Abstract
Repetitive transcranial magnetic stimulation (rTMS) has been used to improve language behavior, including naming, in stroke patients with chronic, nonfluent aphasia. Part 1 of this paper reviews functional imaging studies related to language recovery in aphasia. Part 2 reviews the rationale for using rTMS to treat nonfluent aphasia (based on functional imaging); and presents our current rTMS protocol. We present language results from our rTMS studies, and imaging results from overt naming fMRI scans obtained pre- and post- a series of rTMS treatments. Part 3 presents results from a pilot study where rTMS treatments were followed immediately by constraint-induced language therapy. Part 4 reviews our diffusion tensor imaging study that examined possible connectivity of arcuate fasciculus to different parts of Broca’s area (pars triangularis, PTr; pars opercularis, POp); and to ventral premotor cortex (vPMC). The potential role of mirror neurons in R POp and vPMC in aphasia recovery is discussed.
Introduction
Electrical stimulation techniques including transcranial magnetic stimulation (TMS) have examined brain-behavior relationships across many cognitive domains (attention, neglect, motor and language systems). TMS can be used to temporarily facilitate or inhibit neural activity to examine intact systems, examine the presence of residual capacity in an injured system, or to accelerate natural recovery mechanisms.
We review how TMS has been applied to help re-organize neural networks during language recovery in aphasia. There are four parts: Part 1 reviews functional imaging studies related to recovery of language in aphasia. Part 2 reviews the rationale for use of repetitive TMS (rTMS) in treating nonfluent aphasia based on results from functional imaging studies; and reviews our current rTMS treatment protocol with nonfluent aphasia patients. We present language results from our rTMS studies with nonfluent aphasia patients, and imaging results from overt naming fMRI scans obtained pre- and post- a series of TMS treatments. Part 3 presents results from a pilot study where rTMS treatments were followed immediately by constraint-induced language therapy. Part 4 reviews our diffusion tensor imaging (DTI) study that examined possible connectivity of arcuate fasciculus (AF) to different parts of Broca’s area (pars triangularis, PTr; pars opercularis, POp); and to ventral premotor cortex (vPMC). Part 4 also addresses the potential role of mirror neurons in R POp and vPMC in relationship to our rTMS results with nonfluent aphasia patients, and in aphasia recovery.
Part 1. Functional Imaging Studies with Aphasia Patients
Brain re-organization supporting recovery of language in aphasia is unclear. Both the left hemisphere (LH) and the right hemisphere (RH) are thought to support language recovery after stroke [1–3]. Factors including time poststroke when patients are studied (acute or chronic), lesion location and the specific language tasks examined may affect the mechanisms involved in recovery [3, 4]. The most rapid recovery occurs in the first 6 months poststroke onset. Approximately 20% of stroke patients have speech and language problems, which include hesitant, poorly articulated, agrammatic speech with word-finding problems (nonfluent aphasia) [5]. When functional imaging studies have focused on patients with nonfluent aphasia, an increased activation in RH language homologues has often been observed [6–9].
The LH may be important for better language recovery after stroke [7–12]. Heiss & Thiel (2006) have suggested that for long-term recovery, RH recruitment may be less efficient than restoring the LH network. Patients with better recovery were observed to have higher activation in L superior temporal gyrus (STG) and L supplementary motor area (SMA) [11, 14].
Recovery of naming has been associated with reperfusion of L Brodmann Area (BA) 37 in acute stroke cases studied with perfusion weighted imaging [15]. Winhuisen et al., 2005, also observed as early as 2 weeks poststroke onset, that better performance on a verbal fluency test (and better recovery) was associated with the L inferior frontal gyrus (IFG). Constraint-induced language therapy (CILT) is an intensive therapy program that has been observed to improve object and action naming after 10 treatments, where patients may only respond with verbal output (no gestures, writing, sound effects) [16]. An opaque screen is placed on a table where the Speech-Language Pathologist is seated on one side, and the patient, on the other; there is eye contact above the screen. Each treatment lasts for 3 hours. Richter et al., 2008, observed therapeutic success following treatment with constraint-induced aphasia therapy to be correlated with a relative decrease of activation in RH areas, including the IFG/insular cortex. After speech therapy with chronic stroke patients, new LH activation has been associated with improvement in language [19–22].
