Summary
Seasonally breeding mammals such as sheep use photoperiod, encoded by the nocturnal secretion of the pineal hormone melatonin, as a critical cue to drive hormone rhythms and synchronise reproduction to the most optimal time of year [1, 2]. Melatonin acts directly on the pars tuberalis (PT) of the pituitary, regulating expression of thyrotropin (TSH) which then relays messages back to the hypothalamus to control reproductive circuits [3, 4]. In addition, a second local intra-pituitary circuit controls seasonal prolactin (PRL) release via a currently uncharacterised low molecular weight peptide(s) termed tuberalin(s) of PT origin [5–7]. Studies in birds identified the transcription factor Eya3 as the first molecular response activated by long photoperiods (LP) [8]. Using arrays and in situ hybridization studies, we show Eya3 as the strongest LP activated gene in sheep, revealing a common photoperiodic molecular response in birds and mammals. We also identified TAC1 (encoding the tachykinins Substance P and Neurokinin A; NKA) to be strongly activated by LP within the sheep PT. We show that these PRL secretagogues act on primary pituitary cells, and are thus candidates for the elusive PT-expressed “tuberalin” seasonal hormone regulator.
Keywords: Photoperiod, Pituitary, Pars tuberalis, circadian, Eya3, Tachykinins
Results and Discussion
Seasonal neuroendocrine regulation by the Pars Tuberalis
In birds and mammals, the PT operates as a crucial relay conveying thyroid-dependent effects of photoperiod change to local hypothalamic pathways governing reproductive changes [3]. Recent studies in the Japanese quail examined the pattern of gene activation following exposure to reproductive stimulatory LP. This revealed that TSHβ and the transcriptional co-factor eyes absent-3 (Eya3) were the first genes activated in the PT on Day 1 following LP exposure [8]. In seasonal mammals, the PT is also known to play a direct role in the regulation of annual prolactin (PRL) cycle. This hormone is strongly photoperiodic, with secretion rising over the first few days of LP exposure [9]. Studies in both sheep and seasonal rodent models (Syrian hamsters) show that ex vivo PT cultures secrete a low molecular weight (1–10 kDa) peptidergic PRL releasing factor (termed “tuberalin(s)”), which drives seasonal PRL secretion from dispersed cultures of anterior pituitary cells [5–7], and “tuberalin” activity is modified by the prior photoperiodic regime which animals were exposed to (augmented during LP exposure [7]). Further, hypothalamo-disconnection experiments in sheep demonstrate robust photoperiodic rhythms of PRL following severance of the anatomical links between the pituitary and secretory nerve terminals of the hypothalamus [10]. Thus, seasonal PRL response depends on a local paracrine PRL-stimulating factor of PT origin acting on distal lactotroph cells. This second circuit has so far only been described in mammals, and the identity of tuberalin(s) has remained enigmatic.
Array studies reveal novel PT activated gene in sheep following LP stimulation
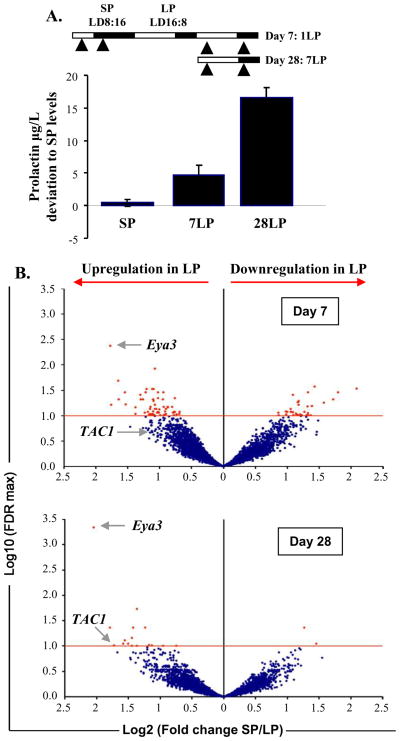
