Abstract
Triacylglycerol (TAG) stored in adipose tissue can be rapidly mobilized by the hydrolytic action of lipases, with the release of fatty acids (FA) that are used by other tissues during times of energy deprivation. Unlike synthesis of TAG, which occurs not only in adipose tissue but also in other tissues such as liver for very-low-density lipoprotein formation, hydrolysis of TAG, lipolysis, predominantly occurs in adipose tissue. Until recently, hormone-sensitive lipase was considered to be the key rate-limiting enzyme responsible for regulating TAG mobilization. However, recent studies on hormone-sensitive lipase-null mice have challenged such a concept. A novel lipase named desnutrin/ATGL has been recently discovered to play a key role in lipolysis in adipocytes. Lipolysis is under tight hormonal regulation. Although opposing regulation of lipolysis in adipose tissue by insulin and catecholamines is well understood, autocrine/paracrine factors may also participate in its regulation. Intricate cooperation of these endocrine and autocrine/paracrine factors leads to a fine regulation of lipolysis in adipocytes, needed for energy homeostasis. In this review, we summarize and discuss the recent progress made in the regulation of adipocyte lipolysis.
Keywords: fatty acids, desnutrin/ATGL, hormone-sensitive lipase, catecholamines, insulin
Adipocytes Play a Critical role in energy homeostasis by hydrolysis (lipolysis) of their triacylglycerol (TAG) reserves to provide fatty acids (FA) that are important oxidative fuels for other tissues during times of energy deprivation such as fasting and exercise. A dysregulation of lipolysis may lead to metabolic abnormalities. Reduced lipolytic activity may contribute to accumulation of TAG in adipose tissue and thus obesity. On the other hand, excessive lipolysis may contribute to lipodystrophy syndromes, which are characterized by a significant loss or redistribution of TAG in adipose tissue depots, which may lead to higher circulating FA and ectopic storage of TAG. These abnormalities are associated with the development of insulin resistance. Thus fine regulation of lipolysis is crucial for maintenance of body energy homeostasis as well as for the prevention of metabolic diseases.
Regulation of HSL
During the lipolytic process, one molecule of glycerol and three molecules of FA are produced by the hydrolysis of one molecule of TAG. FA are released into the circulation to be taken up by other tissues. FA can also be oxidized or used for reesterification in adipocytes to produce TAG. Lipolysis is under tight regulation by hormones, i.e., catecholamines and insulin, of which secretion is under nutritional regulation. For many years, hormone-sensitive lipase (HSL) has been considered to be the regulatory enzyme, hydrolyzing FA the from sn-1 and sn-3 position of TAG to generate 2-monoacylglycerol (MAG), which subsequently requires monoacylglycerol lipase for complete hydrolysis (11). According to this model, adipocyte lipolysis is activated by catecholamines, in times of energy need such as fasting and exercise. Hormone binding to Gαs coupled receptors results in increased adenylate cyclase activity. The stimulation of adenylate cyclase leads to an increase in intracellular cAMP concentrations, resulting in activation of cAMP-dependent PKA. PKA phosphorylates HSL at three serine residues (563, 659, and 660) in a 150-amino acid stretch, termed the regulatory module. This regulatory module is found within the COOH-terminal domain of HSL, which also contains the catalytic triad (Ser-423, Asp-703, and His-733). Phosphorylation of HSL results in increased hydrolytic activity, translocation of HSL from cytosol to the lipid droplet surface, and enhanced TAG breakdown in the cell. In addition to the PKA-mediated phosphorylation, HSL may be phosphorylated by other kinases such as ERK1/2 through the PMA/PKC/MAPK pathway and AMP-activated protein kinase (AMPK), a kinase proposed to be a cellular energy sensor, as well as cGMP-dependent kinase. In vitro, prior phosphorylation of HSL by AMPK prevented subsequent phosphorylation by PKA and vice versa. Therefore AMPK-mediated phosphorylation of HSL may have an antilipolytic effect.