Since 1877, it has been suggested that the RH can support some language after LH stroke [23, 24]. Some functional imaging studies have observed RH activation during different language tasks in a variety of aphasia patients [25–27]. In these studies, the RH activation was considered to be compensatory. Additionally, new RH activation has been observed following speech therapy with some aphasia patients [28–30]. Fernandez et al., 2004, suggested that RH participation in the acute recovery stage of LH stroke may be followed later, by LH activation corresponding to further recovery and that the RH may play a larger role in supporting recovery when there is greater damage to LH language areas. It is possible that high RH activation, may be ‘maladaptive’ and lead to a ‘dead-end’, inefficient strategy for recovery, particularly in nonfluent aphasia patients [3, 6–8, 32, 33].
Whether recovery in aphasia is mediated primarily from LH undamaged language or perilesional regions, or from RH language homologues (or both), the above-mentioned studies suggest there is potential for brain re-organization and improved language in post-stroke aphasia [3].
Part 2. Rationale for use of rTMS with Nonfluent Aphasia
Transcranial Magnetic Stimulation (TMS)
Repetitive TMS (rTMS) allows painless, noninvasive stimulation of human cortex (approximately 1 cc in size), from outside of the skull. It utilizes magnetic fields to create electrical currents in cortical regions of interest (ROIs). Repetitive TMS can be used to produce changes in cortical excitability [32]. When delivered to the same cortical region, slow (1 Hz) rTMS appears to decrease excitability in the targeted cortical ROI that lasts beyond the duration of the train itself [34] leading to measurable behavioral effects. Conversely, rapid rTMS (≥5 Hz) increases cortical excitability [35]. Repetitive TMS has been observed to effect language, ranging from facilitation of naming [36] to speech arrest [37s] depending on rTMS parameters and location of the coil.
As reviewed in Part 1, several functional imaging studies with chronic, nonfluent aphasia patients have observed high activation, possibly “over-activation” during language tasks, in parts of R Broca’s area and other R perisylvian language homologues which may be ‘maladaptive’ [6–9, 12]. When applied to a specific ROI in the undamaged hemisphere, low-frequency (1 Hz) rTMS may suppress the inhibitory process of that ROI, permitting reactivation of some areas within the damaged hemisphere, promoting some functional recovery [32]. This is similar to the phenomenon of ‘Paradoxical Functional Facilitation’ or PFF [38]. The phenomenon of PFF suggests that direct or indirect neural ‘damage’ to a specific area in the central nervous system may result in facilitation of behavior [38]. Thus, suppressing a cortical ROI in the RH of a nonfluent aphasia patient using 1 Hz rTMS may result in a decrease in over-activation of that ROI, thus promoting less inhibition exerted by that ROI on other adjacent or distant areas resulting in an overall modulation of the bilateral neural network for naming.
Review of our rTMS Treatment Protocol with Nonfluent Aphasia Patients Inclusion Criteria
Our studies have included chronic aphasia patients who were at least 6 months post- single, unilateral LH stroke. They were R-handed, native English speakers, ranging in age from 40–80. They had not had a seizure for at least one year. Patients had nonfluent speech, with a 1–4 word phrase length as measured with elicited propositional speech on the Cookie Theft Picture, Boston Diagnostic Aphasia Exam (BDAE). Patients named at least 3 pictures from the first 20 pictures on the Boston Naming Test (BNT). Patients were requested not to receive any individualized speech therapy throughout their first year of participation in our rTMS studies.
Baseline Naming Testing
At Entry, the baseline naming ability for Snodgrass & Vanderwart (S&V) (1980) pictures was established for each patient. This baseline S&V naming score was used during Phase 1 rTMS sessions (explained below), to establish the best RH cortical ROI to suppress with rTMS, in order to improve naming. Ten, 20 item S&V lists were administered across three separate testing sessions during baseline testing. For each patient, the baseline mean and SD for response time (RT) and for number of S&V pictures named correctly across the ten lists were calculated.
rTMS Treatment (Two Phases)
Each patient participated in two phases of rTMS treatment. The intensity of rTMS was adjusted for each TMS session and applied at 90% of motor threshold (MT). Motor threshold is the intensity of magnetic stimulation needed to elicit a muscle twitch in the thumb, L first dorsal interosseus muscle (L FDI), in 5 out of 10 trials when using single-pulse TMS applied to the primary motor cortex of the contralateral hemisphere. Published guidelines for safety parameters of rTMS are based on stimulation intensities expressed as a percent of the individual’s MT [40].