To identify circuits and possible PRL regulating genes activated in the PT following LP stimulation, we collected sheep PT tissue 4h after lights on (ZT4) or off (ZT20) after 7 or 28 days of LP exposure and compared transcript levels with those of PT from SP-housed sheep culled at the same time points (ZT4 and ZT12) (Figure 1A). These times of collection were selected to match to the earliest time when robust PRL responses are detected (Day 7), and when PRL responses are close to maximal (Day 28) [4, 9, 10] as shown in Figure 1A. Using a bovine cDNA array previously validated for use in sheep [11], we screened for altered expression of transcripts at each of the above time points following LP exposure, and compared them against SP values. The comparison involved hybridization against a common reference pool (see supplemental experimental procedures) and as a result was only likely to detect strongly differentially expressed genes. Bioinformatic analyses revealed that the majority of significantly displaced genes occurred in the light phase of LP (ZT4) (Table S1). We therefore focused our interest on changes occurring at Day 7 and 28 at ZT4 to annotate significantly altered genes and found 98 known genes altered in expression at Day7 (64 increased, 34 suppressed) and 18 genes on Day 28 (16 increased and 3 suppressed) (Figure 1B, Table S2). We compared our data on transcripts exhibiting altered expression in the PT with data sets of genes known to exhibit strong circadian expression in several different tissues in the mouse (suprachiasmatic nucleus, kidney, liver and aorta) [12, 13]. This sub-set of mouse cycling genes thus represents a core group of clock-controlled genes likely to cycle in most body tissues. A comparison with ovine PT transcripts revealed that approximately 50% of these genes with altered expression on LP are also cyclic in mouse (supplementary Table 2). In mice, the overall proportions of cycling transcripts in different body organs varies, but an extensive recent study using appropriate statistical power to detect cycling revealed that approximately 7% of transcripts cycled in liver, and 1% in pituitary[14]. Our data thus reveal a remarkable enrichment of circadian transcripts in the PT, and highlight the likely critical importance of the circadian clockwork in encoding seasonal time [15].
Figure 1. Transfer from SP to 7 or 28 days in LP significantly alters circulating prolactin blood levels and gene expression in the PT, in sheep.
A. Prolactin (PRL) concentrations in SP and after 7 and 28 days in LP (7LP and 28LP). 30 sheep were maintained under artificial short photoperiod (SP). After 8 weeks, 5 were killed either 4 h after the light onset or 4 h after the light offset (arrowheads). The rest of the cohort (n=20) was transferred to long photoperiod (LP) and groups of 5 animals were killed either 7 or 28 days after the transfer (again 4 h after the light onset and 4 h after the light offset (arrowheads)). B. Volcano plots representing the alteration in gene expression in the PT after 7 and 28 days in LP with a ratio of expression in SP over LP on the x-axis and the significance of the changes with a False Discovery Rate (FDR) threshold (red line) set at 10% on the y-axis. Eya3 and TAC1 indicated by the arrows show significant alteration in expression after 7 and 28 days in LP for Eya3 and after 28 days in LP for TAC1. See also Table S2 for lists of significantly altered genes in LP.
Long Day activation of Eya3 in the ovine PT
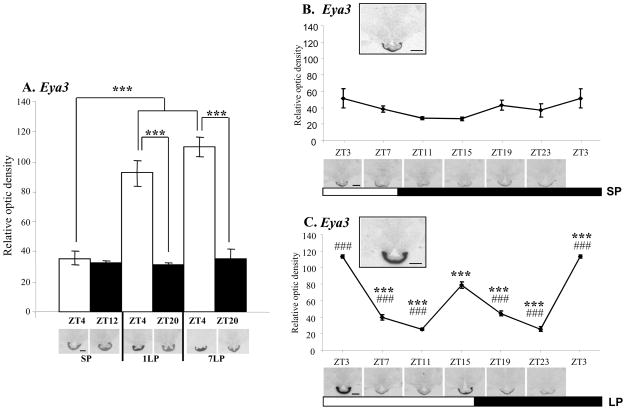