The hydrolytic action of HSL is regulated by perilipin A, a lipid droplet-associated protein. Association of perilipin A with lipid droplet controls the magnitude of lipolysis. Thus perilipin A may act as a barrier to lipases, thereby maintaining a low rate of basal lipolysis. Upon hormonal stimulation, perilipin A undergoes phosphorylation by PKA at six serine residues, and phosphorylation at Ser-517 may globally regulate PKA-stimulated lipolysis in adipocytes (13, 24). PKA-dependent perilipin phosphorylation may facilitate the translocation of HSL to the lipid droplet. However, one recent study showed that PKA-dependent perilipin phosphorylation, although facilitating perilipin interaction with lipid droplet-associated HSL, is not involved in the translocation of HSL to the lipid droplet (14). Regardless, phosphorylation of both perilipin and HSL catalyzes efficient TAG breakdown into diacylglycerol (DAG) and MAG. Lipid droplet-associated proteins include the perilipin-adipophilin-TIP47 (PAT) family of proteins as well as other proteins such as adipocyte fatty acid-binding protein (aFABP/ALBP/aP2) and caveolin 1 (2, 23). PAT family proteins are known to be lipid droplet-associated proteins and also to be integral components of the plasma membrane (16). Although their function and distribution are not clear, it has been proposed that PAT family proteins with their fatty acid cargo from specialized plasma membrane domains transit into lipid droplets as part of the influx mechanism. Regardless, lipid droplets in adipocytes appear to acquire their PAT proteins and caveolin-1 by interaction with specialized plasma membrane domains (16). In this regard, Caveolin-1-null mice exhibit markedly attenuated lipolytic activity in their adipocytes and fail to properly mobilize stored TAG during fasting (2). A complex formation between perilipin, caveolin-1 and the catalytic subunit of PKA was reported be induced by β3AR agonist treatment (2). Thus the PAT and other lipid droplet-associated proteins may constitute a functionally important lipolytic complex in regulating lipolysis. In this regard, CGI-58 [comparative gene identification 58 alpha/beta-hydrolase domain containing protein 5 (CGI-58/ABHD5)], which is associated with Chanarin-Dorfman syndrome with excessive TAG accumulation in various tissues, also interacts with perilipin and thereby localizes to lipid droplets (19). CGI-58 has been shown to activate desnutrin/ATGL, but not HSL, to stimulate lipolysis (12). Molecular mechanisms underlying the activation of lipolysis by CGI-58 may also provide better understanding on the function of perilipin and other lipid droplet-associated proteins.
Desnutrin/ATGL, the Major TAG Lipase in Adipocytes
Studies on HSL-null mice, however, highlighted the importance of other lipases in TAG hydrolysis in adipocytes. HSL-deficient mice fed a high-fat diet exhibited normal body weight and decreased fat mass, but their white adipose tissue still retained ~40% of TAG lipase activity compared with wild-type mice (15, 22). Moreover, lipolytic response to extended fasting appeared normal in HSL-null mice with adequate or even heightened mobilization and oxidation of FA. This suggests that at least one unidentified lipase must exist and is enzymatically active when HSL is absent. In addition, HSL deficiency led to the accumulation of DAG in adipose tissue of HSL knockout mice without significant change in TAG levels, suggesting that HSL is rate limiting for DAG hydrolysis rather than TAG hydrolysis (6). In vitro studies provide additional evidence that HSL may be rate limiting in DAG, but not TAG hydrolysis: HSL has broad substrate specificity and can hydrolyze TAG, DAG, and MAG as well as cholesteryl and retinyl esters. However, HSL is more active against DAG and cholesteryl esters (CEs) than TAG and MAG. For example, HSL has 10-fold higher activity with DAG compared with TAG in vitro. On the other hand, HSL may be the only, and at least, the major CE hydrolase (9); HSL has high activity toward CE in vitro and, in HSL-null mice, hydrolytic capacity toward CE was totally lost. Overall, these findings indicate that another lipase exhibits a preference for the hydrolysis of the first ester bond of the TAG molecule.
Recently, our laboratory and subsequently two other laboratories identified a novel TAG lipase named desnutrin/ATGL (also called PNPLA2, iPLA2ζ, or TTS2.2) that indeed exhibited many predicted properties of the not-yet-identified adipose lipase(s) described above (7, 21, 25). Desnutrin/ATGL is a 486-amino-acid protein with a calculated molecular mass of 54 kDa. Desnutrin/ATGL contains a patatin-like domain in the NH2-terminal region. The domain owes its name to patatin, a storage protein found in potato and other solanaceous plants that display lipid acyl hydrolase and broad esterase activity. The homology of desnutrin/ATGL to the patatin domain-containing protein family suggested desnutrin/ATGL as a lipase. Three highly conserved regions could be identified in the patatin domain of desnutrin/ATGL and related proteins: a glycine-rich GXGXXG nucleotide binding motif, a GXSXG serine hydrolase motif (characteristic of serine esterase), and a DX(G/A) motif containing a conserved aspartate residue. The serine and aspartate residues constitute a catalytic dyad that is required for the lipase activity of patatin. Overexpression of desnutrin/ATGL revealed increase in TAG breakdown and release of FA, demonstrating a role of desnutrin/ATGL as TAG lipase. In vitro lipid hydrolase assays confirmed that desnutrin/ATGL is a TAG hydrolase and, in contrast to HSL, does not hydrolyze cholesteryl or retinyl ester bonds (25). Functional importance of desnutrin/ATGL as TAG hydrolase has also been confirmed by Drosophila melanogaster ortholog, brummer, and Saccharomyces cerevisiae TGL3 (4, 5, 18). In mice, desnutrin/ATGL ablation increased adipose tissue mass by about twofold and caused lipid deposition in other tissues, particularly in heart (5). Ablation or overexpression of desnutrin/ATGL restricted to adipose tissue, however, will help to unravel more clearly the role of desnutrin/ATGL in adipose tissue.