Phase 1 rTMS, Establish Location of Best RH Cortical ROI to Suppress
The location of the single, best RH cortical ROI to suppress with rTMS to improve picture naming was determined individually for each patient during Phase 1, where 1 Hz rTMS was applied at 90% MT for 10 min (600 pulses). A figure 8-shaped TMS coil (7 cm diameter) was used with the Super-Rapid High Frequency MagStim Magnetic Stimulator (MagStim, NY). This rTMS protocol was applied in separate sessions, to different RH cortical ROIs. ROIs examined have included the M1, mouth (orbicularis oris, verified with motor evoked potential), superior temporal gyrus (STG) and subregions within Broca’s area: PTr posterior, and Pop (see Fig. 1). Immediately post-10 minutes of rTMS to suppress an ROI, picture naming was assessed by administering an S&V 20-item picture list. The single RH cortical ROI associated with at least a 2 SD improvement above baseline S&V naming (obtained at Entry) was considered to be the “Best Response” RH cortical ROI for that patient.
Figure 1.
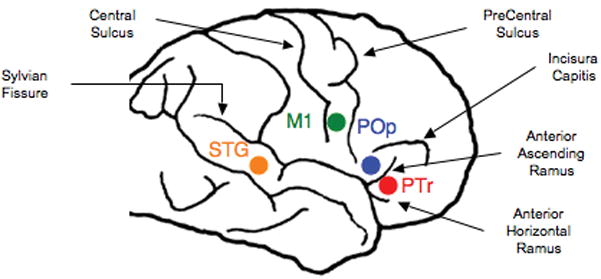
RH cortical ROIs examined during Phase 1 rTMS treatment protocol including M1, mouth (orbicularis oris, verified with motor evoked potential), superior temporal gyrus (STG) and subregions within Broca’s area: PTr posterior, and POp. Phase 1 establishes the location of the Best RH Cortical ROI to Suppress during Phase 2, treatment.
Phase 2 rTMS, Suppressing the Best Response RH ROI Longer, over More Sessions
During Phase 2, the Best Response RH ROI determined for each individual during Phase 1, was suppressed with 1 Hz rTMS (90% MT) for 20 min, 5 days per week, across 2 weeks. On each day of treatment, rTMS was applied using the same MagStim device as in Phase 1. A frameless stereotaxic system (Brainsight, Rogue Industries, Montreal) was used to guide the position of the TMS coil on the patient’s scalp. This enabled on-line monitoring of the specified brain area on the patient’s MRI scan throughout the rTMS session, and from day-to-day. Coil orientation was monitored and held constant across sessions, at approximately 45 degrees. One purpose of Phase 2 was to investigate the long-term effects on naming, following a series of 10 rTMS treatments. Each patient received follow-up language testing at 2 months and up to 8 months following the 10th rTMS treatment. There were no negative side effects.
Results for 6 patients in Phase 1
We observed a site-specific effect of rTMS for number of pictures named correctly (F=14.63; df 3, 5; p=0.0001) and RT (F=5.63; df 3, 15; p=0.009), including a double dissociation within parts of Broca’s area. In 6 aphasia patients, suppression of R PTr with 1 Hz rTMS resulted in patients becoming more accurate, naming more pictures, and having a faster reaction time (RT). However, suppression of R POp with 1 Hz rTMS, resulted in patients becoming less accurate, naming fewer items, and showing an increased RT. Patients named significantly more items after 1 Hz rTMS to suppress R PTr than to R POp (Fisher’s PLSD post-hoc P<0.001), R M1, orbicularis oris (p<0.01), and R STG (p<0.005) [41].
Results for 4 Patients in Phase 2
We observed in 4 aphasia patients at 2 months post- ten rTMS treatments to suppress R PTr, significant improvement on three naming tests: 1) the BNT, first 20 items (p=.003); 2) the BDAE subtest, Animals (p=.02); and 3) the BDAE subtest, Tools/Implements (p=.04) [42]. At 8 months post-TMS, all three naming test scores continued to improve relative to pre-TMS testing, but only Tools/Implements was significant (p=.003). BNT and naming Animals failed to reach significance because of one patient.
Overt Naming Functional MRI Pre- and Post-TMS in Two Nonfluent Aphasia Patients
Functional MRI was utilized to examine brain activation during overt naming, pre- and post- ten, 20-minute, 1 Hz rTMS treatments to suppress part of R PTr, to improve naming, in two chronic nonfluent aphasia patients [9]. One patient was a ‘good responder’ with improved naming and phrase length in propositional speech, lasting out to almost 4 years post-TMS. The other patient was a ‘poor responder’ with no change in naming or propositional speech post-TMS.
Overt Naming fMRI Block Design Paradigm
Overt naming fMRIs were obtained in the same manner as Martin et al., 2005. The continuous sample, block design, overt naming fMRI paradigm took advantage of the hemodynamic response delay where increased blood flow remains for 4–8 seconds after the task [44]. Task-related information is obtained after the task, minimizing motion artifact [45].