On Day 7 and 28, the most strongly up-regulated gene in the array was Eya3 (Figure 1B). In order to define tissue expression with higher resolution quantification and to determine the time course of Eya3 up-regulation in the sheep PT, we examined its expression by in situ hybridization in sheep. For this purpose, we set up another cohort of animals kept in SP and then exposed to 1 or 7 days of LP stimulation, and culled 4h into the light phase (ZT4) or 4h into the dark phase (ZT12 or 20 for SP or LP housed animals respectively; Figure 2A). Eya3 expression was specific to the PT region, with no detectable hybridization signal in the adjacent hypothalamus (Figure 2A–C) or distal pituitary (data not shown). On SP, a hybridization signal was detected, with no change between ZT4 and ZT12. Upon transfer to LP, we observed an abrupt increase in expression at ZT4 on Day 1, which was sustained at Day 7 (Figure 2A). In order to define more precisely the temporal expression of Eya3, we collected samples over a 24h period at 4 hours intervals from SP-housed sheep, or 28 days after LP exposure. On SP, Eya3 expression showed no significant variation over 24h (Figure 2B). In contrast, on LP, we observed a strong early day peak (as seen above on Days 1 and 7) as well as a second peak in the late photophase (Figure 2C). These results confirmed the array results on Days 7 and 28, but also provided the information that Eya3 acts as an acute response to photoperiod with an up-regulation on Day 1 onwards (Figure 2A). These data parallel an earlier study in quail in which Eya3 was identified as a first long day response gene in the avian PT [8]. Comparison of LP-activated genes here with the quail data revealed that Eya3 was the only gene in common with both data sets. In our study, Eya3 was the most strongly differentially expressed transcript on the entire array (over 3 to 4 fold; Figure 1B, Table S2). Unlike in the quail, we show that Eya3 is not only activated during the first days of exposure to LP in sheep but continues to provide a sustained marker for LP activation many weeks later and interestingly we also identified a strong biphasic pattern of expression in LP with a peak in the early and late photophase (Figure 2A, 2C).
Figure 2. Quantification of Eya3 mRNA expression in the sheep PT.
Quantification and representative expression of Eya3 expression in the sheep PT on coronal 20μM cryostat sections, Scale bar: 200μm. A. Eya3 shows a significant up-regulation in the sheep PT in the light phase (ZT4) on Day 1 and Day 7 of LP exposure. B. Eya3 expression in the PT shows low levels of expression in the PT under SP conditions with no significant variation over 24 h. Insert panel shows expression at ZT3. C. On Day 28 of LP, sampling over a 24 h period shows that Eya3 expression is biphasic with a peak at the early and late light phase (ZT3 and ZT15). Insert panel shows expression at ZT3.
Statistical significance was assessed using two-ways ANOVA followed by Bonferroni post-hoc test for time of the day and photoperiodic effect with ***p<0.001 (A.) and one-way ANOVA followed by Bonferroni post-hoc test for ZT3 (***p<0.001 ) and ZT15 (###p<0.001 ) compared to the other time points in SP (B.) and in LP (C.).
Identification of a prolactin-regulating pathway
Since the identification of factors released by the PT driving the seasonal PRL pituitary secretion in seasonal mammals, the existence of “tuberalin(s)” has remained elusive. We next screened our array data for differentially expressed genes coding for putative PRL secretagogues. We identified TAC1 (Tachykinin1, Figure 1B, Table S2) as the only potential candidate gene showing significant up-regulation on Day 28. TAC1 encodes for tachykinins (reviewed in [16]), bioactive peptides including Substance P and Neurokinin A (NKA), which have been shown to be capable of regulating PRL release in vivo (reviewed in [17]). Moreover these peptides are in the correct molecular weight range (1–10 kDa) to act as candidate tuberalin(s) [6].