Although expressed predominantly at a high level only in adipose tissue, desnutrin/ATGL mRNA is also found at a low level in a variety of tissues. Desnutrin/ATGL expression is upregulated during adipocyte differentiation (21). The favored expression of desnutrin/ATGL in adipocytes clearly confirms the involvement of the desnutrin in a function preferential but not exclusive to adipocytes. Desnutrin/ATGL expression is induced by glucocorticoids whose levels also increase during fasting (21). On the other hand, desnutrin/ATGL expression was downregulated by refeeding and insulin (8). Thus desnutrin/ATGL, together with HSL, may increase hydrolysis of adipose TAG in fasting. Interestingly, desnutrin/ATGL mRNA was downregulated in db/db and ob/ob mice, suggesting that desnutrin/ATGL may contribute to the development of obesity by reducing TAG accumulation due to stimulation of lipolysis in adipose tissue (21). The regulation of desnutrin/ATGL activity appears quite different compared with that described for HSL. First, desnutrin/ATGL could be a phosphoprotein but its phosphorylation does not appear to be mediated by PKA (25). Second, desnutrin/ATGL is localized on the lipid droplet in the basal and hormone-stimulated state of the cell, suggesting that desnutrin/ATGL may not be activated by translocation to the lipid droplet as demonstrated for HSL (25). Thus it is not known whether the phosphorylation of desnutrin affects its localization or catalytic activity. Additional studies are also needed to fully understand the role of perilipin in desnutrin/ATGL regulation and its relationship with HSL in TAG hydrolysis in adipocytes.
In addition to HSL and desnutrin/ATGL, additional enzymes have been implicated in adipocyte lipolysis: they include TAG hydrolases as well as other patatin-like domain-containing proteins including adiponutrin (iPLA2ε/PNPLA3), GS2 (iPLA2η/PNPLA4), and GS2-like (PNPLA5), which all have been reported to have hydrolase activity in vitro (7). GS2 and GS2-like protein, but not adiponutrin, increased lipolysis when overexpressed (10). This suggests that GS2 and GS2-like protein may play a role in lipolysis whereas adiponutrin may have a distinct function in adipocytes. Interestingly, adiponutrin is specifically expressed in adipocytes, is not detectable in the fasted state, but is highly induced in fed or in obese states (10). Complicating the matter further, adiponutrin and GS2 as well as the now-identified TAG lipase, desnutrin/ATGL, were all reported to have transacylase as well as phospholipase activity in vitro (7). Regardless, desnutrin/ATGL and HSL are quantitatively the most important lipases found in adipose tissue (17).