Functional Imaging Results for the ‘Good Responder’
We hypothesized that in chronic nonfluent aphasia, after rTMS treatment to suppress R PTr, a shift in activation from RH frontal areas to new activation in LH perilesional, perisylvian areas and L SMA would occur, if there were good response with improved naming. P1, who was a ‘good responder’, showed activation in R and L sensorimotor cortex (mouth area), R IFG, and in R and L SMA, pre-TMS as well as at 3 months and at 16 months post-TMS. At 16 mo. post-TMS, however, there was a significant change in SMA activation, where P1 showed significant increase in activation in the L SMA, compared to pre-, and to 3 mo. post-TMS (p<.02; p<.05, respectively). There was also a trend towards significantly greater activation in L SMA than R SMA at 16 mo. and 46 mo. post-TMS (p<.08; p<.09, respectively). Pre-TMS there had been no difference between L and R SMA activation. It is unknown exactly when, post-TMS, the shift to the stronger L SMA activation occurred for this patient during overt naming, however, it was first observed at 16 mo. post-TMS (his highest accuracy rate; 58% named). There were no intervening overt speech fMRI scans between 3 and 16 mo. post-TMS. The new LH activation remained present, even at 46 mo. post-TMS (nearly 4 years post-TMS) when the patient was 13 yr. 11 mo. poststroke. On the language outcome measures, P1 improved on the BNT from 11 pictures named pre-TMS, to scores ranging from 14–18 pictures, post-TMS (2 mo. to 43 mo. post-TMS). His longest phrase length improved from 3 words pre-TMS, to 5–6 words post-TMS.
Functional Imaging Results for the ‘Poor Responder’
Pre-TMS (1.5 yr. poststroke), P2 had significant activation in R IFG (3% pictures named). At 3 and 6 mo. post-TMS, there was no longer significant activation in R IFG, but significant activation was present in R sensorimotor cortex. Although P2 had significant activation in both the L and R SMA on all three fMRI scans (pre-TMS, and at 3 and 6 mo. post-TMS), ROI analyses showed no difference across sessions in the L or R SMA activation.
For P2, who was a ‘poor responder’, suppression of R PTr with rTMS resulted in no new, lasting perilesional LH activation across sessions. His naming remained only at 1–2 pictures during all three fMRI scans. His BNT score and longest phrase length remained at 1 word, post-TMS.
Lesion site may play a role in each patient’s fMRI activation pattern and response to TMS treatment. P2 had an atypical frontal lesion in the L motor and premotor cortex that extended high, near brain vertex, with deep white matter lesion near L SMA. P2 also had frontal lesion in the posterior middle frontal gyrus at the junction of the premotor cortex, an area important for naming [46]. Additionally, P2 had lesion inferior and posterior to Wernicke’s area, in parts of BA 21 and 37. P1 had no lesion in these three areas.
Part 3. Pilot Study: TMS plus Constraint-Induced Language Therapy
Results for a Severe Nonfluent Aphasia Patient
Constraint-induced language therapy (CILT) is a program observed to improve object and action naming, where patients may only respond with verbal output (no gestures, writing, sound effects) [16]. A severe nonfluent aphasia patient participated in our original rTMS protocol at 6.5 years poststroke. At that time she had improved on the BNT, where her score increased from 4, pre-TMS, to 7 and 12 pictures named, at 2 and 8 Mo. post-TMS [33].
At 5 years, 10 months (12.5 years poststroke) after the first rTMS series, she participated our pilot study where she underwent a second series of ten, identical rTMS treatments to suppress R PTr, however, each rTMS treatment was followed immediately by three hours of CILT (5 days/week for 2 weeks) [47].
Prior to this intervention, her object naming was tested on a set of 250 color pictures, 3 times. During CILT, one-third of the color pictures presented as stimulus items for therapy had never been named on pre-testing (0/3); one-third, she sometimes named (1–2/3); and one-third, she always named (3/3).
To examine changes that might occur during intervention, BDAE naming subtests (Actions, Animals, Tools/Implements), and BNT were administered 12 times pre-TMS; daily, immediately post- each CILT session; and 10 times post-TMS. These time-series data were later analyzed using a double bootstrap method: http://www.stat.wmich.edu/slab/Software/Timeseries.html. Language outcome measures utilized to examine long-term effects included BDAE and BNT examined at baseline, pre-TMS (3 times); and at 1 and 6 Mo. post-TMS. Significant improvement was defined >2 SD above baseline.