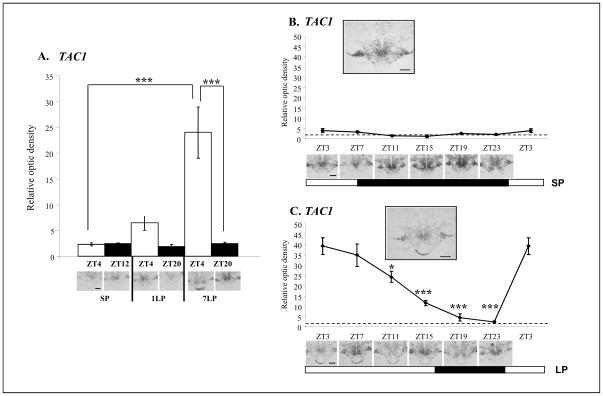
We used in situ hybridisation for the ovine TAC1 in the PT to determine more precisely the onset of the effect of LP exposure on its expression. TAC1 has been previously reported to be expressed in the hypothalamus at the mRNA level (in rat, human or monkey [18, 19]) and this was confirmed here in sheep (Figure 3A–C) with no significant variation across daytime or photoperiod. In the PT, we did not observe a detectable hybridization signal under SP conditions (Figure 3A). However, following exposure to LP we observed induction in the photophase, moderate on Day 1 and significantly stronger (4–5 fold induction) on Day 7 in the PT (Figure 3A). We next investigated the temporal expression of TAC1 in the PT, using sheep kept in SP or for 28 days in LP as described above. There, TAC1 expression exhibited a 10-fold induction at the onset of the day, with gradual decrease of expression over the photophase (Figure 3C). These data reveal that TAC1 is induced in the ovine PT by LP exposure with a gradually augmented response from Day 1 to Day 7 (Figure 3A) and Day 28 (Figure 3C), matching the rise in PRL secretion over the same time course.
Figure 3. Quantification of TAC1 mRNA expression in the sheep PT.
Quantification and representative expression of TAC1 expression in the sheep PT on coronal 20μM cryostat sections, Scale bar: 200μm. A. TAC1 expression is up-regulated in the light phase (ZT4) in LP compared to SP with a progressive rise from one day in LP (1LP) to seven days in LP (7LP). B. TAC1 expression could not be detected in the PT under SP conditions. The dotted line represents the non-specific background expression as observed with a sense probe. Insert panel shows expression at ZT3. C. TAC1 is strongly expressed in the photophase on Day 28 of LP exposure with a peak in the early light phase and a progressive decline towards the end of the day. Insert panel shows expression at ZT3.
Statistical significance was assessed using two-ways ANOVA followed by Bonferroni post-hoc test for time of the day and photoperiodic effect with ***p<0.001 (A.) and one-way ANOVA followed by Bonferroni post-hoc test for ZT3 (*p<0.05, ***p<0.001 ) compared to the other time points in SP (B.) and in LP (C.).
Pituitary responses to tachykinins
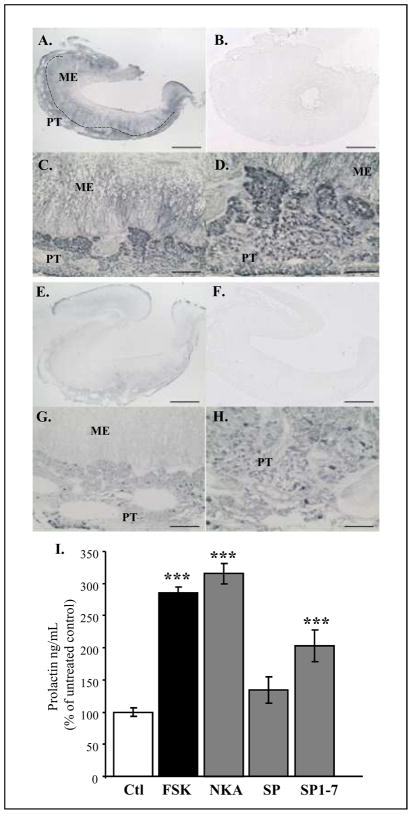
Earlier studies have reported Substance P and NKA in the ovine PT and had suggested that these peptides could act as PRL secretagogues [20, 21]. Previous studies in rodents showed that Substance P and NKA can act as potent regulators of pituitary hormone secretion. Using sheep as a model, we first confirmed the localisation of both peptides in the PT by immunohistochemistry (Figure 4A–H) and then investigated their effects on the PD cells. We collected ovine pituitary tissue, and treated dispersed cells with NKA or Substance P, using the adenylase cyclase activator forskolin as a positive control (Figure 4I). As Substance P is known to be cleaved by endopeptidases, we tested here a known bioactive cleavage product of Substance P (Substance P 1-7) [22] (Figure 4I). NKA treatment induced a strong induction of PRL secretion, and of similar magnitude to that observed following forskolin treatment. Although our data showed no significant effect of Substance P on PRL release, SP1-7 caused a significant stimulatory effect (Figure 4I). The action of SP1-7 compared to SP itself suggests the importance of Substance P pre-processing by endopeptidases within the PT. We have recently undertaken a screen for mRNA expressed in the PT using “deep-sequencing” Illumina NGS technology and have identified endopeptidase 24.11, responsible for the cleavage of Substance P into Substance P1-7 [22], as being expressed in this tissue (Burt, Yu, Dupre, McNeilly and Loudon unpublished). This implies that Substance P could be activated in the PT itself. Tachykinins have been described in the hypothalamus and the PD in mammals and their role in PRL regulation has been the focus of several studies (reviewed in [17]). Our data now provide the evidence for specific photoperiod-dependant expression of TAC1 in the PT, consistent with the hypothesis that products of the TAC1 gene (Substance P and NKA) may act as a seasonal PRL regulator on distal lactotroph cells.