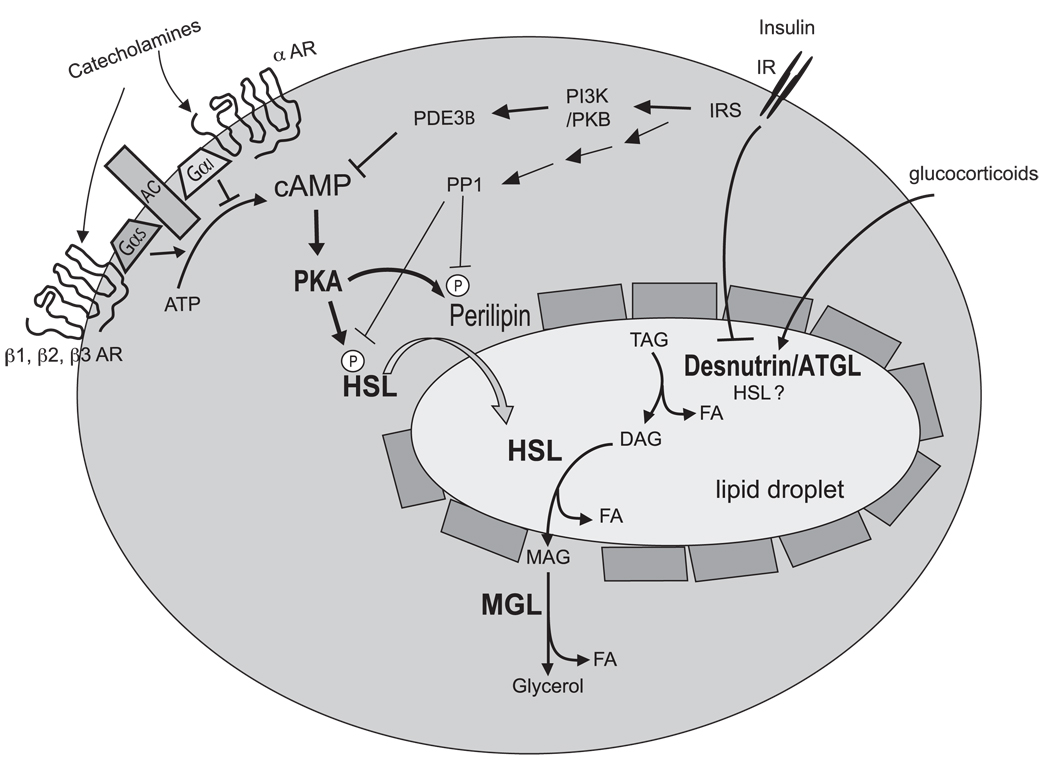
Thus, in the current proposed model of the lipolytic cascade, lipolysis is catalyzed by at least three enzymes: Desnutrin/ATGL catalyzes predominantly the hydrolysis of the first ester bond in TAG (although a fraction of TAG hydrolysis may be catalyzed by HSL). Then, the resulting DAG is hydrolyzed by the HSL-catalyzed reaction to generate MAG. Finally, the hydrolysis of MAG is catalyzed by monoacylglycerol lipase, which is abundant and not regulated (see Fig. 1).
Fig. 1.
Regulation of lipolysis in adipocytes. Lipolysis is under tight hormonal control; it is stimulated during fasting by increased catecholamines (via increased cAMP) and glucocorticoids but is suppressed in the fed state by insulin action. Lipolysis is catalyzed by 3 lipases. Desnutrin/ATGL predominantly performs the initial step in triacylglycerol (TAG) hydrolysis resulting in the formation of diacylglycerol (DAG) and fatty acid (FA). Hormone-sensitive lipase (HSL) catalyzes hydrolysis of TAG, DAG, and monoacylglycerol (MAG) at a ratio of 1:10:1 in vitro. Monoacylglycerol lipase (MGL) catalyzes hydrolysis of MAG to form glycerol and FA. AC: adenylate cyclase; αAR: alpha-adrenergic receptor; Gαs: stimulatory GTP-binding protein alpha subunit; Gα1: inhibitory GTP-binding protein alpha subunit.
Hormonal Regulation of Lipolysis
Opposing regulation of lipolysis in adipose tissue by catecholamines and insulin has been well documented. During fasting, catecholamines are the major hormones to markedly stimulate lipolysis, especially in humans (1, 11). These hormones may reach adipose tissue via circulation (mainly epinephrine) or via sympathetic innervation (norepinephrine). Lipolytic action of catecholamines is mediated by three different β-adrenergic receptor subtypes: β1AR, β2AR, and β3AR. Whereas β1AR and β2AR are broadly expressed throughout tissues of the body, β3AR is found predominantly in white and brown adipocytes in rodents and is poorly expressed in human adipocytes. Each of these receptors is coupled to Gαs, and their activation by catecholamines leads to the lipolytic cascade (as discussed above). In contrast to rodents, human adipocytes express significant levels of α2AR. Catecholamines may exert an antilipolytic effect in human adipocytes by binding to Gαi-coupled α2AR, leading to a decrease in intracellular cAMP levels. Thus α/βAR balance may be important in regulation of adipocyte lipolysis and obesity in humans. In this regard, α2AR transgenic mice on a β3AR knockout background showed reduced catecholamine-stimulated lipolysis and developed obesity (20). Along with catecholamines, glucocorticoids are also elevated in fasted state. As indicated above, by inducing desnutrin/ATGL expression, glucocorticoids may also participate in stimulation of lipolysis in adipose tissue during fasting (21). On the other hand, insulin is by far the most potent antilipolytic hormone. In the fed state, insulin inhibits lipolysis, which brings about dephosphorylation of HSL as well as activation of phosphodiesterase that reduces cAMP levels (1, 11). Insulin induces phosphorylation and activation of PDE3B, which appear to be associated with caveolae in adipocytes. PDE3B has been shown to be inhibited by a dominant-negative PKB/Akt, suggesting that the PI3K/Akt pathway is necessary for insulin-induced phosphorylation and activation of PDE3B. Phosphorylation at serine-273 of PDE3B by PKB/Akt may be responsible for the insulin mediated activation of PDE3B. Insulin may also suppress lipolysis by activation of protein phosphatase-1 through phosphorylation of its regulatory subunit. Activated protein phosphatase-1 rapidly dephosphorylates and deactivates HSL, decreasing lipolysis.