Results for the time-series analysis showed significant improvement on BDAE Action Naming (p=.035); and Tools/Implements (p=.01). On language outcome measures, there was >2 SD improvement on BDAE Action Naming, Tools/Implements and Single Word Repetition. Her improvement in verb action naming was only observed following the second rTMS series, where CILT was included.
Part 4. Diffusion Tensor Imaging (DTI) Study of Arcuate Fasciculus Connections to Parts of Broca’s Area
In our rTMS studies with aphasia patients, we have observed long-term improved naming in chronic nonfluent aphasia patients following a series of rTMS treatments to suppress the R PTr [33, 42]. During Phase 1 rTMS when the R POp was suppressed for 10 minutes, we observed a temporary impairment in naming. Suppression of R POp has never been observed to be a Best Response ROI to improve naming in our nonfluent aphasia patients. In order to better understand these results, we conducted a study with DTI involving the arcuate fasciculus (AF) connections to these subregions within Broca’s area. The AF is a pathway connecting Broca’s area and posterior language zones that has previously been studied extensively in the LH. Connections of the AF to parts of Broca’s area, and the vPMC, however, have not been determined, nor have they been studied in the RH. We utilized DTI in eight healthy subjects (5 M) to track the mid-portion of the AF to parts of Broca’s area (PTr, POp), and the vPMC [Kaplan et al., submitted].
We observed a significantly greater volume of fibers in L AF than R AF, in agreement with previous studies [for review, see Catani & Mesulam, 2008]. Within parts of L Broca’s area, we observed 8/8 subjects to have L AF connections to POp (one subject also had L AF connections to PTr). Within parts of R Broca’s homologue, we observed 5/8 subjects to have R AF connections to POp (one subject also had R AF connections to PTr). All 8 subjects had L AF connections to vPMC; and 7/8 subjects had R AF connections to vPMC. Our study showed limited AF connections with PTr; Frey et al., 2008 observed L anterior Broca’s area connections to STG, to be via the extreme capsule, not via the AF.
Results from our DTI study showed in the LH, connections from L AF are primarily to L POp and that in the RH, connections from R AF are primarily to R POp. Similarly, there are multiple connections from L AF to L vPMC, and from R AF to R vPMC.
Possible Relevance of the Mirror Neuron System to Aphasia Recovery
The POp and vPMC are part of the bilateral, mirror neuron system [50] [see Iacoboni 2008, for review]. Mirror neurons are cells that fire during both production and perception of similar actions. They are important in child language acquisition [51] and are bilateral, thus, they may have special relevance in promoting recovery in aphasia, where the R POp and R vPMC are always spared in aphasia cases with unilateral LH lesion.
The notion that POp is part of the mirror neuron system, could help to clarify why suppression of R POp in our aphasia patients (during Phase 1) would impair naming, directly interrupting activation of mirror neurons, thus interfering with verbal production of names for pictures. Frey et al., 2008, observed direct connections from L posterior Broca’s area, via AF, to the supramarginal gyrus (SMG). Suppression of R POp with 1 Hz rTMS consistently produced impairment in naming, perhaps primarily due to direct suppression of the mirror neurons located there and secondarily, due indirectly to impaired connections from POp via AF, to R posterior language zones, and possibly to LH posterior language zones.
In addition, the R vPMC may also play a similar role in aphasia recovery both primarily, as part of the mirror neuron system, and secondarily, from connections with the AF to posterior language zones. A recent rTMS study by Meister et al., 2007 has supported the role of vPMC mirror neurons as necessary for phonemic categorization in speech perception, where there is a functional interplay between vPMC and the L STG. Although we have no rTMS data regarding the effect of suppression of R vPMC on naming in aphasia patients, we would posit that suppression of R vPMC would impair naming in nonfluent aphasia patients in a manner similar to suppression of R POp.
Conversely, we observed that suppression of R PTr with rTMS improves naming in nonfluent aphasia. Although the mechanism for this beneficial effect is unknown, we have posited the following: In cases with nonfluent aphasia where LH lesion is present in L inferior frontal cortical and/or subcortical white matter areas, hyperactivity of neurons in R PTr may be present due to interhemispheric disinhibition from the damaged L frontal lobe. Furthermore, prior to rTMS, the hyperactivity of R PTr could be excessively suppressing R POp, and thus possibly hindering recovery from aphasia. Therefore, suppression of R PTr with 1 Hz rTMS may promote less inhibition of R POp from R PTr, via U-fibers, directly permitting better modulation of R POp, an important part of the mirror neuron system. Better modulation of R POp may also indirectly support better modulation of R vPMC, as well as other parts of the bilateral neural network for naming including RH and LH temporo-parietal areas [53].