Figure 4. Substance P and Neurokinin A are present in the ovine PT and act as potential secretagogues on pituitary cells.
Immunohistochemistry for Substance P (A–D) and Neurokinin A (NKA) (E–H) showing that both peptide are expressed in the PT. Pre-adsorption of the antibodies with blocking peptide for Substance P (B.) or NKA (F.) show no specific staining. The dotted line in (A.) represents the limit between the PT (pars tuberalis) and the ME (median eminence). Scale bar = 500μm (A,B,E,F), 125μm (C,G), 72.5 μm (D,H).
I. Radio-immuno assays for PRL secretion from ovine pituitary dispersed cells after 1h treatment with Forskolin 10μM (FSK), NKA, Substance P (SP) and a short fragment for Substance P (SP1-7) at 10−9M. Statistical significance against untreated control (Ctl) was set at *p<0.05, **p<0.01, ***p<0.001 using one-way ANOVA followed by Bonferroni post-hoc test.
Expression of Neurokinin receptors
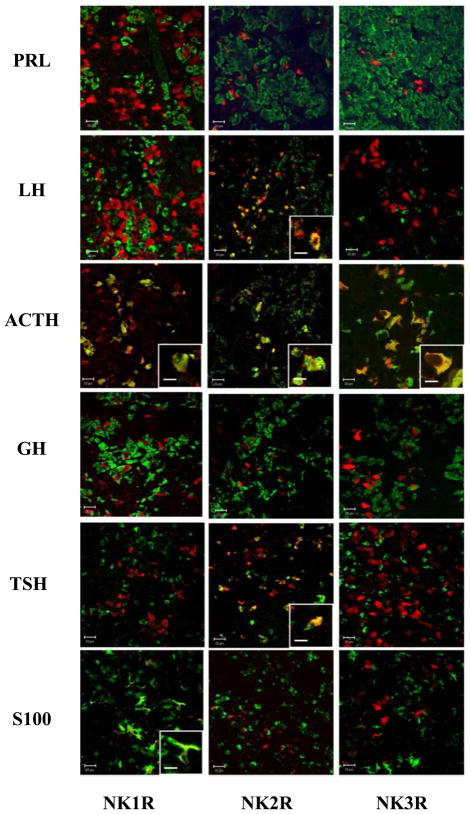
Three cognate G-protein receptors have been described for tachykinins (NK1-3R binding with differing affinities to Substance P and NKA in the order NK1R: Substance P> NKA; NK2R: NKA>Substance P and NK3R: NKA>Substance P [23]. Using immunohistochemistry, we examined expression patterns for these receptors in the sheep PD (Figure 5). This revealed that NK1-3R were all expressed in the hormone-secreting cells of the sheep pars distalis (PD) (Figure 5). However co-localisation patterns for each of the main pituitary secretory cell types (lactotroph, somatotroph, gonadotroph, thyrotroph and corticotroph) and folliculo-stellate cells, revealed that the major receptor for NKA (NK2R) was localised clearly in corticotrophs, gonadotrophs and at a low level in thyrotrophs, and the major receptor for Substance P (NK1R) in corticotrophs and folliculostellate cells. Although expression of NK2R has previously been reported in a subset of lactotrophs cells in rat PD cultures [24], we were unable to identify co-localisation of NK1-3R in lactotrophs, indicating that the effect of these peptides on PRL secretion is likely to be indirect. Our results also indicate co-localisation for NK1R with corticotroph and folliculo-stellate cells, and also NK3R with gonadotroph cells (Table S3).
