benign prostatic hyperplasia and prostate cancer.
Supplementary Material
Phase contrast images of ALDH hi cells (A) and ALDH lo cells (B) show that the cell populations having different ALDH activities have little or no difference in size. (Scale bars = 10 μm).
Cell digests from the proximal region of prostatic ducts (A, B) or the whole prostate (all regions of ducts) (C, D) were incubated with Aldefluor in the presence (A, C) or absence (B, D) of the inhibitor, DEAB. High levels of ALDH activity were expressed by 18.6% of proximal cells (B). Plots of proximal prostate cells are representative of 4 experiments. Cell digests from the whole prostate that contained 8.9% ALDH hi cells (D). Plots of whole prostate are representative of 7 experiments.
Cells were incubated with Aldefluor substrate (this substrate is converted into a green fluorescent product by ALDH that can be measured by FACS in the FL1 channel) and subsequently incubated with biotinylated antibodies against EpCam, Sca-1, CD9, CD200, CD24 or prominin, followed by SA-APC. Controls, using appropriate biotinylated antibodies, were included for each antigen. The ALDH hi and ALDH lo populations were gated and analysed for antigen expression. Dot plots indicate the percentage of ALDH hi or ALDH lo cells that express the indicated antigens and include their respective isotype controls. Each plot is a representative example of more than one experiment.
Cells were incubated with Aldefluor substrate and subsequently FACS sorted into ALDH hi and ALDH lo cells. The cells from each population were then permeabilized and incubated with polyclonal antibodies against CK5, ALDH1/2, ALDH3A1, Bcl-2, Oct 3/4, ABCG2 or nestin, followed by goat anti-rabbit APC or monoclonal antibodies against CK8, followed by rabbit anti-mouse RPE-Cy5. Appropriate isotype control antibodies were included for each antigen. Histograms indicate the percentage of ALDH hi or ALDH lo cells that express the indicated antigens. Each plot is a representative example of more than one experiment.
Acknowledgments
Acknowledgement of grants: This work was supported by the University of Cape Town Staff Research Fund, the South African Medical Research Council, National Institutes of Health CA132641, NYS Department of Health, Amgen Inc. and the Helen L and Martin S Kimmel Center for Stem Cell Biology at NYU School of Medicine.
We are grateful to Ronnie Dreyer and Peter Lopez for help with FACS sorting.
Footnotes
Author Contribution: Patricia Burger: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Rashmi Gupta: Collection and assembly of data, final approval of manuscript.
Xiaozhong Xiong: Collection and assembly of data, final approval of manuscript.
Christopher Ontiveros: Collection and assembly of data, final approval of manuscript.
Sarah Salm: Collection and assembly of data, final approval of manuscript.
David Moscatelli: Manuscript writing, final approval of manuscript.
E. Lynette Wilson: Conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Conflict of interests: None
References
- 1.Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev. 2004;36:279–299. doi: 10.1081/dmr-120034001. [DOI] [PubMed] [Google Scholar]
- 2.Cai J, Weiss ML, Rao MS. In search of “stemness”. Exp Hematol. 2004;32:585–598. doi: 10.1016/j.exphem.2004.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douville J, Beaulieu R, Balicki D. ALDH1 as a Functional Marker of Cancer Stem and Progenitor Cells. Stem Cells Dev. 2008;23:23. doi: 10.1089/scd.2008.0055. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong L, Stojkovic M, Dimmick I, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 5.Corti S, Locatelli F, Papadimitriou D, et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- 6.Fallon P, Gentry T, Balber AE, et al. Mobilized peripheral blood SSCloALDHbr cells have the phenotypic and functional properties of primitive haematopoietic cells and their number correlates with engraftment following autologous transplantation. Br J Haematol. 2003;122:99–108. doi: 10.1046/j.1365-2141.2003.04357.x. [DOI] [PubMed] [Google Scholar]
- 7.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 8.Corti S, Locatelli F, Papadimitriou D, et al. Transplanted ALDHhiSSClo neural stem cells generate motor neurons and delay disease progression of nmd mice, an animal model of SMARD1. Hum Mol Genet. 2006;15:167–187. doi: 10.1093/hmg/ddi446. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 10.Gentry T, Foster S, Winstead L, et al. Simultaneous isolation of human BM hematopoietic, endothelial and mesenchymal progenitor cells by flow sorting based on aldehyde dehydrogenase activity: implications for cell therapy. Cytotherapy. 2007;9:259–274. doi: 10.1080/14653240701218516. [DOI] [PubMed] [Google Scholar]
- 11.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 12.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde Dehydrogenase 1 Is a Marker for Normal and Malignant Human Colonic Stem Cells (SC) and Tracks SC Overpopulation during Colon Tumorigenesis. Cancer Res. 2009;69:1–8. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu C, Yao Z, Dai J, et al. Aldehyde dehydrogenase is a marker of prostate cancer stem cell-like cells in cell lines. [abstract 1300]. 98th American Assiociation for Cancer Research Annual Meeting; Los Angeles, CA. Apr 14–18th, 2007. Proceedings. [Google Scholar]