Other Transcranial Electrical Stimulation Studies
The effect of high-frequency rTMS on naming actions and objects in mild and moderate-severe Alzheimer’s disease (AD) has been recently studied [54]. The 20 Hz rTMS was applied to the L or R dorsolateral prefrontal cortex areas for 500 msec while the subject was asked to name a picture on the screen. The mild cases showed significant improvement in naming actions, only; while the moderate-severe cases showed improvement in naming actions and objects.
Another electrical stimulation intervention, which holds promise for improvement in chronic aphasia patients, includes transcranial direct current stimulation (tDCS) [55]. Although the effect of only one session was examined, improved naming was observed with an increase of 33.6% (SEM 13.8%) immediately post-tDCS treatment, in eight chronic nonfluent aphasia patients. These results are similar to our rTMS studies, in that they suggest a neural plasticity is present in chronic stroke patients with aphasia.
Conclusion
Transcranial electrical stimulation with either slow or fast rTMS, or tDCS, when placed on the proper cortical target area with specific treatment parameters, can induce changes in language behavior, particularly naming. Our rTMS results with chronic, nonfluent aphasia patients support the notion behind PFF [37], where a new, temporary “virtual” lesion (as with 1 Hz rTMS) can improve behavior in chronic stroke.
With the exception of our rTMS studies with nonfluent aphasia patients, studies with transcranial electrical stimulation have applied only a single session of treatment. It is suggested that more treatment sessions be applied over longer periods of time. Functional imaging studies pre- and post-TMS contribute information toward understanding of the plasticity of neural networks for language and recovery. The overt speech fMRI data with our nonfluent patient with good response to TMS support the hypothesis that restoration of parts of the LH language network is linked, at least in part, to better recovery of naming and phrase length in nonfluent aphasia. Additional fMRI studies pre- and post-TMS are warranted. Also, the combination of electrical stimulation (rTMS or tDCS) with speech therapy sessions provided immediately afterwards, might promote further language improvement in a variety of chronic aphasia patients.
Figure 2.
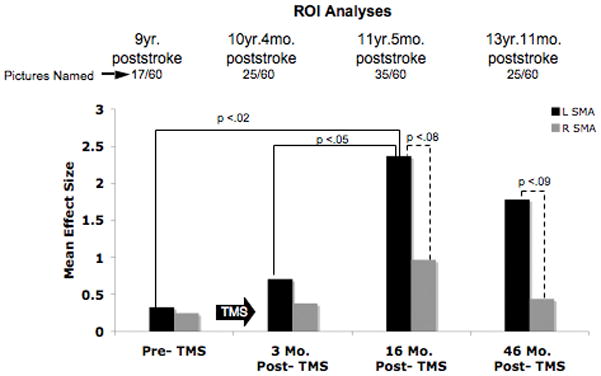
Overt naming fMRI results for ROI analysis pre- and post-Phase 2 rTMS for P1, good responder. Results shown for scans pre-TMS, 3 mo. post-TMS, 16 mo. post-TMS, and 46 mo. post-TMS. Bar graph displays the mean effect size for the L and R SMA activation. There was a significant shift to greater L SMA activation at 16 mo. post-TMS compared to pre-TMS and to 3 mo. post-TMS. Greater L SMA activation still remained at 46 mo. post-TMS.
Acknowledgments
Research supported by NIH grant RO1 DC05672 from the National Institute on Deafness and Other Communication Disorders (NIDCD), Bethesda, MD, and a grant from the Medical Research Service, Department of Veterans Affairs, Washington, D.C. (to M.A.N.); a K24 NIH award (RRO18875, to A.P.-L) and the Harvard-Thorndike General Clinical Research Center (NCRR MO1 RR01032); and a P30 DC05207 NIDCD grant to the Harold Goodglass BU Aphasia Research Center.
Footnotes
Website
Timeseries software, S. McKnight, J.W. McKean and B. Huitema. Available at http://www.stat.wmich.edu/slab/Software/Timeseries.html. Accessed July 2009.