Figure 5. Characterisation of the cell types expressing Neurokinin receptors in the ovine PD.
Double immunofluorescence showing expression of NK1, 2 and 3R (red) in the main pituitary cell types (green): lactotrophs (PRL), gonadotrophs (LH), corticotrophs (ACTH), somatotrophs (GH), thyrotrophs (TSH) and folliculo-stellate cells (S100). NK1R is co-localised with ACTH and S100, NK2R with LH, ACTH and TSH and NK3R with ACTH as shown in insert panels. Scale bar =20μm (10μm in the insert panels).
See table S3 for percentage of pituitary cell types co-expressing NK1-3R.
Lack of co-localisation of receptors for tachykinins (NK1-3R) to cells of the lactotroph cell lineage suggest that seasonal PRL control may involve an additional intra-pituitary signaling pathway, with NKA and activated Substance P acting on other secretory cell types, which may release paracrine factors controlling hormone secretion from neighboring lactotrophs (Figure 6). Several studies in sheep have shown that prolactin is secreted in a diurnal pattern, with elevated levels in the late day/nocturnal period [25]. Our data here imply that the prolactin-releasing action of tachykinins is likely mediated via intermediate cell types, with unknown temporal dynamics in terms of de-novo RNA transcription and/or local hormone release in target cells of the pars distalis. Thus, rhythmic TAC1 transcripts in the PT over the light phase may be phase-lagged relative to circulating patterns of prolactin. Several NKR-positive cell types are attractive candidates as intermediate prolactin-regulating cells. For instance, corticotroph cells strongly co-express all 3 NKR and secrete a complex range of POMC-derived gene products, some capable of driving PRL secretion. Further, folliculostellate cells (NK1R-positive) are widely distributed throughout the PD and also PT, and have been implicated in serving a role both as local pituitary stem cells and paracrine regulators [26]. Our data are thus compatible with a model in which seasonal PRL regulation represents a distinctive pituitary circuit, which is separate from that controlling TSH and reproductive changes (Figure S1). Whether such a circuit operates in birds is yet to be ascertained.
Dual role of PT thyrotrophs in controlling seasonal hormone release
Our study now places Eya3 as the first common long-day molecular signature for birds and mammals in the PT. EYA operates both as a transcriptional co-factor and as an enzyme (tyrosine phosphatase) [27]. The regulatory potential of such a bifunctional protein is considerable; Eya3 has an important role in photoreceptor differentiation and operates as part of an evolutionarily-conserved gene network in Drosophila and vertebrates, controlling tissue development including the eye. EYA3 has recently been shown to act as a key regulator of DNA break-point repair [28]; it is plausible to propose that long-term seasonal changes in neuroendocrine control may in part depend on local tissue photoperiod-driven re-modelling events in the PT (as demonstrated in the ependymal region of quail [29]), which within the PT may be controlled by an EYA3-driven pathway. The extent to which EYA3 might be directly involved in regulating of TAC1 and TSHβ gene expression is yet to be ascertained (Figure S1).
PRL has a unique seasonal signature in all vertebrate species as it is consistently elevated by LP stimulation [30]. As such, it operates as the only true LP-regulated hormone. In contrast, pituitary hormones regulating reproductive responses are tuned to the breeding biology of the species and may be activated in spring (rodents) or autumn (sheep). PRL is known to control the moult cycle of hair and feather follicle growth in mammals and birds, and these are strictly seasonal events with similar timing in most habitats irrespective of breeding season (Figure S1). Accordingly, the system we have defined here may operate as a form of seasonal hour-glass, driving annual cycles of PRL to track predictable photoperiod changes. Earlier studies predicted that a photoperiod regulated PRL secreting pathway should be characterized by the identification of a low molecular peptide within the PT region of the pituitary [6], and that the gene encoding this factor should be activated in a PT specific manner by photoperiodic stimulation, and in a pattern consistent with the natural rise in PRL secretion detected in peripheral blood in the weeks following stimulation by LP. A further prediction is that this candidate peptide should be capable of eliciting PRL release from primary pituitary cells in the same species. The data we present are therefore consistent with the hypothesis that tachykinins may serve as the elusive seasonal “tuberalins” driving PRL secretion. Testing the biological action of tachykinins in vivo is likely to be highly technically demanding, given the short half-life of these peptides in circulation and the need for local enzymic processing, and may require direct in vivo targeting of pituitary structures.