- 16.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seigel GM, Campbell LM, Narayan M, et al. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–737. [PubMed] [Google Scholar]
- 18.Tsujimura A, Koikawa Y, Salm S, et al. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol. 2002;157:1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger PE, Xiong X, Coetzee S, et al. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci U S A. 2005;102:7180–7185. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha GR, Donjacour A. Mesenchymal-epithelial interactions: technical considerations. Prog Clin Biol Res. 1987;239:273–282. [PubMed] [Google Scholar]
- 21.Norman JT, Cunha GR, Sugimura Y. The induction of new ductal growth in adult prostatic epithelium in response to an embryonic prostatic inductor. Prostate. 1986;8:209–220. doi: 10.1002/pros.2990080302. [DOI] [PubMed] [Google Scholar]
- 22.Xin L, Ide H, Kim Y, et al. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci U S A. 2003;100:11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salm SN, Takao T, Tsujimura A, et al. Differentiation and stromal-induced growth promotion of murine prostatic tumors. Prostate. 2002;51:175–188. doi: 10.1002/pros.10075. [DOI] [PubMed] [Google Scholar]
- 24.Winter MJ, Nagtegaal ID, van Krieken JH, et al. The epithelial cell adhesion molecule (Ep-CAM) as a morphoregulatory molecule is a tool in surgical pathology. Am J Pathol. 2003;163:2139–2148. doi: 10.1016/S0002-9440(10)63570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Hayward S, Cao M, et al. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- 26.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 28.Oka M, Tagoku K, Russell TL, et al. CD9 is associated with leukemia inhibitory factor-mediated maintenance of embryonic stem cells. Mol Biol Cell. 2002;13:1274–1281. doi: 10.1091/mbc.02-01-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanatsu-Shinohara M, Toyokuni S, Shinohara T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol Reprod. 2004;70:70–75. doi: 10.1095/biolreprod.103.020867. [DOI] [PubMed] [Google Scholar]
- 30.Klassen H, Schwartz MR, Bailey AH, et al. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci Lett. 2001;312:180–182. doi: 10.1016/s0304-3940(01)02215-7. [DOI] [PubMed] [Google Scholar]
- 31.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 32.Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: Overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191:253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15:82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- 34.Salm SN, Burger PE, Coetzee S, et al. TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol. 2005;170:81–90. doi: 10.1083/jcb.200412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiberio R, Marconi A, Fila C, et al. Keratinocytes enriched for stem cells are protected from anoikis via an integrin signaling pathway in a Bcl-2 dependent manner. FEBS Lett. 2002;524:139–144. doi: 10.1016/s0014-5793(02)03040-5. [DOI] [PubMed] [Google Scholar]
- 36.Kloepper JE, Tiede S, Brinckmann J, et al. Immunophenotyping of the human bulge region: the quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp Dermatol. 2008;17:592–609. doi: 10.1111/j.1600-0625.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 37.Ohyama M, Terunuma A, Tock CL, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawasaki BT, Mistree T, Hurt EM, et al. Co-expression of the toleragenic glycoprotein, CD200, with markers for cancer stem cells. Biochem Biophys Res Commun. 2007;364:778–782. doi: 10.1016/j.bbrc.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim M, Turnquist H, Jackson J, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 40.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 41.Tai MH, Chang CC, Kiupel M, et al. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 42.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 43.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 44.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 45.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leong KG, Wang BE, Johnson L, et al. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 47.Richardson GD, Robson CN, Lang SH, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 48.Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4:193–201. doi: 10.1007/s12015-008-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 50.Goto K, Salm SN, Coetzee S, et al. Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis. Stem Cells. 2006;24:1859–1868. doi: 10.1634/stemcells.2005-0585. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 52.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 53.Grey BR, Oates JE, Brown MD, et al. Cd133: a marker of transit amplification rather than stem cell phenotype in the prostate? BJU Int. 2009;103:856–858. doi: 10.1111/j.1464-410X.2008.08250.x. [DOI] [PubMed] [Google Scholar]