References
*of importance
**of outstanding importance
- *1.Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang YL, Moore AB, Raymer AM, Briggs RW, Sherod MG, et al. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev. 2007;17(2):157–177. doi: 10.1007/s11065-007-9024-z. This article reviews fMRI studies with aphasia patients and examines the question of the role of each hemisphere in language recovery. It also examines various methodological challenges in aphasia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35(4):803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 3.Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18(4):429–434. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- 4.Thiel A, Habedank B, Herholz K, Kessler J, Winhuisen L, Haupt WF, Heiss WD. From the left to the right: How the brain compensates progressive loss of language function. Brain Lang. 2006;98(1):57–65. doi: 10.1016/j.bandl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17 (1):35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- 6.Belin P, Van Eeckhout P, Zilbovicius M, Remy P, Francois C, Guillaume S, Chain F, Rancurel G, Samson Y. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47(6):1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- 7.Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Dromerick AW, Fiez JA, Corbetta MD. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 8.Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, Palumbo CL, Goodglass H, Wingfield A, Samaraweera R, et al. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22(1):29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- **9.Martin PI, Naeser MA, Ho M, Doron K, Kurland J, Kaplan J, Wang Y, Nicholas M, Baker E, Fregni F, et al. Overt Naming pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post- TMS. Brain Lang. 2007;103:248–249. doi: 10.1016/j.bandl.2009.07.007. [abstract] In Press, Brain Lang 2009. This fMRI study examined brain activation for overt naming pre- and post- ten 1Hz TMS treatments in two patients; one ‘good responder and one ‘poor responder’. It contributes information toward understanding of the plasticity of neural networks for language and recovery. The fMRI data for the ‘good responder’ supports the hypothesis that restoration of parts of the LH language network is linked at least in part, to better recovery of naming and phrase length in nonfluent aphasia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold BT, Kertesz A. Right hemisphere semantic processing of visual words in an aphasic patient: an fMRI study. Brain Lang. 2000;73(3):456–465. doi: 10.1006/brln.2000.2317. [DOI] [PubMed] [Google Scholar]
- 11.Karbe H, Thiel A, Weber-Luxenburger G, Herholz K, Kessler J, Heiss WD. Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang. 1998;64(2):215–230. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- 12.Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, Basso A, Fazio F. A fMRI study of word retrieval in aphasia. Brain Lang. 2003;85 (3):357–368. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- 13.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98(1):118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt 6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 15.Hillis AE, Kleinman JT, Newhart M, Heidler-Gary J, Gottesman R, Barker PB, Aldrich E, Llinas R, Wityk R, Chaudhry P. Restoring cerebral blood flow reveals neural regions critical for naming. J Neurosci. 2006;26(31):8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher LM, Kendall D, Swearengin JA, Rodriguez A, Leon SA, Pingel K, et al. A pilot study of use-dependent learning in the context of Constraint Induced Language Therapy. J Int Neuropsychol Soc. 2006;12(6):843–852. doi: 10.1017/S1355617706061029. [DOI] [PubMed] [Google Scholar]
- 17.Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36(8):1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- 18.Richter M, Miltner WH, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 2008;131(Pt 5):1391–1401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- 19.Small SL, Flores DK, Noll DC. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain Lang. 1998;62 (2):298–308. doi: 10.1006/brln.1998.1951. [DOI] [PubMed] [Google Scholar]
- 20.Leger A, Demonet JF, Ruff S, Aithamon B, Touyeras B, Puel M, Boulanouar K, Cardebat D. Neural substrates of spoken language rehabilitation in an aphasic patient: an fMRI study. Neuroimage. 2002;17 (1):174–183. doi: 10.1006/nimg.2002.1238. [DOI] [PubMed] [Google Scholar]
- 21.Cornelissen K, Laine M, Tarkiainen A, Jarvensivu T, Martin N, Salmelin R. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. 2003;15 (3):444–461. doi: 10.1162/089892903321593153. [DOI] [PubMed] [Google Scholar]
- 22.Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, Rockstroh B. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39(4):2038–2046. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Barlow T. Brit Med J. 1877:103. doi: 10.1136/bmj.2.865.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsbourne M. The Minor Cerebral hemisphere as a Source of Aphasic Speech. Archives of Neurology. 1971;25(4):302–306. doi: 10.1001/archneur.1971.00490040028003. [DOI] [PubMed] [Google Scholar]
- 25.Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, Dutschka K, Woods RP, Noth J, Diener HC. Recovery from Wernicke’s aphasia: a positron emission tomographic study. Ann Neurol. 1995;37(6):723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- 26.Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30(4):749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 27.Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36(1):159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- 28.Crosson B, Moore AB, Gopinath K, White KD, Wierenga CE, Gaiefsky ME, Fabrizio KS, Peck KK, Soltysik D, Milsted C, et al. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J Cogn Neurosci. 2005;17(3):392–406. doi: 10.1162/0898929053279487. [DOI] [PubMed] [Google Scholar]