Highlights
Photoperiodically regulated genes at a melatonin target (PT) were identified by array
TAC1 and Eya3 were strongly induced in the PT by long photoperiods
TAC1 products (NKA, Substance P) drove prolactin release in lactotroph cells
These peptides may serve as the elusive seasonal prolactin regulator (tuberalin).
Supplementary Material
Acknowledgments
We thank Joan Docherty and her staff at the Marshall Building, University of Edinburgh for the animal care; Richard Talbot and Alison Downing the ARK-genomics facility, Roslin Institute for their support in conducting the microarray experiment; Alexander C West, University of Manchester, for his assistance with tissue collection and Ben RC Saer, University of Manchester, for his help with tissue dissection and in situ hybridization experiments. The work was supported by a grant from the Biotechnology and Biological Sciences Research Council (UK) awarded to AL, JD and DB and by a National Institute of Health grant (USA) awarded to RLG.
Funding: This work was supported by the BBSRC (grant G003033/1 to AL, JRE and DB) and by the NIH (grant to RLG, NIH RO1 HD017864).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 2.Morgan PJ, Hazlerigg DG. Photoperiodic signalling through the melatonin receptor turns full circle. J Neuroendocrinol. 2008;20:820–826. doi: 10.1111/j.1365-2826.2008.01724.x. [DOI] [PubMed] [Google Scholar]
- 3.Hazlerigg D, Loudon A. New insights into ancient seasonal life timers. Curr Biol. 2008;18:R795–R804. doi: 10.1016/j.cub.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- 5.Graham ES, Webster CA, Hazlerigg DG, Morgan PJ. Evidence for the biosynthesis of a prolactin-releasing factor from the ovine pars tuberalis, which is distinct from thyrotropin-releasing hormone. J Neuroendocrinol. 2002;14:945–954. doi: 10.1046/j.1365-2826.2002.00848.x. [DOI] [PubMed] [Google Scholar]
- 6.Morgan PJ, Webster CA, Mercer JG, Ross AW, Hazlerigg DG, MacLean A, Barrett P. The ovine pars tuberalis secretes a factor(s) that regulates gene expression in both lactotropic and nonlactotropic pituitary cells. Endocrinology. 1996b;137:4018–4026. doi: 10.1210/endo.137.9.8756579. [DOI] [PubMed] [Google Scholar]
- 7.Stirland JA, Johnston JD, Cagampang FRA, Morgan PJ, Castro MG, White MRH, Davis JRE, Loudon ASI. Photoperiodic regulation of prolactin gene expression in the Syrian hamster by a pars tuberalis-derived factor. J Neuroendocrinol. 2001;13:147–157. doi: 10.1046/j.1365-2826.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- 9.Hazlerigg DG, Andersson H, Johnston JD, Lincoln G. Molecular characterization of the long-day response in the Soay sheep, a seasonal mammal. Curr Biol. 2004;14:334–339. doi: 10.1016/j.cub.2004.01.057. [DOI] [PubMed] [Google Scholar]
- 10.Lincoln GA, Clarke IJ. Photoperiodically-induced cycles in the secretion of prolactin in hypothalamo-pituitary disconnected rams: evidence for translation of the melatonin signal in the pituitary gland. J Neuroendocrinol. 1994;6:251–260. doi: 10.1111/j.1365-2826.1994.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 11.Dupre SM, Burt DW, Talbot R, Downing A, Mouzaki D, Waddington D, Malpaux B, Davis JR, Lincoln GA, Loudon AS. Identification of melatonin-regulated genes in the ovine pituitary pars tuberalis, a target site for seasonal hormone control. Endocrinology. 2008;149:5527–5539. doi: 10.1210/en.2008-0834. [DOI] [PubMed] [Google Scholar]
- 12.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 13.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626. [DOI] [PubMed] [Google Scholar]