- 54.Shmelkov SV, Butler JM, Hooper AT, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Curr Stem Cell Res Ther. 2008;3:237–246. doi: 10.2174/157488808786734006. [DOI] [PubMed] [Google Scholar]
- 56.Wilson JG, Warkany J. Malformations in the genito-urinary tract induced by maternal vitamin A deficiency in the rat. Am J Anat. 1948;83:357–407. doi: 10.1002/aja.1000830303. [DOI] [PubMed] [Google Scholar]
- 57.Vezina CM, Allgeier SH, Fritz WA, et al. Retinoic acid induces prostatic bud formation. Dev Dyn. 2008;237:1321–1333. doi: 10.1002/dvdy.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aboseif SR, Dahiya R, Narayan P, et al. Effect of retinoic acid on prostatic development. Prostate. 1997;31:161–167. doi: 10.1002/(sici)1097-0045(19970515)31:3<161::aid-pros3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 59.Lohnes D, Kastner P, Dierich A, et al. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- 60.Pasquali D, Thaller C, Eichele G. Abnormal level of retinoic acid in prostate cancer tissues. J Clin Endocrinol Metab. 1996;81:2186–2191. doi: 10.1210/jcem.81.6.8964849. [DOI] [PubMed] [Google Scholar]
- 61.Okuducu AF, Janzen V, Ko Y, et al. Cellular retinoic acid-binding protein 2 is down-regulated in prostate cancer. Int J Oncol. 2005;27:1273–1282. [PubMed] [Google Scholar]
- 62.Huang J, Powell WC, Khodavirdi AC, et al. Prostatic intraepithelial neoplasia in mice with conditional disruption of the retinoid X receptor alpha allele in the prostate epithelium. Cancer Res. 2002;62:4812–4819. [PubMed] [Google Scholar]
- 63.Kim H, Lapointe J, Kaygusuz G, et al. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res. 2005;65:8118–8124. doi: 10.1158/0008-5472.CAN-04-4562. [DOI] [PubMed] [Google Scholar]
- 64.Richter F, Joyce A, Fromowitz F, et al. Immunohistochemical localization of the retinoic Acid receptors in human prostate. J Androl. 2002;23:830–838. [PubMed] [Google Scholar]
- 65.Quash G, Fournet G, Courvoisier C, et al. Aldehyde dehydrogenase inhibitors: alpha, beta-acetylenic N-substituted aminothiolesters are reversible growth inhibitors of normal epithelial but irreversible apoptogens for cancer epithelial cells from human prostate in culture. Eur J Med Chem. 2008;43:906–916. doi: 10.1016/j.ejmech.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Njar VC, Gediya L, Purushottamachar P, et al. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg Med Chem. 2006;14:4323–4340. doi: 10.1016/j.bmc.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 67.Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Purton LE, Dworkin S, Olsen GH, et al. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203:1283–1293. doi: 10.1084/jem.20052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Everts HB, King LE, Jr, Sundberg JP, et al. Hair cycle-specific immunolocalization of retinoic acid synthesizing enzymes Aldh1a2 and Aldh1a3 indicate complex regulation. J Invest Dermatol. 2004;123:258–263. doi: 10.1111/j.0022-202X.2004.23223.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phase contrast images of ALDH hi cells (A) and ALDH lo cells (B) show that the cell populations having different ALDH activities have little or no difference in size. (Scale bars = 10 μm).
Cell digests from the proximal region of prostatic ducts (A, B) or the whole prostate (all regions of ducts) (C, D) were incubated with Aldefluor in the presence (A, C) or absence (B, D) of the inhibitor, DEAB. High levels of ALDH activity were expressed by 18.6% of proximal cells (B). Plots of proximal prostate cells are representative of 4 experiments. Cell digests from the whole prostate that contained 8.9% ALDH hi cells (D). Plots of whole prostate are representative of 7 experiments.
Cells were incubated with Aldefluor substrate (this substrate is converted into a green fluorescent product by ALDH that can be measured by FACS in the FL1 channel) and subsequently incubated with biotinylated antibodies against EpCam, Sca-1, CD9, CD200, CD24 or prominin, followed by SA-APC. Controls, using appropriate biotinylated antibodies, were included for each antigen. The ALDH hi and ALDH lo populations were gated and analysed for antigen expression. Dot plots indicate the percentage of ALDH hi or ALDH lo cells that express the indicated antigens and include their respective isotype controls. Each plot is a representative example of more than one experiment.
Cells were incubated with Aldefluor substrate and subsequently FACS sorted into ALDH hi and ALDH lo cells. The cells from each population were then permeabilized and incubated with polyclonal antibodies against CK5, ALDH1/2, ALDH3A1, Bcl-2, Oct 3/4, ABCG2 or nestin, followed by goat anti-rabbit APC or monoclonal antibodies against CK8, followed by rabbit anti-mouse RPE-Cy5. Appropriate isotype control antibodies were included for each antigen. Histograms indicate the percentage of ALDH hi or ALDH lo cells that express the indicated antigens. Each plot is a representative example of more than one experiment.