- 29.Cherney LR, Small SL. Task-dependent changes in brain activation following therapy for nonfluent aphasia: discussion of two individual cases. J Int Neuropsychol Soc. 2006;12(6):828–842. doi: 10.1017/S1355617706061017. [DOI] [PubMed] [Google Scholar]
- 30.Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bezy C, Demonet JF, Cardebat D. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008;70(4):290–298. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez B, Cardebat D, Demonet JF, Joseph PA, Mazaux JM, Barat M, Allard M. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke. 2004;35(9):2171–2176. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- *32.Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS) Neurophysiol Clin. 2006;36(3):105–115. doi: 10.1016/j.neucli.2006.08.011. This article reviews how TMS has been applied to examine post-stroke recovery and neural plasticity in the domains of motor recovery, neglect, and aphasia, suggesting that TMS may be utilized effectively as a clinical rehabilitation tool. [DOI] [PubMed] [Google Scholar]
- 33.Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, et al. Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase. 2005;11(3):182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(5):800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- 35.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15(4):333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Mottaghy FM, Hungs M, Brugmann M, Sparing R, Boroojerdi B, Foltys H, Huber W, Topper R. Facilitation of picture naming after repetitive transcranial magnetic stimulation. Neurology. 1999;53(8):1806–1812. doi: 10.1212/wnl.53.8.1806. [DOI] [PubMed] [Google Scholar]
- 37.Epstein CM, Meador KJ, Loring DW, Wright RJ, Weissman JD, Sheppard S, Lah JJ, Puhalovich F, Gaitan L, Davey KR. Localization and characterization of speech arrest during transcranial magnetic stimulation. Clin Neurophysiol. 1999;110(6):1073–1079. doi: 10.1016/s1388-2457(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 38.Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review Brain. 1996;119 (Pt 5):1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- 39.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 40.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 41.Naeser MA, Martin PI, Fregni F, Theoret H, Kobayashi M, Nicholas M, Baker EH, Maria-Tormos J, Steven M, Pascual-Leone A. Suppression of Part of Right Anterior Broca’s Area Improves Naming in Chronic Nonfluent Aphasia. NeuroImage; Presented at The 8th Internation Conference on Functional Mapping of the Human Brain; June 2–6: 2002; Sendai, Japan. 2002. Abstract #133. [Google Scholar]
- 42.Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Martin PI, Naeser MA, Doron KW, Bogdan A, Baker EH, Kurland J, Renshaw P, Yurgelun-Todd D. Overt naming in aphasia studied with a functional MRI hemodynamic delay design. Neuroimage. 2005;28(1):194–204. doi: 10.1016/j.neuroimage.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 44.Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–177. [Google Scholar]
- 45.Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23(3):1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 46.Duffau H, Capelle L, Denvil D, Gatignol P, Sichez N, Lopes M, Sichez JP, Van Effenterre R. The role of dominant premotor cortex in language: a study using intraoperative functional mapping in awake patients. Neuroimage. 2003;20(4):1903–1914. doi: 10.1016/s1053-8119(03)00203-9. [DOI] [PubMed] [Google Scholar]
- 47.Naeser MA, Martin PI, Treglia E, Ho M, Baker E, Kaplan E, Bashir S, Pascual-Leone A. Improved action naming in a severe, nonfluent aphasia case following transcranial magnetic stimulation plus constraint-induced language therapy. Brain and Language; To be presented as a poster at The 47th Annual Meeting of the Academy of Aphasia; October18–20, 2009; Boston, MA. 2009. [abstract, in press] [Google Scholar]
- 48.Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44 (8):953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Iacoboni M. The role of premotor cortex in speech perception: evidence from fMRI and rTMS. J Physiol Paris. 2008;102(1–3):31–34. doi: 10.1016/j.jphysparis.2008.03.003. This article discusses fMRI and rTMS data that suggest a direct involvement of premotor cortical areas (mirror neurons) in speech perception, thus advocating an active role of motor structures in speech perception. These ideas support the Liberman et al., 1957, 1967 “motor theory of speech perception.”. [DOI] [PubMed] [Google Scholar]
- 51.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 52.Meister IG, Wilson SM, Deblieck C, Wu AD, Iacoboni M. The essential role of premotor cortex in speech perception. Curr Biol. 2007;17(19):1692–1696. doi: 10.1016/j.cub.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Cotelli M, Manenti R, Cappa SF, Zanetti O, Miniussi C. Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur J Neurol. 2008;15(12):1286–1292. doi: 10.1111/j.1468-1331.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 55.Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, Vergari M, Zago S, Priori A. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79 (4):451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]