- 14.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lincoln GA, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals--a unifying hypothesis. J Endocrinol. 2003;179:1–13. doi: 10.1677/joe.0.1790001. [DOI] [PubMed] [Google Scholar]
- 16.Page NM. New challenges in the study of the mammalian tachykinins. Peptides. 2005;26:1356–1368. doi: 10.1016/j.peptides.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Debeljuk L, Lasaga M. Tachykinins and the control of prolactin secretion. Peptides. 2006;27:3007–3019. doi: 10.1016/j.peptides.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Gautreau A, Duval P, Kerdelhue B. Variations in levels of substance P-encoding beta-, gamma-preprotachykinin and substance P receptor NK-1 transcripts in the rat hypothalamus throughout the estrous cycle: a correlation between amounts of beta-preprotachykinin and NK-1 mRNA. Brain Res Mol Brain Res. 1997;49:157–164. doi: 10.1016/s0169-328x(97)00140-x. [DOI] [PubMed] [Google Scholar]
- 19.Hurd YL, Keller E, Sotonyi P, Sedvall G. Preprotachykinin-A mRNA expression in the human and monkey brain: An in situ hybridization study. J Comp Neurol. 1999;411:56–72. doi: 10.1002/(sici)1096-9861(19990816)411:1<56::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Skinner DC, Lang AL, Pahl L, Wang Q. Substance P-immunoreactive cells in the ovine pars tuberalis. Neuroendocrinology. 2009;89:3–8. doi: 10.1159/000167797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skinner DC. Rethinking the stalk effect: a new hypothesis explaining suprasellar tumor-induced hyperprolactinemia. Med Hypotheses. 2009;72:309–310. doi: 10.1016/j.mehy.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall ME, Miley F, Stewart JM. The role of enzymatic processing in the biological actions of substance P. Peptides. 1989;10:895–901. doi: 10.1016/0196-9781(89)90132-0. [DOI] [PubMed] [Google Scholar]
- 23.Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Pisera D, Candolfi M, De Laurentiis A, Seilicovich A. Characterization of tachykinin NK2 receptor in the anterior pituitary gland. Life Sci. 2003;73:2421–2432. doi: 10.1016/s0024-3205(03)00650-7. [DOI] [PubMed] [Google Scholar]
- 25.Wallace JM, Martin GB, McNeilly AS. Changes in the secretion of LH pulses, FSH and prolactin during the preovulatory phase of the oestrous cycle of the ewe and the influence of treatment with bovine follicular fluid during the luteal phase. J Endocrinol. 1988;116:123–135. doi: 10.1677/joe.0.1160123. [DOI] [PubMed] [Google Scholar]
- 26.Devnath S, Inoue K. An insight to pituitary folliculo-stellate cells. J Neuroendocrinol. 2008;20:687–691. doi: 10.1111/j.1365-2826.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 27.Rebay I, Silver SJ, Tootle TL. New vision from Eyes absent: transcription factors as enzymes. Trends Genet. 2005;21:163–171. doi: 10.1016/j.tig.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Lukas J, Bartek J. DNA repair: New tales of an old tail. Nature. 2009;458:581–583. doi: 10.1038/458581a. [DOI] [PubMed] [Google Scholar]
- 29.Yamamura T, Yasuo S, Hirunagi K, Ebihara S, Yoshimura T. T(3) implantation mimics photoperiodically reduced encasement of nerve terminals by glial processes in the median eminence of Japanese quail. Cell Tissue Res. 2006;324:175–179. doi: 10.1007/s00441-005-0126-8. [DOI] [PubMed] [Google Scholar]
- 30.Curlewis JD. Seasonal prolactin secretion and its role in seasonal reproduction: a review. Reprod Fertil Dev. 1992;4:1–23. doi: 10.1071/rd9920001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.