Although opposing regulation of lipolysis by insulin and catecholamines is well characterized, not much is known about regulation of lipolysis by other hormones and autocrine/paracrine factors. It has been reported that thyroid hormone, growth hormone, natriuretic peptide, alpha-melanocyte-stimulating hormone (α-MSH), as well as TNF-α stimulate lipolysis whereas adenosine and neuropeptide Y inhibit lipolysis (11). PGE2 has been reported to have a biphasic effect: PGE2 at nanomolar concentrations suppressed lipolysis, whereas at micromolar concentrations it stimulated lipolysis (3). On the other hand, PGI2 showed no effect in general or exerted a biphasic effect: PGI2 at nanomolar concentrations stimulated lipolysis, whereas at micromolar concentrations it suppressed lipolysis. Along with primary hormones that regulate lipolysis, insulin and catecholamines, autocrine/paracrine factors may participate for the precise regulation of lipolysis in adipocytes to meet the physiologic and metabolic changes. The signal transduction pathways and molecular mechanisms underlying regulation of lipolysis in response to various hormones and autocrine/paracrine factors need to be elucidated in the future.
In conclusion, adipocyte lipolysis is a complex process that is tightly controlled through integration of multiple and diverse hormonal and biochemical signals. Breakdown of this regulation may contribute to the development of obesity and associated pathologies. Many exciting advances have been made recently, including the discovery of major lipases that catalyze adipocyte lipolysis. However, questions remain such as the regulation of lipolytic enzymes and their coordinate interaction as well as the cooperation between endocrine and autocrine/paracrine signals regulating lipolysis in adipocytes. As genetic mouse models of lipases are generated, it is likely that our understanding of adipocyte lipolysis will be achieved in the near future.
Acknowledgments
GRANTS
The work from the authors’ laboratory was supported by National Institutes Health grants to H. S. Sul.
REFERENCES
- 1.Carmen GY, Victor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18:401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE, Lisanti MP. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Luria RRG. Prostaglandin E2 can bimodally inhibit and stimulate the epididymal adipocyte adenylyl cyclase activity. Cell Signal. 1992;4:331–335. doi: 10.1016/0898-6568(92)90073-h. [DOI] [PubMed] [Google Scholar]
- 4.Gronke S, Mildner A, Fellert S, Tennagels N, Petry S, Muller G, Jackle H, Kuhnlein RP. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1:323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 6.Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 8.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- 9.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 10.Lake AC, Sun Y, Li JL, Kim JE, Johnson JW, Li D, Revett T, Shih HH, Liu W, Paulsen JE, Gimeno RE. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J Lipid Res. 2005;46:2477–2487. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi H, Perfield JW, 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS. Control of ATGL action by serine 517 of perilipin A globally regulates PKA-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem. 2006;281:15837–15844. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
- 15.Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robenek H, Robenek MJ, Buers I, Lorkowski S, Hofnagel O, Troyer D, Severs NJ. Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem. 2005;280:26330–26338. doi: 10.1074/jbc.M413312200. [DOI] [PubMed] [Google Scholar]
- 17.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 18.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7:106–113. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 20.Valet P, Grujic D, Wade J, Ito M, Zingaretti MC, Soloveva V, Ross SR, Graves RA, Cinti S, Lafontan M, Lowell BB. Expression of human alpha 2-adrenergic receptors in adipose tissue of beta 3-adrenergic receptor-deficient mice promotes diet-induced obesity. J Biol Chem. 2000;275:34797–34802. doi: 10.1074/jbc.M005210200. [DOI] [PubMed] [Google Scholar]
- 21.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 22.Wang SP, Laurin N, Himms-Hagen J, Rudnicki MA, Levy E, Robert MF, Pan L, Oligny L, Mitchell GA. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obes Res. 2001;9:119–128. doi: 10.1038/oby.2001.15. [DOI] [PubMed] [Google Scholar]
- 23.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3–12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–19155. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HH, Souza SC, Muliro KV, Kraemer FB, Obin MS, Greenberg AS. Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J Biol Chem. 2003;278:51535–51542. doi: 10.1074/jbc.M309591200. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]